Chemistry Lab Report: A Practical Investigation of Physical Properties
VerifiedAdded on 2023/06/13
|11
|2673
|211
Practical Assignment
AI Summary
This chemistry lab report details a practical investigation into the physical properties of various substances. The experiments cover the effect of a charged rod on liquids, miscibility of liquids, and the solubility of iodine, powdered graphite, and calcium chloride in different solvents (water, ethanol, and hexane). The report discusses the principles behind polarity, volatility, and solubility, relating them to the observed behaviors of the substances. Key findings include the deflection of polar liquids by a charged rod, the miscibility of polar liquids, and the solubility of different substances in polar and non-polar solvents. The report concludes by emphasizing the importance of accurate measurements and proper procedures in achieving reliable results and highlights the connection between a substance's physical properties and its molecular structure and bonding.

1
Practical
Investigation of physical properties of selected substances
Chemistry Lab Report
Objective
The main objective of this experiment is to investigate the physical properties of different
substances using different tests. Moreover, the test will examine the effect of the substances
on the solubility of these substances, the effect of charged rod upon the streams substance.
Each experiment is discussed in detail.
Aim
To perform some key tests to test both reducing and non-reducing sugars and classify
indentified samples of substances.
Introduction
Different substances have different physical and chemical properties which make
them have different reactions with water, ethanol and hexane. Investigating different physical
properties of the given substance helps to understand their ability to bear these different
reactions. With the help of different equipments and substances this experiment will help to
classify the different substances through the different results for the different substances. The
experiments in this practical will be able to investigate polarity, volatility and solubility of
some of these specific substances and help to understand the differences of the different
substances.
The physical properties of any substances depend on its structure and bonding which
is present in them. When investigating the physical properties measurement of the solubility
of substances in different liquids, volatility and polarity are usually carried out. Different
compounds have different bonding such as sodium chloride has ionic bond in them. Some
compounds have covalent bonds. In the case of covalent bond is the degree of polarity can
Practical
Investigation of physical properties of selected substances
Chemistry Lab Report
Objective
The main objective of this experiment is to investigate the physical properties of different
substances using different tests. Moreover, the test will examine the effect of the substances
on the solubility of these substances, the effect of charged rod upon the streams substance.
Each experiment is discussed in detail.
Aim
To perform some key tests to test both reducing and non-reducing sugars and classify
indentified samples of substances.
Introduction
Different substances have different physical and chemical properties which make
them have different reactions with water, ethanol and hexane. Investigating different physical
properties of the given substance helps to understand their ability to bear these different
reactions. With the help of different equipments and substances this experiment will help to
classify the different substances through the different results for the different substances. The
experiments in this practical will be able to investigate polarity, volatility and solubility of
some of these specific substances and help to understand the differences of the different
substances.
The physical properties of any substances depend on its structure and bonding which
is present in them. When investigating the physical properties measurement of the solubility
of substances in different liquids, volatility and polarity are usually carried out. Different
compounds have different bonding such as sodium chloride has ionic bond in them. Some
compounds have covalent bonds. In the case of covalent bond is the degree of polarity can
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
2
affect the properties of the substances (Zumdahl & Zumdahl, 2013). These bonds are able to
bring out the different results when these substances react with water, ethanol and hexane.
The sharing of electrons is able to affect the bonding of the substances, which in turn affect
their properties.
Substances which are provided are water, hexane, ethanol, calcium chloride, powder
graphite and iodine. Solubility of the different substances is checked and results will be
mentioned. Affects of charge rod is also measured and results will be mentioned.
Materials and Equipments
The purpose of taking these substances is that they are related to plastic and rubber family
and hence the study and effect of these substances is very easy to see the effect.
Equipments Materials
Polythene Rod and fur – for investigating
charge conductivity
Water – used for solubility purposes
Three spatulas – used to scoop and measure
the substances
Hexane – substance to be tested for
3 burettes – pipeting the solubility liquid
used.
Ethanol – substance to be tested
3 beakers (100ml) – for mixing up the
substances
Iodine
Timer (stop clock ) – for recording the time
of reaction of substances
Powder graphite
Methods
Experiment 1: The Effect of a Charged Rod on Thin Streams of Liquid
Procedure:
First fill the clean burettes with the liquids such as water, hexane and ethanol in
different burettes.
affect the properties of the substances (Zumdahl & Zumdahl, 2013). These bonds are able to
bring out the different results when these substances react with water, ethanol and hexane.
The sharing of electrons is able to affect the bonding of the substances, which in turn affect
their properties.
Substances which are provided are water, hexane, ethanol, calcium chloride, powder
graphite and iodine. Solubility of the different substances is checked and results will be
mentioned. Affects of charge rod is also measured and results will be mentioned.
