Report on Physics Practical
VerifiedAdded on 2020/04/15
|21
|3167
|52
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

PHYSICS
STUDENT ID:
[Pick the date]
STUDENT ID:
[Pick the date]
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

PHYSICS
Question 1
a) A basic thermometer consists of a liquid measure in the form of alcohol, mercury etc. which
is held in a glass tube and vacuum sealed. Based on the underlying temperature, this liquid
tends to expand or cool which forms the basis of thermometer. Calibration of thermometer is
essential so that the reading in the scale of the thermometer actually represents the
temperature. The calibration of thermometer is usually carried out using the reference points
that relate to the 1000C and 00C that tend to serve as reference points based on which the
marking on the scale are engraved. The range of readings represented varies with the use of
the underlying thermometer and after the scratching of the numbers, a hydrofluoric acid dip is
provided for sealing the engravings (Bejan, 2016).
b) i) Ice point is known as the freezing point whereas steam point is known as boiling point in
thermodynamics.
ii) The 122 F corresponds to 500C on the thermometer.
iii) The number 80 on temperature would correspond to 26.670C.
Question 2
a) Absolute zero in layman terms refers to the coldest temperature that can be achieved
theoretically. As a result, it is not possible to cool any object to a lower temperature.
b) “Triple point is the particular temperature and pressure at which the solid, liquid and gaseous
phases of a given substance are all at equilibrium with one another”
The triple point of water comes out to be 0.010 C or 273.16K
c) Similarities and differences between Celsius and Kelvin scales are given below (Kutz, 2005).
Definition
Celsius: The temperature scale on which 0° C means melting point (MP) of ice and 100° Cmean
boiling point (BP) of water.
Question 1
a) A basic thermometer consists of a liquid measure in the form of alcohol, mercury etc. which
is held in a glass tube and vacuum sealed. Based on the underlying temperature, this liquid
tends to expand or cool which forms the basis of thermometer. Calibration of thermometer is
essential so that the reading in the scale of the thermometer actually represents the
temperature. The calibration of thermometer is usually carried out using the reference points
that relate to the 1000C and 00C that tend to serve as reference points based on which the
marking on the scale are engraved. The range of readings represented varies with the use of
the underlying thermometer and after the scratching of the numbers, a hydrofluoric acid dip is
provided for sealing the engravings (Bejan, 2016).
b) i) Ice point is known as the freezing point whereas steam point is known as boiling point in
thermodynamics.
ii) The 122 F corresponds to 500C on the thermometer.
iii) The number 80 on temperature would correspond to 26.670C.
Question 2
a) Absolute zero in layman terms refers to the coldest temperature that can be achieved
theoretically. As a result, it is not possible to cool any object to a lower temperature.
b) “Triple point is the particular temperature and pressure at which the solid, liquid and gaseous
phases of a given substance are all at equilibrium with one another”
The triple point of water comes out to be 0.010 C or 273.16K
c) Similarities and differences between Celsius and Kelvin scales are given below (Kutz, 2005).
Definition
Celsius: The temperature scale on which 0° C means melting point (MP) of ice and 100° Cmean
boiling point (BP) of water.

PHYSICS
Kelvin: It is an absolute temperature scale on which 0 K means -273.15 ° C (absolute zero) and
273.15 K means 0 ° C .
Similarities
Both are used to represent the temperature.
Both the scales are part of SI unit system.
One formula can be used to change the temperature scale from Celsius to Kelvin i.e.
T ( K )=273.15+T ( ° C )
Both scales are precisely the same.
Differences
Kelvin scale is based on the molecular energy which defines the extremes of cold and hot
while Celsius scale is based on the MP and BP of water under standard normal atmospheric
conditions.
Kelvin scale is represented as K while Celsius scale is represented as ° C .
For scientific work Kelvin scale is more preferable than Celsius scale.
Zero degrees Celsius represent the MP of ice while the zero Kelvin means absolute zero.
Kelvin: It is an absolute temperature scale on which 0 K means -273.15 ° C (absolute zero) and
273.15 K means 0 ° C .
Similarities
Both are used to represent the temperature.
Both the scales are part of SI unit system.
One formula can be used to change the temperature scale from Celsius to Kelvin i.e.
T ( K )=273.15+T ( ° C )
Both scales are precisely the same.
Differences
Kelvin scale is based on the molecular energy which defines the extremes of cold and hot
while Celsius scale is based on the MP and BP of water under standard normal atmospheric
conditions.
Kelvin scale is represented as K while Celsius scale is represented as ° C .
For scientific work Kelvin scale is more preferable than Celsius scale.
Zero degrees Celsius represent the MP of ice while the zero Kelvin means absolute zero.

