Analysis of Crystallization Kinetics in Metallic Glasses
VerifiedAdded on 2022/09/14
|21
|3046
|25
Report
AI Summary
This report presents a comparative study on the crystallization kinetics of Zr55All0Ni30Pd5, Zr60Al5Ni30Pd5, and Zr60All0Ni25Pd5 metallic glasses. The research utilizes Differential Scanning Calorimetry (DSC) to investigate thermal behavior and non-isothermal crystallization kinetics at varying heating rates. The study employs various thermal models, including Kissinger, Mahadevan et al., Augis and Bennett, and Ozawa-Chen methods, to calculate activation energies. The report details the experimental procedures, including alloy preparation via arc melting and melt-spinning methods. Results indicate that Zr55All0Ni30Pd5 exhibits two-stage crystallization, while the other two alloys show one-stage crystallization. Local activation energies are computed using Kissinger-Akahira-Sunose (KAS), Ozawa-Flynn-Wall (OFW), and Tang methods. The report also covers the theoretical background of non-isothermal crystallization kinetics, including the Johnson-Mehl-Avrami (JMA) model and Avrami constant calculations. The findings provide valuable insights into the glass-forming ability and crystallization behavior of these metallic glasses, which are crucial for applications in biomedical, engineering, and electronic fields. The report also includes a detailed study methodology and discussion of experimental results, highlighting the significance of crystallization kinetics in materials science.

1
PHYSICS
By Name
Course
Instructor
Institution
Location
Date
PHYSICS
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
A comparative study of glass-forming ability, crystallization kinetics Zr55 All0
Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 metallic glasses
Abstract
Differential scanning calorimetry (DSC) was used to investigate the thermal behavior
and non-isothermal crystallization kinetics of Zr55All0Ni30Pd5, Zr60Al5 Ni30Pd5 and Zr60
All0 Ni25Pd5 glassy alloy ribbons at different heating rates. It is found that
Zr55All0Ni30Pd5 metallic glass exhibits two-stage crystallization on heating while
Zr60Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 glassy alloys exhibit one-stage crystallization on
heating. Various thermal models are employed in order to calculate the activation
energy.
A comparative study of glass-forming ability, crystallization kinetics Zr55 All0
Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 metallic glasses
Abstract
Differential scanning calorimetry (DSC) was used to investigate the thermal behavior
and non-isothermal crystallization kinetics of Zr55All0Ni30Pd5, Zr60Al5 Ni30Pd5 and Zr60
All0 Ni25Pd5 glassy alloy ribbons at different heating rates. It is found that
Zr55All0Ni30Pd5 metallic glass exhibits two-stage crystallization on heating while
Zr60Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 glassy alloys exhibit one-stage crystallization on
heating. Various thermal models are employed in order to calculate the activation
energy.

3
Table of Contents
Abstract.......................................................................................................................1
Introduction................................................................................................................1
Theoretical background..............................................................................................2
Non-isothermal crystallization kinetics......................................................................2
Johnson-Mehl-Avrami (JMA) model..........................................................................4
Kissinger method........................................................................................................4
Augis and Bennett approximation method.................................................................5
Ozawa-Chen method..................................................................................................5
Local activation energy..............................................................................................6
Kissinger-Akahira-Sunose (KAS) method..................................................................6
Ozawa-Flynn-Wall (OFW) method............................................................................6
Tang method...............................................................................................................6
Avrami constant..........................................................................................................6
Non- isothermal crystallization kinetics.....................................................................6
Inconversional techniques............................................................................................................7
Study Methodology....................................................................................................8
Experimental study.....................................................................................................8
RESULTS AND DISCUSSIONS..............................................................................9
Conclusion................................................................................................................12
Table of Contents
Abstract.......................................................................................................................1
Introduction................................................................................................................1
Theoretical background..............................................................................................2
Non-isothermal crystallization kinetics......................................................................2
Johnson-Mehl-Avrami (JMA) model..........................................................................4
Kissinger method........................................................................................................4
Augis and Bennett approximation method.................................................................5
Ozawa-Chen method..................................................................................................5
Local activation energy..............................................................................................6
Kissinger-Akahira-Sunose (KAS) method..................................................................6
Ozawa-Flynn-Wall (OFW) method............................................................................6
Tang method...............................................................................................................6
Avrami constant..........................................................................................................6
Non- isothermal crystallization kinetics.....................................................................6
Inconversional techniques............................................................................................................7
Study Methodology....................................................................................................8
Experimental study.....................................................................................................8
RESULTS AND DISCUSSIONS..............................................................................9
Conclusion................................................................................................................12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
Introduction
Recently metallic glasses have received much interest due to their broad applications
in the biomedical, engineering and electronic fields. Magnetic resistance sensors,
computer memories, making surgical instruments and reinforcing elements in
concrete are some applications of these metallic glasses. The preparation of metallic
glasses can be done in a variety of ways including melt spinning, electrodeposition,
sputtering and Ion Implantation (Chiavaro, 2017). Rapid removal of heat from the
metal to produce crystallization in the alloy is the key of successfully fabricating
metallic glasses.