Materials and Equipments
The purpose of taking these substances is that they are related to plastic and rubber family
and hence the study and effect of these substances is very easy to see the effect.
Equipments Materials
Polythene Rod and fur – for investigating
charge conductivity
Water – used for solubility purposes
Three spatulas – used to scoop and measure
the substances
Hexane – substance to be tested for
3 burettes – pipeting the solubility liquid
used.
Ethanol – substance to be tested
3 beakers (100ml) – for mixing up the
substances
Iodine
Timer (stop clock ) – for recording the time
of reaction of substances
Powder graphite
Methods
Experiment 1: The Effect of a Charged Rod on Thin Streams of Liquid
Procedure:
First fill the clean burettes with the liquids such as water, hexane and ethanol in
different burettes.

3
Then rub the polythene rod on the fur, the fur will charge the rod.
Then place the rod near the opening of the burette of water, watch closely if the thin
layer of water show any respond to the charge rod of polythene.
Repeat the above three activities with the burettes filled with hexane and ethanol.
Experiment 2: Miscibility of liquids
Procedure:
First prepare the mixture of substance with equal amount of liquids 1cm3 in test tube.
Samples are: water and ethanol, water and hexane, hexane and ethanol.
Watch closely, if the pair of the liquids is miscible.
NB: Time will be a key factor to record the different changes which will happen on
the liquids at different times.
Experiment 3: The Solubility of Iodine in Different Liquids
Procedure:
First with the help of spatula, put small crystal of iodine in the test tube.
Then add distilled water in the test tube about 5cm3 and place a stopper on the test
tube.
Shake the test tube and note the time of shaking, let’s say 20 seconds.
Some of the iodine will dissolve in the water, note the colour if changed.
Repeat the above steps using hexane and ethanol as a liquid, instead of water.
Time will be essential to help determine time taken for iodine to dissolve in the
different liquids. Record the time when the iodine dissolves completely in the specific
liquids.
Experiment 4: The Solubility of Powdered Graphite in Different Liquids
Procedure:
Then rub the polythene rod on the fur, the fur will charge the rod.
Then place the rod near the opening of the burette of water, watch closely if the thin
layer of water show any respond to the charge rod of polythene.
Repeat the above three activities with the burettes filled with hexane and ethanol.
Experiment 2: Miscibility of liquids
Procedure:
First prepare the mixture of substance with equal amount of liquids 1cm3 in test tube.
Samples are: water and ethanol, water and hexane, hexane and ethanol.
Watch closely, if the pair of the liquids is miscible.
NB: Time will be a key factor to record the different changes which will happen on
the liquids at different times.
Experiment 3: The Solubility of Iodine in Different Liquids
Procedure:
First with the help of spatula, put small crystal of iodine in the test tube.
Then add distilled water in the test tube about 5cm3 and place a stopper on the test
tube.
Shake the test tube and note the time of shaking, let’s say 20 seconds.
Some of the iodine will dissolve in the water, note the colour if changed.
Repeat the above steps using hexane and ethanol as a liquid, instead of water.
Time will be essential to help determine time taken for iodine to dissolve in the
different liquids. Record the time when the iodine dissolves completely in the specific
liquids.
Experiment 4: The Solubility of Powdered Graphite in Different Liquids
Procedure:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
With the help of spatula, put small amount of powder graphite in the test tube.
Add distilled water in the test tube about 5cm3 and place a stopper on the test tube.
Shake the test tube and note the time of shaking, let’s say 20 seconds.
Powder graphite will dissolve in the water, note the colour if changed.
Repeat the procedure using hexane and ethanol as a liquid, instead of water.
Time will be an essential factor in determining the time taken by graphite to dissolve
in different liquids.
Experiment 5: The Solubility of Calcium Chloride in Different Liquids
Procedure:
With the help of spatula, put small amount of calcium chloride in the test tube.
Add distilled water in the test tube about 5cm3 and place a stopper on the test tube.
Shake the test tube and note the time of shaking, let’s say 20 seconds.
Calcium chloride will dissolve in the water and the solution is colourless.
Repeat the procedure using hexane and ethanol as a liquid, the solution with hexane
and ethanol is colourless too.
To measure the solubility, pour the solutions in new test tubes, left the excess amount
of calcium chloride in it, and add 2cm3 of 0.1M silver nitrate place the stopper and
shake the test tube.
The amount of silver nitrate precipitated, will provide the results of the solubility of
the calcium nitrate in the solutions.
Time is a key factor on the formation of silver nitrate precipitate. Different amount
will be formed at different times and this will determine the peak of the solubility
reaction.
Experiment 6: (Sublime) from Solid to Gas and it changed to purple suplane
With the help of spatula, put small amount of powder graphite in the test tube.