PHYSICS
d) The basic relationship between the two temperature scales is as highlighted below.
(Temperature in Celsius × 9/5) + 32 = Temperature in Fahrenheit
The temperature in F is given as 100
Hence, (Temperature in Celsius × 9/5) + 32 = 100
Solving the above, temperature in Celsius = 37.78
The above temperature in Celsius can now be converted into Kelvin scale using the following
formula.
Temperature in Kelvin = Temperature in Celsius + 273.15
Hence, temperature in Kelvin = 37.78 + 273.15 = 310.93 K
Question 3
Similarity and difference between heat energy and temperature is given below (Oakes, 2012).
Definition
Heat energy: “It describes the transfer of thermal energy between molecules within a system.”
Temperature: “It describes the average kinetic energy of molecules within a material or system.”
Similarity
Temperature and heat energy both are thermodynamic properties or state functions.
Both are physical quantities of matter.
Difference
Heat energy mainly depends on the total speed of the molecules, number, size, and mass of
molecules and also on the nature of the molecule. However, temperature is not a function of
size or nature of molecule.
There can be two different systems with same temperature and different heat energies.
d) The basic relationship between the two temperature scales is as highlighted below.
(Temperature in Celsius × 9/5) + 32 = Temperature in Fahrenheit
The temperature in F is given as 100
Hence, (Temperature in Celsius × 9/5) + 32 = 100
Solving the above, temperature in Celsius = 37.78
The above temperature in Celsius can now be converted into Kelvin scale using the following
formula.
Temperature in Kelvin = Temperature in Celsius + 273.15
Hence, temperature in Kelvin = 37.78 + 273.15 = 310.93 K
Question 3
Similarity and difference between heat energy and temperature is given below (Oakes, 2012).
Definition
Heat energy: “It describes the transfer of thermal energy between molecules within a system.”
Temperature: “It describes the average kinetic energy of molecules within a material or system.”
Similarity
Temperature and heat energy both are thermodynamic properties or state functions.
Both are physical quantities of matter.
Difference
Heat energy mainly depends on the total speed of the molecules, number, size, and mass of
molecules and also on the nature of the molecule. However, temperature is not a function of
size or nature of molecule.
There can be two different systems with same temperature and different heat energies.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
PHYSICS
The unit of measurement for Heat energy is Joule while degree (C or F) or K is used to
measure the temperature.
Heat energy would provide the ability to system to do work while the temperature is simple a
measured of level of heat possess by the system or material.
Question 4
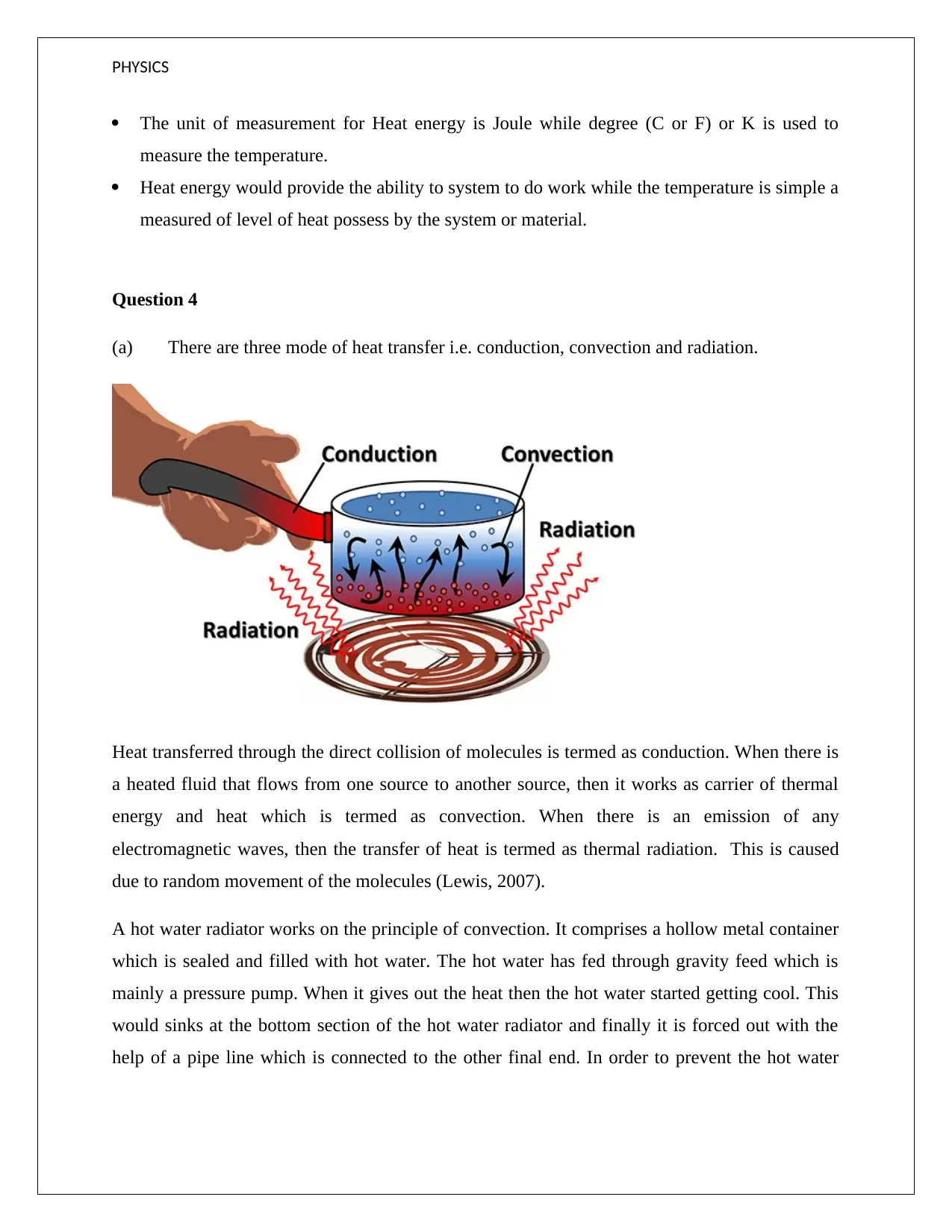
(a) There are three mode of heat transfer i.e. conduction, convection and radiation.
Heat transferred through the direct collision of molecules is termed as conduction. When there is
a heated fluid that flows from one source to another source, then it works as carrier of thermal
energy and heat which is termed as convection. When there is an emission of any
electromagnetic waves, then the transfer of heat is termed as thermal radiation. This is caused