The motivation for using metallic glasses is due to high strength, larger elasticity,
excllent corrosion resistance, hardness and lower elastic modulus. However, some
drawbacks of metallic glasses could be a tensile ductility at room temperature, some
metallic glasses do not show glass transition and they are considered quasi-brittle
materials.
Introduction
Recently metallic glasses have received much interest due to their broad applications
in the biomedical, engineering and electronic fields. Magnetic resistance sensors,
computer memories, making surgical instruments and reinforcing elements in
concrete are some applications of these metallic glasses. The preparation of metallic
glasses can be done in a variety of ways including melt spinning, electrodeposition,
sputtering and Ion Implantation (Chiavaro, 2017). Rapid removal of heat from the
metal to produce crystallization in the alloy is the key of successfully fabricating
metallic glasses.
The motivation for using metallic glasses is due to high strength, larger elasticity,
excllent corrosion resistance, hardness and lower elastic modulus. However, some
drawbacks of metallic glasses could be a tensile ductility at room temperature, some
metallic glasses do not show glass transition and they are considered quasi-brittle
materials.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
The crystallization kinetics of amorphous alloys are studied by thermo-analytical
skills, which include differential scanning calorimetry (DSC), dilatometry (DIL) ,
thermogravimetry (TG) , and differential thermal analysis (DTA).
To detect crystallization, two methods can be basically applied, i.e. isothermal
crystallization and non-isothermal crystallization process. In isothermal case, samples
are heated up to a particular temperature and held for a particular time period for
crystallization (Calorimetry, 2016). On the other hand, in non-isothermal
crystallization, samples are continuously heated up above the crystallization
temperature at a particular rate of heating.
In this work, the kinetics parameters of Zr55 All0 Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0
Ni25Pd5 amorphous alloys have been worked out using various theoretical approaches,
which include Kissinger, Mahadevan et al., Ozawa – chen and Augis - Bennett
approximation models for non-isothermal crystallization. In addition, local activation
energies have been calculated using various approaches such as Kissinger-Akahira-
Sunose (KAS), Ozawa-Flynn-Wall (OFW) and Tang methods (Deutsche
Physikalische Gesellschaft, 2017).
Theoretical background
Non-isothermal crystallization kinetics
Metallic glasses that are bulk in nature, having unique glass-forming ability (GFA)
offer a study opportunity of crystallization kinetics in the while region of the
undercooled. The process of crystallization can be examined under isothermal and
non-isothermal circumstances.
Non-isothermal research can be done faster and quite easily than the isothermal
researches. Furthermore, they offer smaller ratio on signal-tonoise for kinetic
researches. Therefore, in order to study the process of crystallization under non-
The crystallization kinetics of amorphous alloys are studied by thermo-analytical
skills, which include differential scanning calorimetry (DSC), dilatometry (DIL) ,
thermogravimetry (TG) , and differential thermal analysis (DTA).