Add distilled water in the test tube about 5cm3 and place a stopper on the test tube.
Shake the test tube and note the time of shaking, let’s say 20 seconds.
Powder graphite will dissolve in the water, note the colour if changed.
Repeat the procedure using hexane and ethanol as a liquid, instead of water.
Time will be an essential factor in determining the time taken by graphite to dissolve
in different liquids.
Experiment 5: The Solubility of Calcium Chloride in Different Liquids
Procedure:
With the help of spatula, put small amount of calcium chloride in the test tube.
Add distilled water in the test tube about 5cm3 and place a stopper on the test tube.
Shake the test tube and note the time of shaking, let’s say 20 seconds.
Calcium chloride will dissolve in the water and the solution is colourless.
Repeat the procedure using hexane and ethanol as a liquid, the solution with hexane
and ethanol is colourless too.
To measure the solubility, pour the solutions in new test tubes, left the excess amount
of calcium chloride in it, and add 2cm3 of 0.1M silver nitrate place the stopper and
shake the test tube.
The amount of silver nitrate precipitated, will provide the results of the solubility of
the calcium nitrate in the solutions.
Time is a key factor on the formation of silver nitrate precipitate. Different amount
will be formed at different times and this will determine the peak of the solubility
reaction.
Experiment 6: (Sublime) from Solid to Gas and it changed to purple suplane
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
Procedure:
In the fume cupboard, heat few crystals of the substance to be sublimed in a test tube.
Make the content contact for about 1-2 min with hot surface of hot plate instrument.
Without using any liquid phase, the Sublime is changed from Solid to Gas
Solid to gas process can be reversed to make it back gas from solid
Results and Discussion
It is seen that the materials used in the experiments have the capability to change
themselves in the gas state without coming into the liquid state because the forces present
inside the material and the spaces of the atoms are not come into the packed state as that in
liquid. Water, hexane and ethanol have different molecules and bonding. These are some of
key characteristics which were able to help in classifying the different substances, since they
will have different reaction with these key liquids. The attraction and separation of the
different substances depend on the molecules which the substances have and the type oftion
of bonding available. In addition, the experiment was able to ensure that different substances
were indentified correct according to their reaction with water, ethanol and hexane.
Moreover, the classification of different molecules was done to enhance the understanding of
the reaction of the different substances. Polar and non-polar molecules were clearly defined
from the experiments.
Experiment 1:
Water molecules were able to produce some deflection while hexane and ethanol did not.
substance Deflection
1 Water Yes
2 Hexane No deflection
3 Ethanol Deflects to the right
Procedure:
In the fume cupboard, heat few crystals of the substance to be sublimed in a test tube.
Make the content contact for about 1-2 min with hot surface of hot plate instrument.
Without using any liquid phase, the Sublime is changed from Solid to Gas
Solid to gas process can be reversed to make it back gas from solid
Results and Discussion
It is seen that the materials used in the experiments have the capability to change
themselves in the gas state without coming into the liquid state because the forces present
inside the material and the spaces of the atoms are not come into the packed state as that in
liquid. Water, hexane and ethanol have different molecules and bonding. These are some of
key characteristics which were able to help in classifying the different substances, since they
will have different reaction with these key liquids. The attraction and separation of the
different substances depend on the molecules which the substances have and the type oftion
of bonding available. In addition, the experiment was able to ensure that different substances
were indentified correct according to their reaction with water, ethanol and hexane.
Moreover, the classification of different molecules was done to enhance the understanding of
the reaction of the different substances. Polar and non-polar molecules were clearly defined
from the experiments.
Experiment 1:
Water molecules were able to produce some deflection while hexane and ethanol did not.