due to random movement of the molecules (Lewis, 2007).
A hot water radiator works on the principle of convection. It comprises a hollow metal container
which is sealed and filled with hot water. The hot water has fed through gravity feed which is
mainly a pressure pump. When it gives out the heat then the hot water started getting cool. This
would sinks at the bottom section of the hot water radiator and finally it is forced out with the
help of a pipe line which is connected to the other final end. In order to prevent the hot water
The unit of measurement for Heat energy is Joule while degree (C or F) or K is used to
measure the temperature.
Heat energy would provide the ability to system to do work while the temperature is simple a
measured of level of heat possess by the system or material.
Question 4
(a) There are three mode of heat transfer i.e. conduction, convection and radiation.
Heat transferred through the direct collision of molecules is termed as conduction. When there is
a heated fluid that flows from one source to another source, then it works as carrier of thermal
energy and heat which is termed as convection. When there is an emission of any
electromagnetic waves, then the transfer of heat is termed as thermal radiation. This is caused
due to random movement of the molecules (Lewis, 2007).
A hot water radiator works on the principle of convection. It comprises a hollow metal container
which is sealed and filled with hot water. The hot water has fed through gravity feed which is
mainly a pressure pump. When it gives out the heat then the hot water started getting cool. This
would sinks at the bottom section of the hot water radiator and finally it is forced out with the
help of a pipe line which is connected to the other final end. In order to prevent the hot water

PHYSICS
knocking in the radiator anti-hammer devices are taken into consideration while designing the
hot water radiator to provide the heat to the room (Morna, 2003).
(b) The three main effective ways that Matilda can be used to minimize the heat loss from
her home are as follows (Bejan, 2016).
Insulation (loft, roof, window, door, walls, floor) it will minimize the heat loss incurred
due to conduction, convection and radiation. In this process, an insulating material such
as glass wool, polymeric material would be filled between the gaps or cavities in order to
prevent the heat loss.
Installing double glazed window. This would minimize the heat loss incurred due to
conduction and convection. In this process, the gap/cavity exists between the two plates
of glass would fill by air.
Sealing and installation of draught excluder. This would also minimize the heat loss
which occurrs due to the minor cavities.
Question 5
(a) The electrical conductivity in any metal is due to the movement of negatively charged
(electrically charged) particles in the metal. Any metal which comprises og free valance
electrons located in the outer shell of the atom would increase the ability of metal to
conduct/transmit the electric current from it. When there is a weak electric resistance or
no resistance then the generated electric field would provide clear path to the free
electrons to move such as billiard balls and show high electric conductivity such as
copper, iron, silver, gold and so forth (Oakes, 2012).