To detect crystallization, two methods can be basically applied, i.e. isothermal
crystallization and non-isothermal crystallization process. In isothermal case, samples
are heated up to a particular temperature and held for a particular time period for
crystallization (Calorimetry, 2016). On the other hand, in non-isothermal
crystallization, samples are continuously heated up above the crystallization
temperature at a particular rate of heating.
In this work, the kinetics parameters of Zr55 All0 Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0
Ni25Pd5 amorphous alloys have been worked out using various theoretical approaches,
which include Kissinger, Mahadevan et al., Ozawa – chen and Augis - Bennett
approximation models for non-isothermal crystallization. In addition, local activation
energies have been calculated using various approaches such as Kissinger-Akahira-
Sunose (KAS), Ozawa-Flynn-Wall (OFW) and Tang methods (Deutsche
Physikalische Gesellschaft, 2017).
Theoretical background
Non-isothermal crystallization kinetics
Metallic glasses that are bulk in nature, having unique glass-forming ability (GFA)
offer a study opportunity of crystallization kinetics in the while region of the
undercooled. The process of crystallization can be examined under isothermal and
non-isothermal circumstances.
Non-isothermal research can be done faster and quite easily than the isothermal
researches. Furthermore, they offer smaller ratio on signal-tonoise for kinetic
researches. Therefore, in order to study the process of crystallization under non-

6
isothermal situation, different theoretical models and approximations have been
suggested (Gabbott, 2017). Crystallization kinetics based on the Zr metallic glasses
can be agreeable by two techniques, which are isokinetic and isoconversional
techniques. Isokinetic techniques, they are also referred to as model-dependent
techniques. Are dependents of various models of reaction, and mechanism of
transformation is constant with temperature and time.
Isoconversional techniques also referred to as model free technique, their mechanisms
of transformation ranges with conversion degree. Various parameters of kinetics can
be assessed by the two methods. From the time Zr-based metallic glasses were
discovered, efforts have been made to agree on their stability on thermal and GFA
against crystallization (Gabbott, 2017). A lot of studies and information about kinetics
’crystallization in isothermal and non-isothermal situations for Zr-based metallic
alloys are obtainable in the writings.
In the writings, Qiao and Pelletier have carried out research on crystallization of Zr55
All0 Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 . metallic glass. They have used
isochronal and isothermal methods. Studies of the above kinetics have also been
conducted showing that the process of crystallization is controlled by diffusion with
three dimensional growths.
Johnson-Mehl-Avrami (JMA) model
The crystallization kinetics of metallic glasses has been investigated through the use
of classical Johnson-Mehl-Avrami (JMA) model in which the crystallization function
(x) can be defined as a function of time to the relationship:
x (t )=1−exp [− ( Kt )n ]
isothermal situation, different theoretical models and approximations have been
suggested (Gabbott, 2017). Crystallization kinetics based on the Zr metallic glasses
can be agreeable by two techniques, which are isokinetic and isoconversional
techniques. Isokinetic techniques, they are also referred to as model-dependent
techniques. Are dependents of various models of reaction, and mechanism of
transformation is constant with temperature and time.
Isoconversional techniques also referred to as model free technique, their mechanisms
of transformation ranges with conversion degree. Various parameters of kinetics can
be assessed by the two methods. From the time Zr-based metallic glasses were
discovered, efforts have been made to agree on their stability on thermal and GFA
against crystallization (Gabbott, 2017). A lot of studies and information about kinetics
’crystallization in isothermal and non-isothermal situations for Zr-based metallic
alloys are obtainable in the writings.
In the writings, Qiao and Pelletier have carried out research on crystallization of Zr55
All0 Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 . metallic glass. They have used
isochronal and isothermal methods. Studies of the above kinetics have also been
conducted showing that the process of crystallization is controlled by diffusion with
three dimensional growths.