substance Deflection
1 Water Yes
2 Hexane No deflection
3 Ethanol Deflects to the right
6
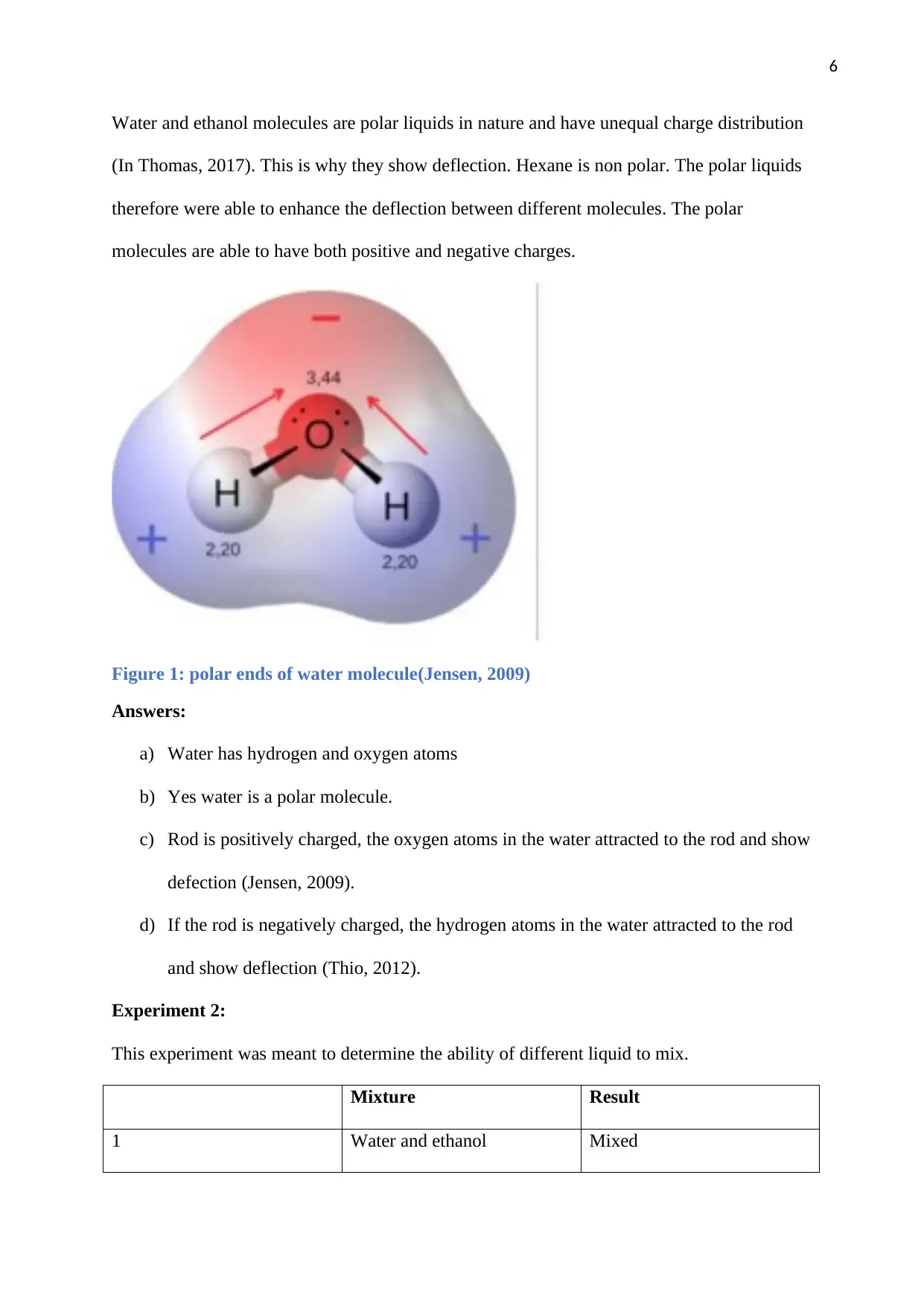
Water and ethanol molecules are polar liquids in nature and have unequal charge distribution
(In Thomas, 2017). This is why they show deflection. Hexane is non polar. The polar liquids
therefore were able to enhance the deflection between different molecules. The polar
molecules are able to have both positive and negative charges.
Figure 1: polar ends of water molecule(Jensen, 2009)
Answers:
a) Water has hydrogen and oxygen atoms
b) Yes water is a polar molecule.
c) Rod is positively charged, the oxygen atoms in the water attracted to the rod and show
defection (Jensen, 2009).
d) If the rod is negatively charged, the hydrogen atoms in the water attracted to the rod
and show deflection (Thio, 2012).
Experiment 2:
This experiment was meant to determine the ability of different liquid to mix.
Mixture Result
1 Water and ethanol Mixed
Water and ethanol molecules are polar liquids in nature and have unequal charge distribution
(In Thomas, 2017). This is why they show deflection. Hexane is non polar. The polar liquids
therefore were able to enhance the deflection between different molecules. The polar
molecules are able to have both positive and negative charges.
Figure 1: polar ends of water molecule(Jensen, 2009)
Answers:
a) Water has hydrogen and oxygen atoms
b) Yes water is a polar molecule.
c) Rod is positively charged, the oxygen atoms in the water attracted to the rod and show
defection (Jensen, 2009).
d) If the rod is negatively charged, the hydrogen atoms in the water attracted to the rod
and show deflection (Thio, 2012).
Experiment 2:
This experiment was meant to determine the ability of different liquid to mix.