(b) Electrical conductivity is directly related with the thermal conductivity of the metal. It is
also depends on the number of free electrons present in the atom. Hence, it can be said
that most of the good electric conductor would also be a good thermal conductor. Yes,
there are other factors also such as boiling and melting point, wavelength, delocalization
are some of the variables which also affect the heat conduction in metals (Kutz, 2005).
knocking in the radiator anti-hammer devices are taken into consideration while designing the
hot water radiator to provide the heat to the room (Morna, 2003).
(b) The three main effective ways that Matilda can be used to minimize the heat loss from
her home are as follows (Bejan, 2016).
Insulation (loft, roof, window, door, walls, floor) it will minimize the heat loss incurred
due to conduction, convection and radiation. In this process, an insulating material such
as glass wool, polymeric material would be filled between the gaps or cavities in order to
prevent the heat loss.
Installing double glazed window. This would minimize the heat loss incurred due to
conduction and convection. In this process, the gap/cavity exists between the two plates
of glass would fill by air.
Sealing and installation of draught excluder. This would also minimize the heat loss
which occurrs due to the minor cavities.
Question 5
(a) The electrical conductivity in any metal is due to the movement of negatively charged
(electrically charged) particles in the metal. Any metal which comprises og free valance
electrons located in the outer shell of the atom would increase the ability of metal to
conduct/transmit the electric current from it. When there is a weak electric resistance or
no resistance then the generated electric field would provide clear path to the free
electrons to move such as billiard balls and show high electric conductivity such as
copper, iron, silver, gold and so forth (Oakes, 2012).
(b) Electrical conductivity is directly related with the thermal conductivity of the metal. It is
also depends on the number of free electrons present in the atom. Hence, it can be said
that most of the good electric conductor would also be a good thermal conductor. Yes,
there are other factors also such as boiling and melting point, wavelength, delocalization
are some of the variables which also affect the heat conduction in metals (Kutz, 2005).
PHYSICS
Question 6
(a) Specific Heat Capacity: The heat required to change the temperature of a unit
quantity/mass by one degree temperature. It is an intensive variable which has unit
energy per mass per degree of temperature i.e. J
kgK .
When the heat is at constant pressure then the specific heat capacity is represented as C pand
when the heat is at constant volume then the specific heat capacity is represented as Cv .