Johnson-Mehl-Avrami (JMA) model
The crystallization kinetics of metallic glasses has been investigated through the use
of classical Johnson-Mehl-Avrami (JMA) model in which the crystallization function
(x) can be defined as a function of time to the relationship:
x (t )=1−exp [− ( Kt )n ]
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
Where n is the Avrami index, K is the reaction constant rate which is generally
expressed by Arrhenius equation
K= Ko exp (−Ec
RT )
where Ko is the frequency factor, Ec is the crystal activation energy, R is the constant
universal gas and T is the absolute temperature.
Established through JMA model, various theoretical methods have been
established to study the crystallization kinetics of amorphous alloys (Mike Reading,
2016).
Herein some of the methods that are used to determine the crystallization kinetics of
Zr55 All0 Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 metallic glasses using the non-
isothermal DSC measurements.
Kissinger method
The Kissinger equation is one of the most often used equations to work out the
energy used in activating crystallization, which is given by
ln ( α
T p
2 ) =−Ec
R T p
+C
Where is the amount of heating applied during the experiment, Tp is the
crystallization ultimate temperature, Ec is the activation energy of the crystallization
and C is a constant.
Mahadevan et al. method
According to the rough calculation of Mahadevan et al. the variation of the
ultimate temperature with the heating level can be expressed as
Where n is the Avrami index, K is the reaction constant rate which is generally
expressed by Arrhenius equation
K= Ko exp (−Ec
RT )
where Ko is the frequency factor, Ec is the crystal activation energy, R is the constant
universal gas and T is the absolute temperature.
Established through JMA model, various theoretical methods have been
established to study the crystallization kinetics of amorphous alloys (Mike Reading,
2016).
Herein some of the methods that are used to determine the crystallization kinetics of
Zr55 All0 Ni30Pd5, Zr60 Al5 Ni30Pd5 and Zr60 All0 Ni25Pd5 metallic glasses using the non-
isothermal DSC measurements.
Kissinger method
The Kissinger equation is one of the most often used equations to work out the
energy used in activating crystallization, which is given by
ln ( α
T p
2 ) =−Ec
R T p
+C
Where is the amount of heating applied during the experiment, Tp is the
crystallization ultimate temperature, Ec is the activation energy of the crystallization
and C is a constant.
Mahadevan et al. method
According to the rough calculation of Mahadevan et al. the variation of the
ultimate temperature with the heating level can be expressed as
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
ln ( α )= −Ec
R T p
+C
Augis and Bennett approximation method
Augis and Bennett have come up with a more precise method for weighing the
energy activation of crystallization and the pre-exponential factor of constant rate, Ko
by reflecting on the temperature dependence of the reaction rate. This resulted into a
linear relationship between ln (/Tp) versus 1/Tp in the following formula:
ln ( α
T p )=−Ec
R T p
+ln ( Ko )
Ozawa-Chen method
Ozawa and Chen came up with the Kissinger relation that is modified to estimate
energy activation of crystallization by taking into account the form of crystallization
peak. In this method the temperature T at any given value of crystallized volume
fraction (x) at different heating rates () has been used instead of peak temperature
Tp.
ln ( α
T ❑
2 )= −Ec
R T❑
+C
where crystallized volume fraction (x) can be determined by taking the ratio between
the partial area and the total area under the peak of crystallization (Williams, 2016).
Local activation energy
The energy of activating the non-isothermal crystallization process is not persistent; it
depends on the volume fraction of crystallization. In order to have better
understanding on the crystallization of kinetics, studying the local activation energy
is extremely useful. There are several methods that are used for obtaining the local
ln ( α )= −Ec
R T p
+C
Augis and Bennett approximation method
Augis and Bennett have come up with a more precise method for weighing the
energy activation of crystallization and the pre-exponential factor of constant rate, Ko
by reflecting on the temperature dependence of the reaction rate. This resulted into a
linear relationship between ln (/Tp) versus 1/Tp in the following formula:
ln ( α
T p )=−Ec
R T p
+ln ( Ko )
Ozawa-Chen method
Ozawa and Chen came up with the Kissinger relation that is modified to estimate
energy activation of crystallization by taking into account the form of crystallization
peak. In this method the temperature T at any given value of crystallized volume
fraction (x) at different heating rates () has been used instead of peak temperature
Tp.
ln ( α
T ❑
2 )= −Ec
R T❑
+C
where crystallized volume fraction (x) can be determined by taking the ratio between
the partial area and the total area under the peak of crystallization (Williams, 2016).