Mixture Result
1 Water and ethanol Mixed
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
7
2 Water and hexane Not mixed
3 Hexane and ethanol Mixed
Water and ethanol are polar liquids in nature, the oxygen in the water attracts the hydrogen
which is present in the ethanol and packed the hydrogen in oxygen thus mixing happen.
Hexane is non polar that’s why it does not mix with water. Hexane and ethanol mix due to
their low concentrations (Jeew-m, 2012).
Experiment 3:
This experiment was able to measure the color changes for different substances are mixed
with water.
Mixture Color change
1 Water and iodine Colour changed to brown when shaken
2 Water and ethanol Colour changes to brown
3 Water and hexane Colour changed to purple
Iodine is non polar and has violet color. When mix with water its complex mixture observe
the light and change the color to brown (Z., 2016). Same thing is happening with the ethanol
and hexane and show different color when observe light. The polar attraction and deflection
are able to imitate the colour changes when reaction takes place.
2 Water and hexane Not mixed
3 Hexane and ethanol Mixed
Water and ethanol are polar liquids in nature, the oxygen in the water attracts the hydrogen
which is present in the ethanol and packed the hydrogen in oxygen thus mixing happen.
Hexane is non polar that’s why it does not mix with water. Hexane and ethanol mix due to
their low concentrations (Jeew-m, 2012).
Experiment 3:
This experiment was able to measure the color changes for different substances are mixed
with water.
Mixture Color change
1 Water and iodine Colour changed to brown when shaken
2 Water and ethanol Colour changes to brown
3 Water and hexane Colour changed to purple
Iodine is non polar and has violet color. When mix with water its complex mixture observe
the light and change the color to brown (Z., 2016). Same thing is happening with the ethanol
and hexane and show different color when observe light. The polar attraction and deflection
are able to imitate the colour changes when reaction takes place.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
8
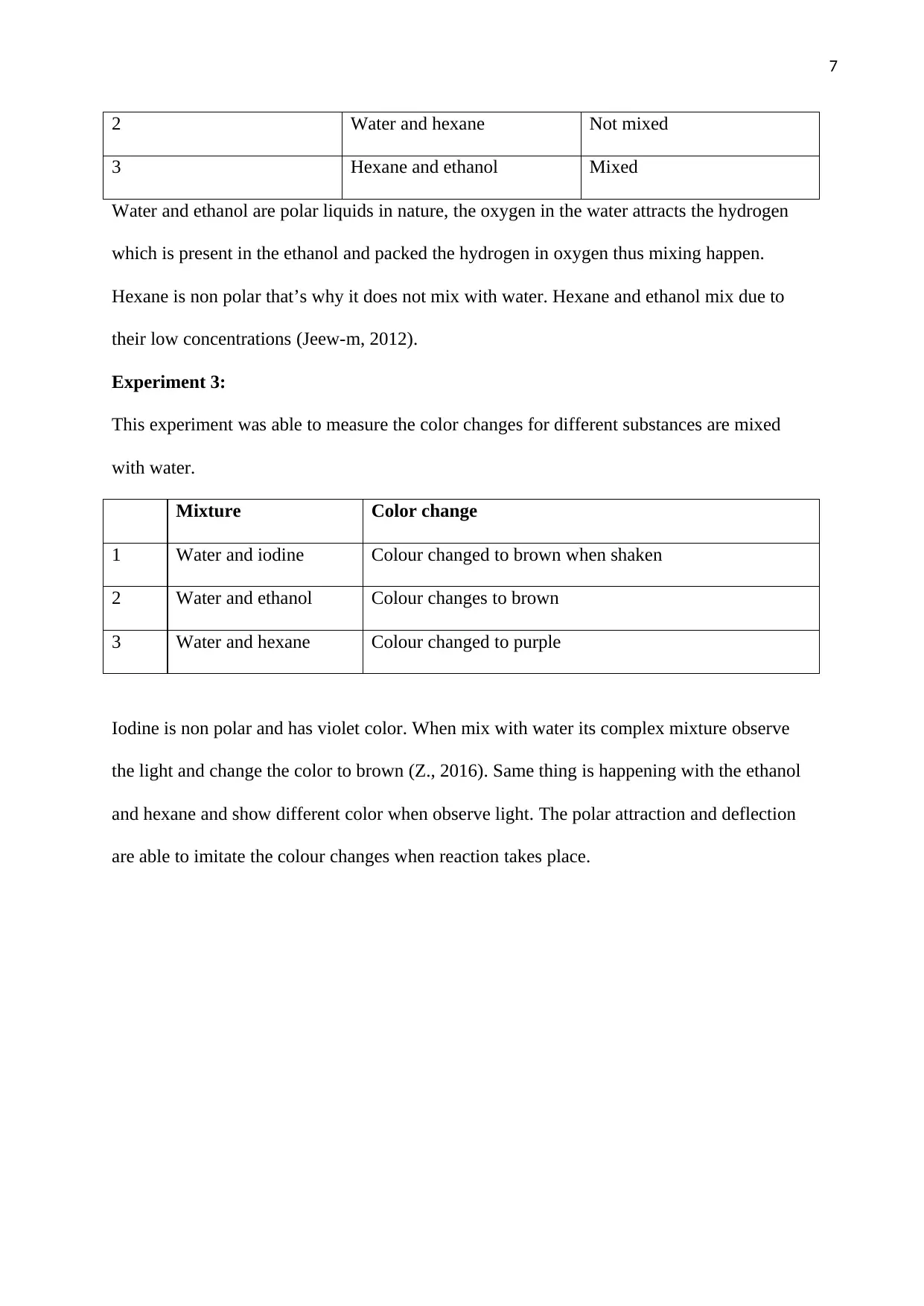
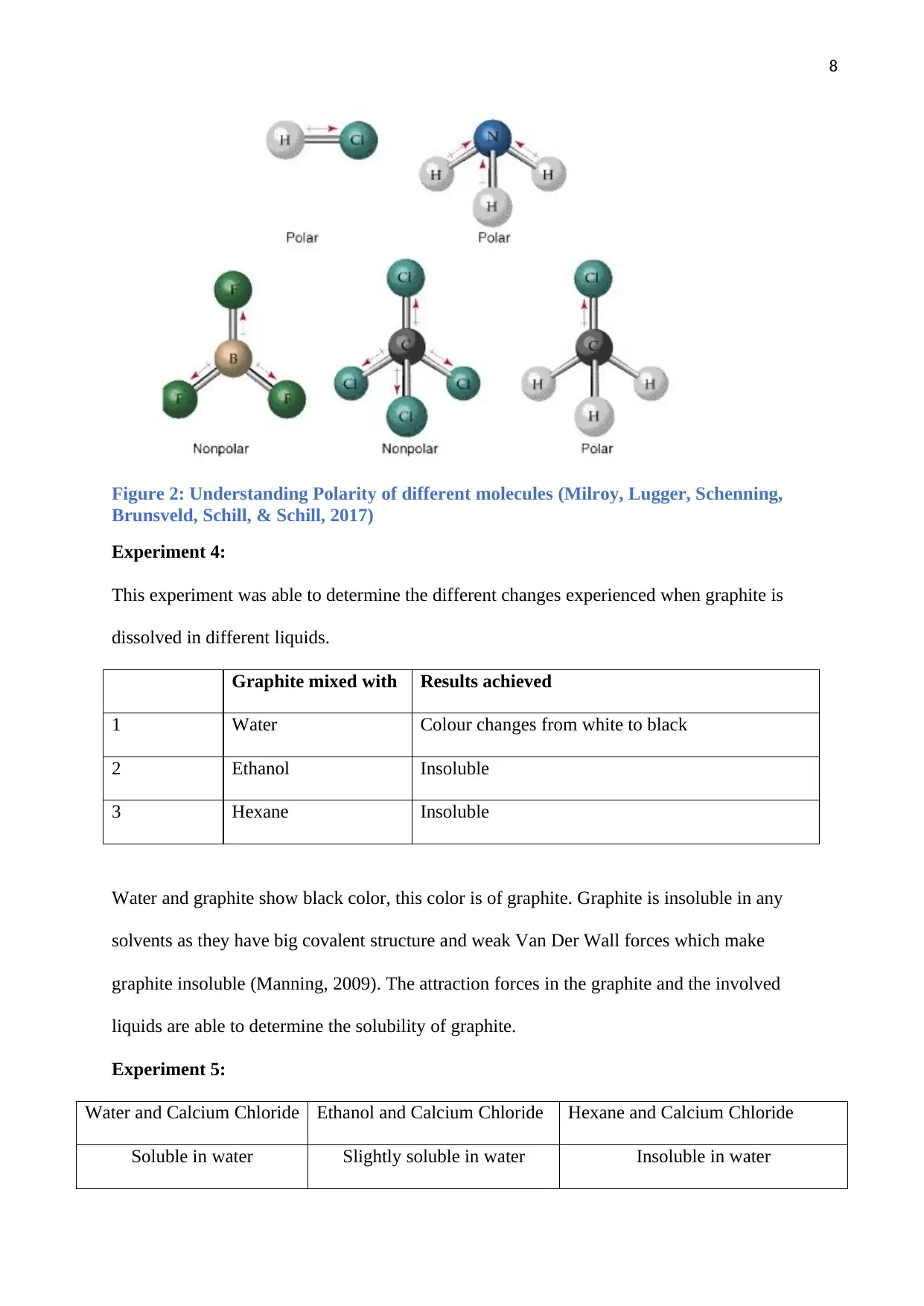
Figure 2: Understanding Polarity of different molecules (Milroy, Lugger, Schenning,
Brunsveld, Schill, & Schill, 2017)
Experiment 4:
This experiment was able to determine the different changes experienced when graphite is
dissolved in different liquids.