Latent Heat of Fusion: The thermal energy required for any solid to melt or for any liquid to
freeze is termed as latent heat of fusion. The unit of latent heat of fusion is kJ
mol ∨ kJ
kg .
Latent Heat of Vaporization: The energy required for the phase change basically from liquid to
gas is termed as latent heat of vaporization. The unit of latent heat of vaporization is kJ
mol ∨ kJ
kg .
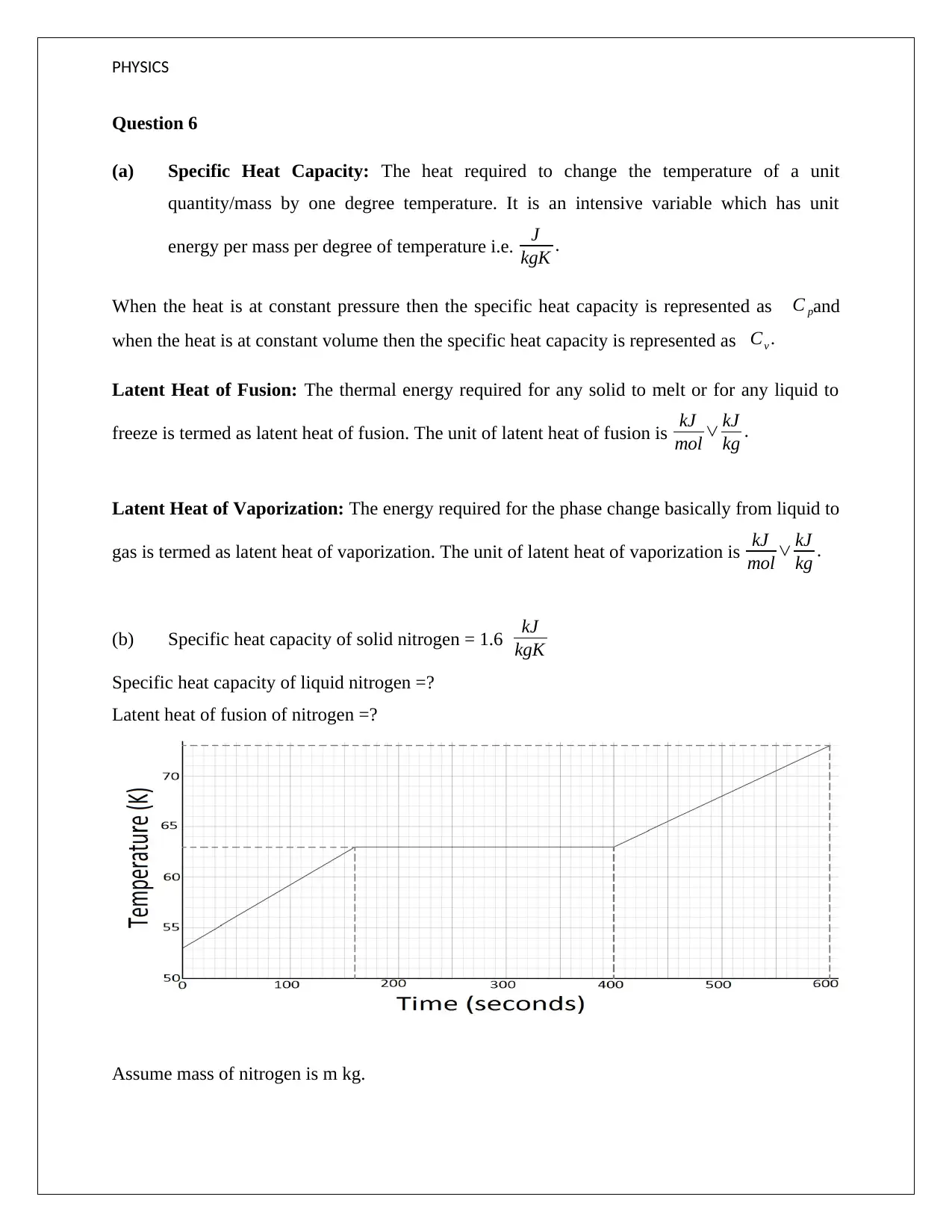
(b) Specific heat capacity of solid nitrogen = 1.6 kJ
kgK
Specific heat capacity of liquid nitrogen =?
Latent heat of fusion of nitrogen =?
Assume mass of nitrogen is m kg.
Question 6
(a) Specific Heat Capacity: The heat required to change the temperature of a unit
quantity/mass by one degree temperature. It is an intensive variable which has unit
energy per mass per degree of temperature i.e. J
kgK .
When the heat is at constant pressure then the specific heat capacity is represented as C pand
when the heat is at constant volume then the specific heat capacity is represented as Cv .
Latent Heat of Fusion: The thermal energy required for any solid to melt or for any liquid to
freeze is termed as latent heat of fusion. The unit of latent heat of fusion is kJ
mol ∨ kJ
kg .
Latent Heat of Vaporization: The energy required for the phase change basically from liquid to
gas is termed as latent heat of vaporization. The unit of latent heat of vaporization is kJ
mol ∨ kJ
kg .
(b) Specific heat capacity of solid nitrogen = 1.6 kJ
kgK
Specific heat capacity of liquid nitrogen =?
Latent heat of fusion of nitrogen =?
Assume mass of nitrogen is m kg.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PHYSICS
Change in heat ∆ Q=m L
Where,
m=mass of nitrogen
L=Latent heat
Now, the following formula is applied for solid nitrogen based on the given graph.
Change in heat ∆ Q=mc ∆ T =m∗1.6∗( 63−53 )
Also , ∆ Q=10 KJ
Thus, m = 1/1.6 = 0.625 kg
Assuming that the heat is being supplied at the same rate
Now,
∆ Q=m L
10=0.625∗L
Hence, L= 16 KJ/Kg
Also, Change in heat ∆ Q=mc ∆ T =0.625∗c∗ (73−63 )
Again , ∆ Q=10 KJ
Thus, c for liquid nitrogen = 2.56 kJkg-1K-1
(c) Specific heat capacity of Cu ¿ 0.394 kJ
kgK
Heat lost ¿ ?
Mass of Cu block ¿ 100 kg
Temperature difference=100 ¿50 ° C
Heat lost Q=mc ∆ T
Q=100∗0.394∗( 323.15−373.15 )
Change in heat ∆ Q=m L
Where,
m=mass of nitrogen
L=Latent heat
Now, the following formula is applied for solid nitrogen based on the given graph.
Change in heat ∆ Q=mc ∆ T =m∗1.6∗( 63−53 )
Also , ∆ Q=10 KJ
Thus, m = 1/1.6 = 0.625 kg
Assuming that the heat is being supplied at the same rate
Now,
∆ Q=m L
10=0.625∗L
Hence, L= 16 KJ/Kg
Also, Change in heat ∆ Q=mc ∆ T =0.625∗c∗ (73−63 )
Again , ∆ Q=10 KJ
Thus, c for liquid nitrogen = 2.56 kJkg-1K-1
(c) Specific heat capacity of Cu ¿ 0.394 kJ
kgK
Heat lost ¿ ?