Local activation energy
The energy of activating the non-isothermal crystallization process is not persistent; it
depends on the volume fraction of crystallization. In order to have better
understanding on the crystallization of kinetics, studying the local activation energy
is extremely useful. There are several methods that are used for obtaining the local

9
activation energy, including Kissinger-Akahira-Sunose (KAS), Ozawa-Flynn-Wall
(OFW) and Tang methods.
Kissinger-Akahira-Sunose (KAS) method
ln ( α
T x
2 ) =− Ex
R T x
+ C
Ozawa-Flynn-Wall (OFW) method
ln ( α ) =−1 ∙ 0516 Ex
R T x
+C
Tang method
ln ( α
T x
1 ∙894661 )=−1∙ 00145033 Ex
R T x
+C
where Ex is local activation energy and Tx is the temperature corresponding to a
certain crystallized volume fraction x.
Avrami constant
Avrami constant was calculated using
ln (−ln (1−x ) )=−nln ( α )+nln(T −T 0 )
Non- isothermal crystallization kinetics
In this case, non-isothermal crystallization kinetic reaction can be solved through the
equation;
activation energy, including Kissinger-Akahira-Sunose (KAS), Ozawa-Flynn-Wall
(OFW) and Tang methods.
Kissinger-Akahira-Sunose (KAS) method
ln ( α
T x
2 ) =− Ex
R T x
+ C
Ozawa-Flynn-Wall (OFW) method
ln ( α ) =−1 ∙ 0516 Ex
R T x
+C
Tang method
ln ( α
T x
1 ∙894661 )=−1∙ 00145033 Ex
R T x
+C
where Ex is local activation energy and Tx is the temperature corresponding to a
certain crystallized volume fraction x.
Avrami constant
Avrami constant was calculated using
ln (−ln (1−x ) )=−nln ( α )+nln(T −T 0 )
Non- isothermal crystallization kinetics
In this case, non-isothermal crystallization kinetic reaction can be solved through the
equation;
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

10
k (T) is the constant rate, b is the rate of heating, a is the conversion degree and f the model of reaction. The main aim of studying crystallization
kinetics is to determine kinetic parameters. Therefore, in determining kinetic triplet, different methods of in conversion and isokinetic ones are
applied.
Inconversional techniques
they do not depend on reaction model. Therefore, they are sometimes referred to as model-free methods. They can be classified further into linear
integral and linear differential techniques. Integral techniques are subjected on the (United States. Department of Energy,
2014)estimation of the temperature integral. Differential techniques depend on transformation rate. Variable separation and integration of
equation gives;
The above integral equation does not have analytical equation that is exact. linear integral isoconversional technique is expressed in rhe general
linear form of equation as;
The K and A parameters depend on temperature integral approximations where C is a constant.
Whereas the conversion degree is a at a particular time, n is the growth exponent and the constant of rate provided by K is k(T), as shown below;
Where the pre-exponential factor is k0, E is representing energy activation, and R the universal constant of gas. fraction transformed can be b, from
the above equation (4) and (5).
k (T) is the constant rate, b is the rate of heating, a is the conversion degree and f the model of reaction. The main aim of studying crystallization
kinetics is to determine kinetic parameters. Therefore, in determining kinetic triplet, different methods of in conversion and isokinetic ones are
applied.