Graphite mixed with Results achieved
1 Water Colour changes from white to black
2 Ethanol Insoluble
3 Hexane Insoluble
Water and graphite show black color, this color is of graphite. Graphite is insoluble in any
solvents as they have big covalent structure and weak Van Der Wall forces which make
graphite insoluble (Manning, 2009). The attraction forces in the graphite and the involved
liquids are able to determine the solubility of graphite.
Experiment 5:
Water and Calcium Chloride Ethanol and Calcium Chloride Hexane and Calcium Chloride
Soluble in water Slightly soluble in water Insoluble in water
Figure 2: Understanding Polarity of different molecules (Milroy, Lugger, Schenning,
Brunsveld, Schill, & Schill, 2017)
Experiment 4:
This experiment was able to determine the different changes experienced when graphite is
dissolved in different liquids.
Graphite mixed with Results achieved
1 Water Colour changes from white to black
2 Ethanol Insoluble
3 Hexane Insoluble
Water and graphite show black color, this color is of graphite. Graphite is insoluble in any
solvents as they have big covalent structure and weak Van Der Wall forces which make
graphite insoluble (Manning, 2009). The attraction forces in the graphite and the involved
liquids are able to determine the solubility of graphite.
Experiment 5:
Water and Calcium Chloride Ethanol and Calcium Chloride Hexane and Calcium Chloride
Soluble in water Slightly soluble in water Insoluble in water

9
Milky white Milky white No change
Results
It is seen that polar dissolves polar substances and non-polar substances dissolves non-polar
substances. Concluding like dissolves like.
Experiment 6:
(Sublime) from Solid to Gas and it changed to purple suplane.
The sublimation is a physical process which show that a solid transformed to gas state
directly without passing at liquid state. The properties of the specific molecules of iodine are
responsible for the sublimation factor. The van der walls forces are responsible for this state
of the substance (Nibler, Garland, Stine, & Kim, 2014). They are much weak and therefore
do not allow the transition of the material in the liquid state.
Conclusion
The experiment was able to achieve the classification of the different substances and
also understanding their reactions with different liquids. Different substances show different
physical properties and treated with the water, hexane and ethanol. These are due to the
nature of polarity and non-polarity. The experiments in this chemistry lab were able to show
the reactions of the substances in different liquids. In conclusion, I was able to learn different
aspects of these substances along with their properties. It is important to note that there were
few strengths & weaknesses for every experiment, but still things were good enough to learn
that what physical properties are carried by these substances. Correct measurement of the
substances is a key error which can affect the experiment. This is because the quantity of the
amount of reacting substance is important when carrying out the experiment. Each practical
experiments can be improved in future experiments by making sure that previous mistakes
are not repeated and steps are followed as per given guidelines. Using other liquids can be
used to improve the understanding of substances reaction. In the end, it is necessary to
Milky white Milky white No change
Results
It is seen that polar dissolves polar substances and non-polar substances dissolves non-polar
substances. Concluding like dissolves like.
Experiment 6:
(Sublime) from Solid to Gas and it changed to purple suplane.
The sublimation is a physical process which show that a solid transformed to gas state
directly without passing at liquid state. The properties of the specific molecules of iodine are
responsible for the sublimation factor. The van der walls forces are responsible for this state
of the substance (Nibler, Garland, Stine, & Kim, 2014). They are much weak and therefore
do not allow the transition of the material in the liquid state.
Conclusion
The experiment was able to achieve the classification of the different substances and
also understanding their reactions with different liquids. Different substances show different
physical properties and treated with the water, hexane and ethanol. These are due to the
nature of polarity and non-polarity. The experiments in this chemistry lab were able to show
the reactions of the substances in different liquids. In conclusion, I was able to learn different
aspects of these substances along with their properties. It is important to note that there were
few strengths & weaknesses for every experiment, but still things were good enough to learn
that what physical properties are carried by these substances. Correct measurement of the
substances is a key error which can affect the experiment. This is because the quantity of the
amount of reacting substance is important when carrying out the experiment. Each practical
experiments can be improved in future experiments by making sure that previous mistakes
are not repeated and steps are followed as per given guidelines. Using other liquids can be
used to improve the understanding of substances reaction. In the end, it is necessary to
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

10
mention that physical properties of these substances are good enough to give certain
classification to them. The results obtained were quite up to the mark and it is seen for the
sublimation process that the substances that show sublimation process are much likely to
show more abrupt change of phase that we cannot see the time in which the liquid state
appears. The use of the known samples was able to help determining the macromolecules on
the unknown samples and therefore helped in classifying the unknown samples.