Mass of Cu block ¿ 100 kg
Temperature difference=100 ¿50 ° C
Heat lost Q=mc ∆ T
Q=100∗0.394∗( 323.15−373.15 )
PHYSICS
Q=−1970 kJ
Therefore, heat lost by Cu is -1970kJ.
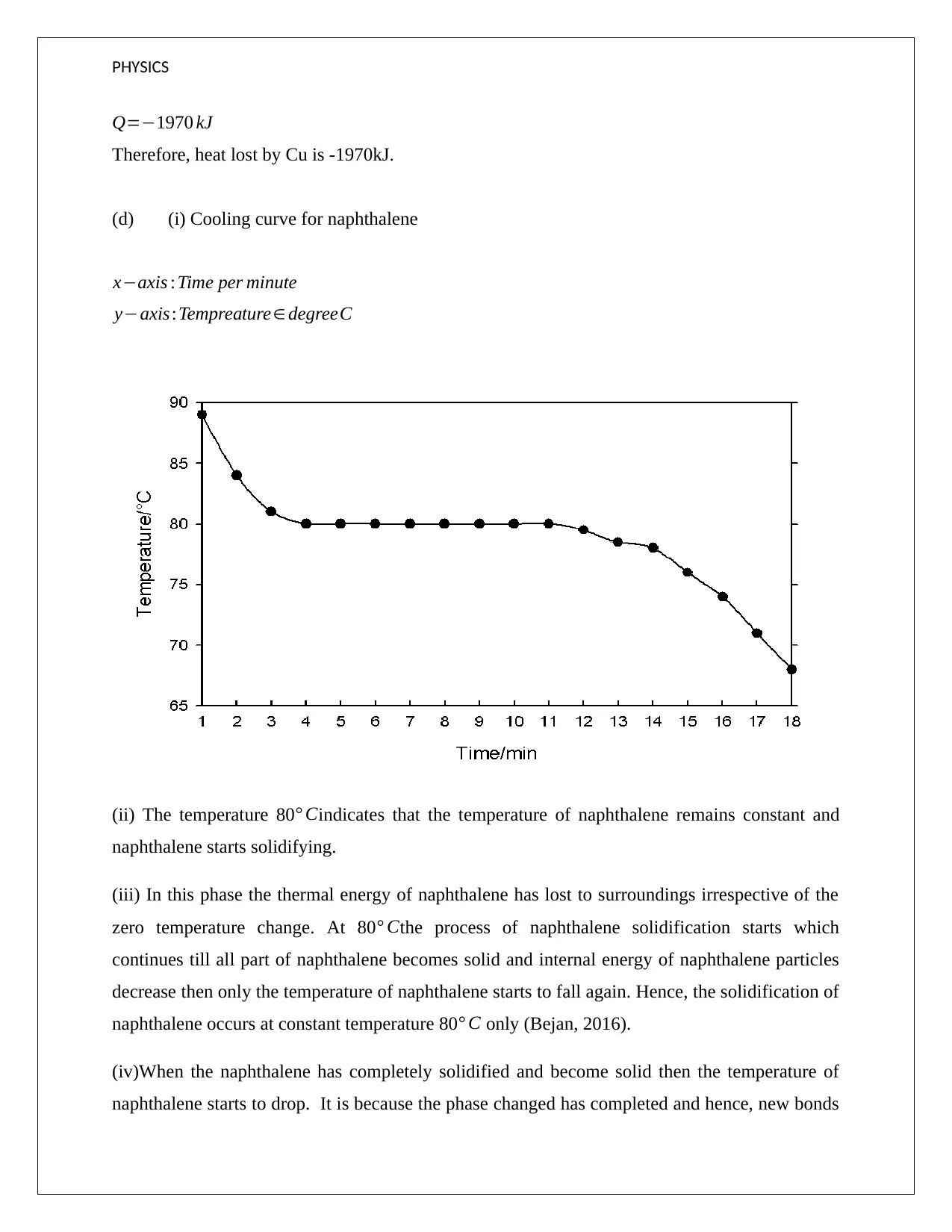
(d) (i) Cooling curve for naphthalene
x−axis :Time per minute
y−axis:Tempreature∈ degreeC
(ii) The temperature 80° Cindicates that the temperature of naphthalene remains constant and
naphthalene starts solidifying.
(iii) In this phase the thermal energy of naphthalene has lost to surroundings irrespective of the
zero temperature change. At 80° Cthe process of naphthalene solidification starts which
continues till all part of naphthalene becomes solid and internal energy of naphthalene particles
decrease then only the temperature of naphthalene starts to fall again. Hence, the solidification of
naphthalene occurs at constant temperature 80° C only (Bejan, 2016).