Inconversional techniques
they do not depend on reaction model. Therefore, they are sometimes referred to as model-free methods. They can be classified further into linear
integral and linear differential techniques. Integral techniques are subjected on the (United States. Department of Energy,
2014)estimation of the temperature integral. Differential techniques depend on transformation rate. Variable separation and integration of
equation gives;
The above integral equation does not have analytical equation that is exact. linear integral isoconversional technique is expressed in rhe general
linear form of equation as;
The K and A parameters depend on temperature integral approximations where C is a constant.
Whereas the conversion degree is a at a particular time, n is the growth exponent and the constant of rate provided by K is k(T), as shown below;
Where the pre-exponential factor is k0, E is representing energy activation, and R the universal constant of gas. fraction transformed can be b, from
the above equation (4) and (5).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
11
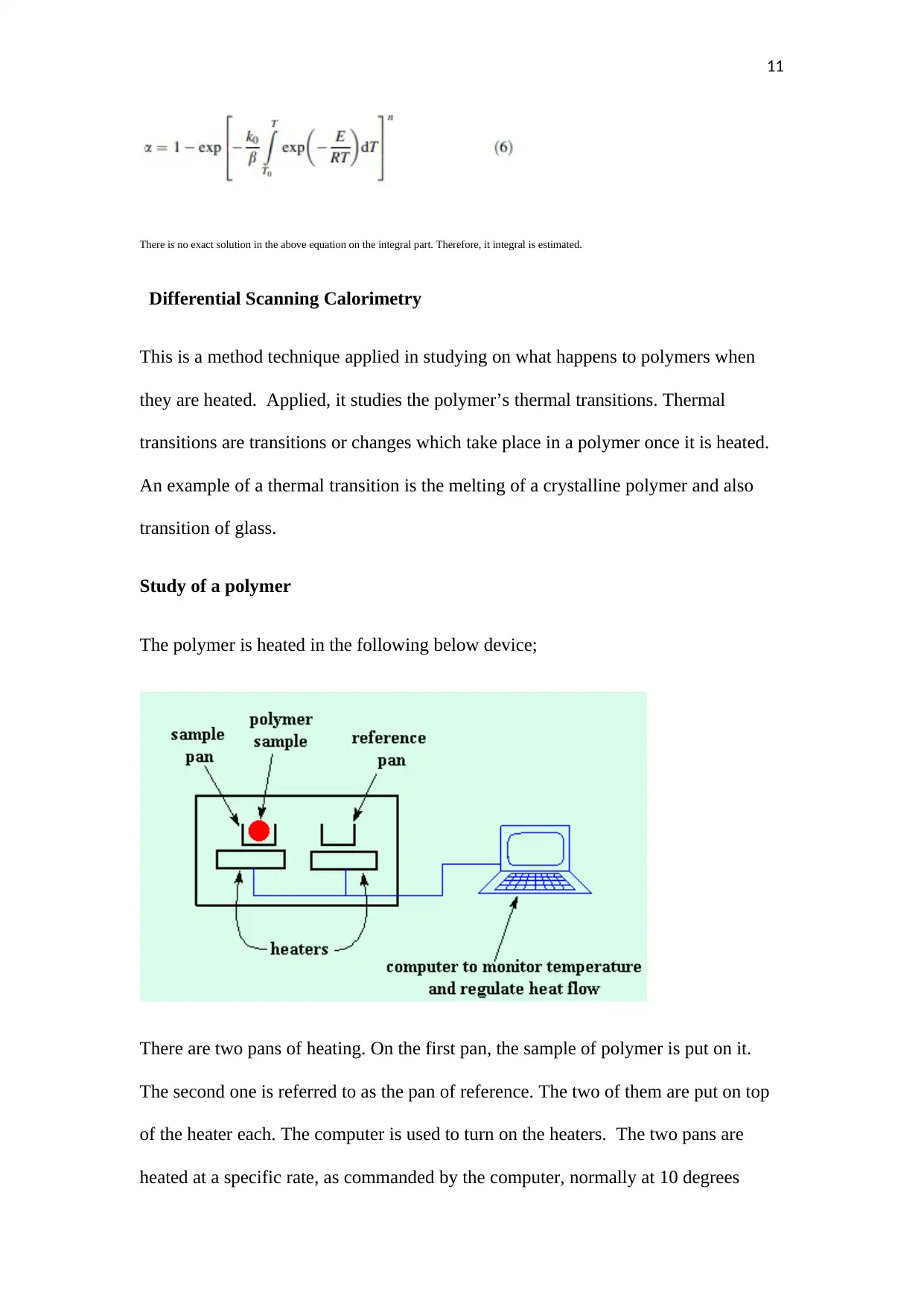
There is no exact solution in the above equation on the integral part. Therefore, it integral is estimated.