mention that physical properties of these substances are good enough to give certain
classification to them. The results obtained were quite up to the mark and it is seen for the
sublimation process that the substances that show sublimation process are much likely to
show more abrupt change of phase that we cannot see the time in which the liquid state
appears. The use of the known samples was able to help determining the macromolecules on
the unknown samples and therefore helped in classifying the unknown samples.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

11
References
In Thomas, T. (2017). Covalent Bonds. Albuquerque, NM : World Weaver Press.
Jeew-m. (2012, Aug 12). why is it when you mix 50mL of water and 50mL of ethanol alcohal it doesn't
equal 100mL?my chemistry teacher asked us for 3 reasons why we think so. Retrieved from
https://www.enotes.com/homework-help/why-when-you-mix-50ml-water-50ml-ethanol-alcohal-
353421
Jensen, W. B. (2009). "The Origin of the "Delta" Symbol for Fractional Charges". (Vol. 86). J. Chem.
Educ.
Manning, P. (2009). Chemical Bonds. New York: Chelsea House.
Milroy, L., Lugger, J., Schenning, A., Brunsveld, L., Schill, J., & Schill, J. (2017). Relationship between
side-chain polarity and the self-assembly characteristics of perylene diimide derivatives in aqueous
solution (Vol. ChemistryOpen vol.6). date: 2017-04-01 nr.2: p.266-272 [ISSN 2191-1363].) .
Nibler, J. W., Garland, C. W., Stine, K. J., & Kim, J. E. (2014). Experiments in physical chemistry (9 ed.).
Boston: McGraw-Hill Education.
Thio, A. (2012, April 11). Why is a stream of water deflected towards a charged rod if the molecules
have an overall neutral charge? Retrieved from https://www.quora.com/Why-is-a-stream-of-water-
deflected-towards-a-charged-rod-if-the-molecules-have-an-overall-neutral-charge
Z., E. (2016, Feb 11). When Iodine (I2) is mixed with water, the water dissolves some iodine by
forming a temporary charge. Does the oxygen from water bond with the Iodine or does the
hydrogen? Retrieved from https://socratic.org/questions/when-iodine-i2-is-mixed-with-water-the-
water-dissolves-some-iodine-by-forming-a-
Zumdahl, S. S., & Zumdahl, S. A. (2013). Lab Manual for Zumdahl/Zumdahl's Chemistry, 9th (9th
Edition ed.). Cengage Learning.
References
In Thomas, T. (2017). Covalent Bonds. Albuquerque, NM : World Weaver Press.
Jeew-m. (2012, Aug 12). why is it when you mix 50mL of water and 50mL of ethanol alcohal it doesn't
equal 100mL?my chemistry teacher asked us for 3 reasons why we think so. Retrieved from
https://www.enotes.com/homework-help/why-when-you-mix-50ml-water-50ml-ethanol-alcohal-
353421
Jensen, W. B. (2009). "The Origin of the "Delta" Symbol for Fractional Charges". (Vol. 86). J. Chem.
Educ.
Manning, P. (2009). Chemical Bonds. New York: Chelsea House.
Milroy, L., Lugger, J., Schenning, A., Brunsveld, L., Schill, J., & Schill, J. (2017). Relationship between
side-chain polarity and the self-assembly characteristics of perylene diimide derivatives in aqueous
solution (Vol. ChemistryOpen vol.6). date: 2017-04-01 nr.2: p.266-272 [ISSN 2191-1363].) .
Nibler, J. W., Garland, C. W., Stine, K. J., & Kim, J. E. (2014). Experiments in physical chemistry (9 ed.).
Boston: McGraw-Hill Education.
Thio, A. (2012, April 11). Why is a stream of water deflected towards a charged rod if the molecules
have an overall neutral charge? Retrieved from https://www.quora.com/Why-is-a-stream-of-water-
deflected-towards-a-charged-rod-if-the-molecules-have-an-overall-neutral-charge
Z., E. (2016, Feb 11). When Iodine (I2) is mixed with water, the water dissolves some iodine by
forming a temporary charge. Does the oxygen from water bond with the Iodine or does the
hydrogen? Retrieved from https://socratic.org/questions/when-iodine-i2-is-mixed-with-water-the-
water-dissolves-some-iodine-by-forming-a-
Zumdahl, S. S., & Zumdahl, S. A. (2013). Lab Manual for Zumdahl/Zumdahl's Chemistry, 9th (9th
Edition ed.). Cengage Learning.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.