(iv)When the naphthalene has completely solidified and become solid then the temperature of
naphthalene starts to drop. It is because the phase changed has completed and hence, new bonds
Q=−1970 kJ
Therefore, heat lost by Cu is -1970kJ.
(d) (i) Cooling curve for naphthalene
x−axis :Time per minute
y−axis:Tempreature∈ degreeC
(ii) The temperature 80° Cindicates that the temperature of naphthalene remains constant and
naphthalene starts solidifying.
(iii) In this phase the thermal energy of naphthalene has lost to surroundings irrespective of the
zero temperature change. At 80° Cthe process of naphthalene solidification starts which
continues till all part of naphthalene becomes solid and internal energy of naphthalene particles
decrease then only the temperature of naphthalene starts to fall again. Hence, the solidification of
naphthalene occurs at constant temperature 80° C only (Bejan, 2016).
(iv)When the naphthalene has completely solidified and become solid then the temperature of
naphthalene starts to drop. It is because the phase changed has completed and hence, new bonds

PHYSICS
are formed which indicates the movement of naphthalene molecules is reduced and hence, the
overall temperature starts decreasing.
Question 7
Title: Determination of Specific Heat Capacity of solid using electrical method
Aim: An experiment to determine the specific heat capacity of metal block.
Introduction
The basis of the electrical method to determine specific heat capacity is to keep on varying the
voltage and thereby taking measurement with regards to the rise of the temperature of the given
solid owing to the heat energy produced. Using the relationship ∆Q=mc∆T, the specific heat
capacity of the specimen provided can be found out. The apparatus required for the given
experiment is highlighted below.
Ammeter, Voltmeter, Power supply, test metal block, Leads, immersion heater, stop clock,
balance, calibrated thermometer, heat proof mat and lagging.
Method
The basic setup of the experiment is as highlighted below.
are formed which indicates the movement of naphthalene molecules is reduced and hence, the
overall temperature starts decreasing.
Question 7
Title: Determination of Specific Heat Capacity of solid using electrical method
Aim: An experiment to determine the specific heat capacity of metal block.
Introduction
The basis of the electrical method to determine specific heat capacity is to keep on varying the
voltage and thereby taking measurement with regards to the rise of the temperature of the given
solid owing to the heat energy produced. Using the relationship ∆Q=mc∆T, the specific heat
capacity of the specimen provided can be found out. The apparatus required for the given