Differential Scanning Calorimetry
This is a method technique applied in studying on what happens to polymers when
they are heated. Applied, it studies the polymer’s thermal transitions. Thermal
transitions are transitions or changes which take place in a polymer once it is heated.
An example of a thermal transition is the melting of a crystalline polymer and also
transition of glass.
Study of a polymer
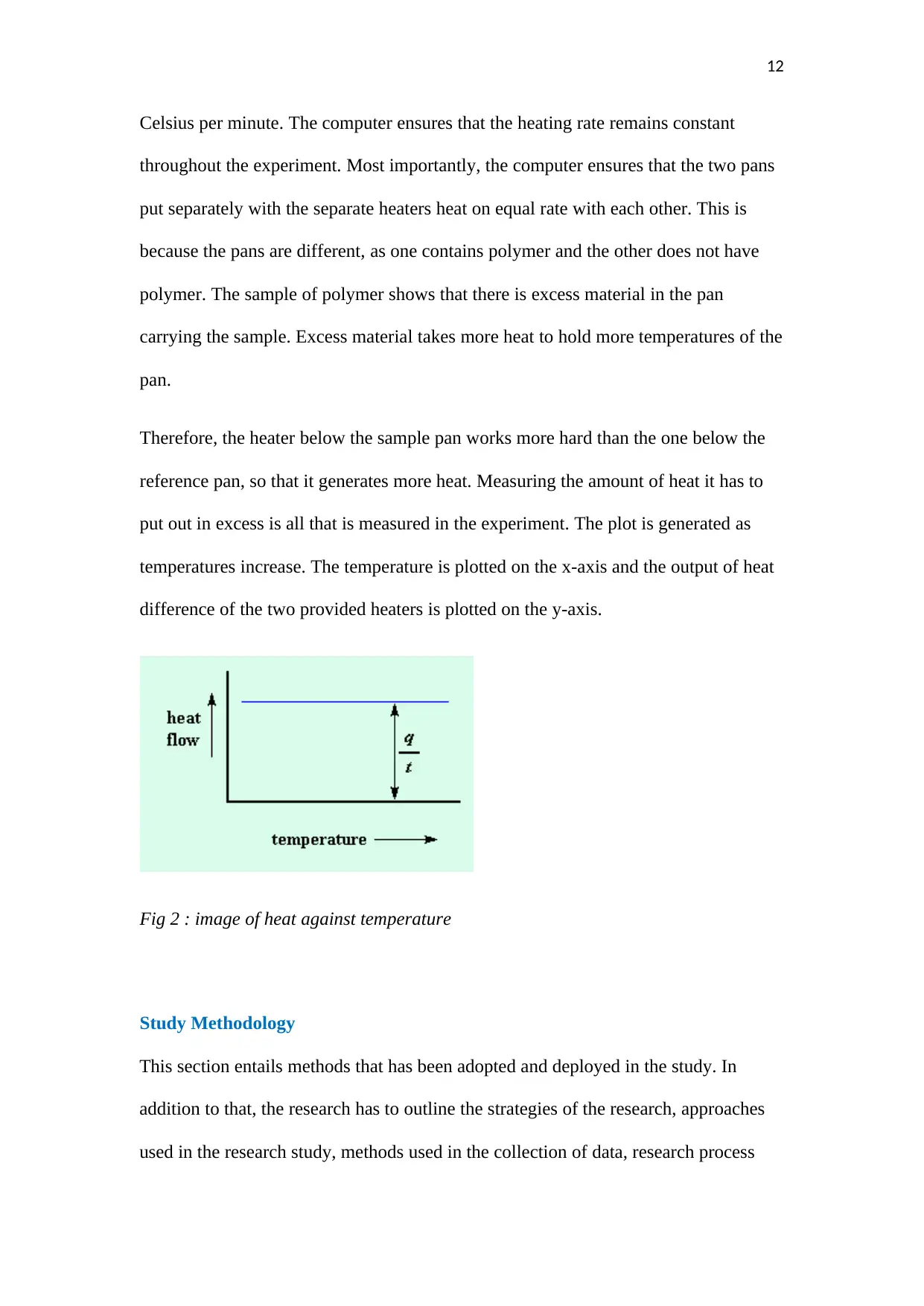
The polymer is heated in the following below device;
There are two pans of heating. On the first pan, the sample of polymer is put on it.
The second one is referred to as the pan of reference. The two of them are put on top
of the heater each. The computer is used to turn on the heaters. The two pans are
heated at a specific rate, as commanded by the computer, normally at 10 degrees
There is no exact solution in the above equation on the integral part. Therefore, it integral is estimated.
Differential Scanning Calorimetry
This is a method technique applied in studying on what happens to polymers when
they are heated. Applied, it studies the polymer’s thermal transitions. Thermal
transitions are transitions or changes which take place in a polymer once it is heated.
An example of a thermal transition is the melting of a crystalline polymer and also
transition of glass.
Study of a polymer
The polymer is heated in the following below device;
There are two pans of heating. On the first pan, the sample of polymer is put on it.
The second one is referred to as the pan of reference. The two of them are put on top
of the heater each. The computer is used to turn on the heaters. The two pans are
heated at a specific rate, as commanded by the computer, normally at 10 degrees
12
Celsius per minute. The computer ensures that the heating rate remains constant
throughout the experiment. Most importantly, the computer ensures that the two pans
put separately with the separate heaters heat on equal rate with each other. This is
because the pans are different, as one contains polymer and the other does not have
polymer. The sample of polymer shows that there is excess material in the pan
carrying the sample. Excess material takes more heat to hold more temperatures of the
pan.
Therefore, the heater below the sample pan works more hard than the one below the
reference pan, so that it generates more heat. Measuring the amount of heat it has to
put out in excess is all that is measured in the experiment. The plot is generated as
temperatures increase. The temperature is plotted on the x-axis and the output of heat
difference of the two provided heaters is plotted on the y-axis.
Fig 2 : image of heat against temperature
Study Methodology
This section entails methods that has been adopted and deployed in the study. In
addition to that, the research has to outline the strategies of the research, approaches
used in the research study, methods used in the collection of data, research process
Celsius per minute. The computer ensures that the heating rate remains constant
throughout the experiment. Most importantly, the computer ensures that the two pans
put separately with the separate heaters heat on equal rate with each other. This is
because the pans are different, as one contains polymer and the other does not have
polymer. The sample of polymer shows that there is excess material in the pan
carrying the sample. Excess material takes more heat to hold more temperatures of the
pan.
Therefore, the heater below the sample pan works more hard than the one below the
reference pan, so that it generates more heat. Measuring the amount of heat it has to
put out in excess is all that is measured in the experiment. The plot is generated as
temperatures increase. The temperature is plotted on the x-axis and the output of heat
difference of the two provided heaters is plotted on the y-axis.
Fig 2 : image of heat against temperature
Study Methodology
This section entails methods that has been adopted and deployed in the study. In
addition to that, the research has to outline the strategies of the research, approaches
used in the research study, methods used in the collection of data, research process
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 21
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.