experiment is highlighted below.
Ammeter, Voltmeter, Power supply, test metal block, Leads, immersion heater, stop clock,
balance, calibrated thermometer, heat proof mat and lagging.
Method
The basic setup of the experiment is as highlighted below.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
PHYSICS
Results
Ammeter reading can be taken as positive and thus, the obervation table is shown below:
Original mass
(gram)
996.29 Original temp. T1 (degree C) 22
x-axis y -axis
T2 T2 - T1 Voltmete
r
Ammeter Time Energy
degree C degree C V A Sec J
25 3 11.08 -4.1 120 5451.36
27 5 11.12 -4.11 180 8226.57
6
32 10 11.04 -4.24 240 11234.3
38 16 10.98 -4.31 300 14197.1
4
41 19 11.05 -4.35 360 17304.3
45 23 11.05 -4.35 420 20188.3
5
49 27 11.04 -4.36 480 23104.5
1
Results
Ammeter reading can be taken as positive and thus, the obervation table is shown below:
Original mass
(gram)
996.29 Original temp. T1 (degree C) 22
x-axis y -axis
T2 T2 - T1 Voltmete
r
Ammeter Time Energy
degree C degree C V A Sec J
25 3 11.08 -4.1 120 5451.36
27 5 11.12 -4.11 180 8226.57
6
32 10 11.04 -4.24 240 11234.3
38 16 10.98 -4.31 300 14197.1
4
41 19 11.05 -4.35 360 17304.3
45 23 11.05 -4.35 420 20188.3
5
49 27 11.04 -4.36 480 23104.5
1
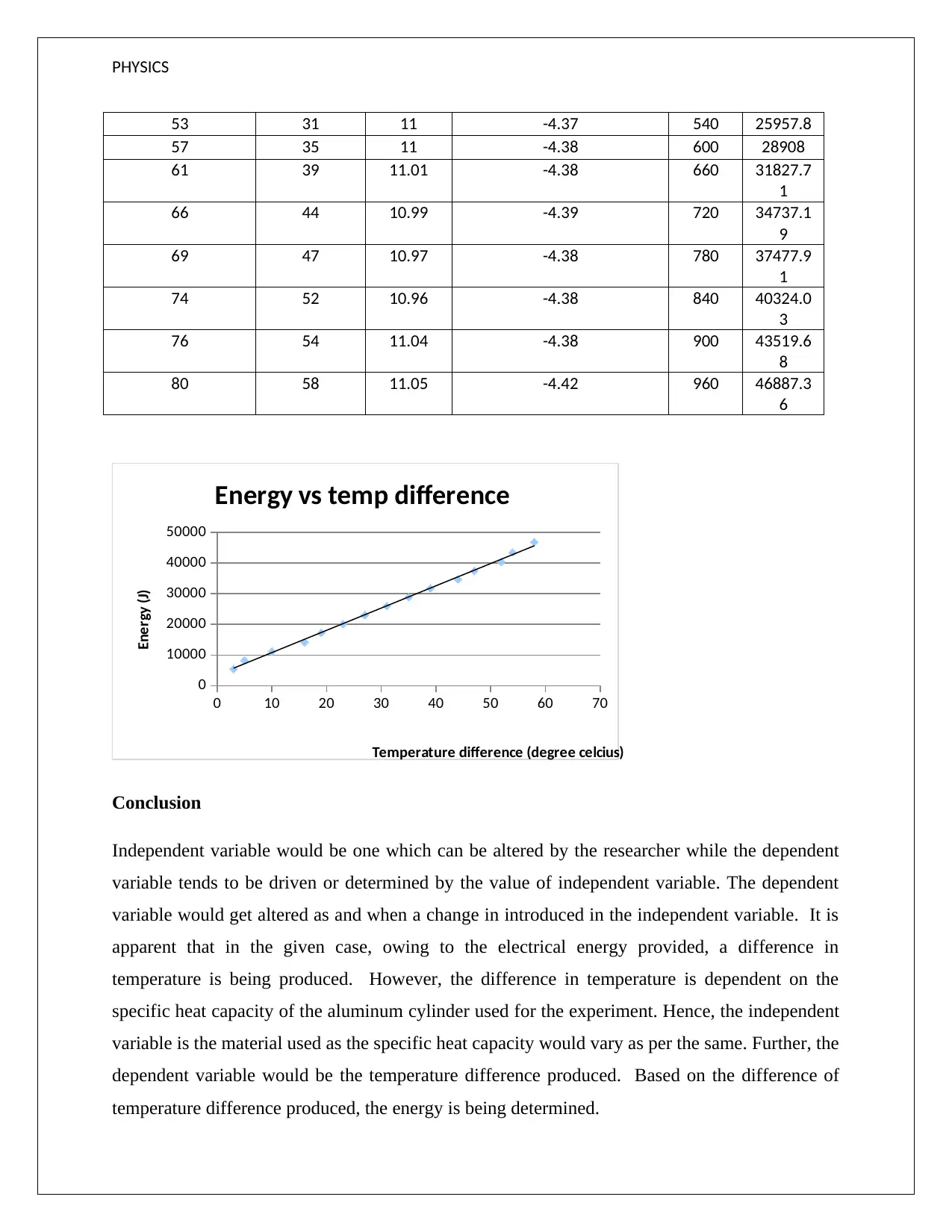
PHYSICS
53 31 11 -4.37 540 25957.8
57 35 11 -4.38 600 28908
61 39 11.01 -4.38 660 31827.7
1
66 44 10.99 -4.39 720 34737.1
9
69 47 10.97 -4.38 780 37477.9
1
74 52 10.96 -4.38 840 40324.0
3
76 54 11.04 -4.38 900 43519.6
8
80 58 11.05 -4.42 960 46887.3
6
0 10 20 30 40 50 60 70
0
10000
20000
30000
40000
50000
Energy vs temp difference
Temperature difference (degree celcius)
Energy (J)
Conclusion
Independent variable would be one which can be altered by the researcher while the dependent
variable tends to be driven or determined by the value of independent variable. The dependent
variable would get altered as and when a change in introduced in the independent variable. It is
apparent that in the given case, owing to the electrical energy provided, a difference in
temperature is being produced. However, the difference in temperature is dependent on the
specific heat capacity of the aluminum cylinder used for the experiment. Hence, the independent
variable is the material used as the specific heat capacity would vary as per the same. Further, the
dependent variable would be the temperature difference produced. Based on the difference of
temperature difference produced, the energy is being determined.
53 31 11 -4.37 540 25957.8
57 35 11 -4.38 600 28908
61 39 11.01 -4.38 660 31827.7
1
66 44 10.99 -4.39 720 34737.1
9
69 47 10.97 -4.38 780 37477.9
1
74 52 10.96 -4.38 840 40324.0
3
76 54 11.04 -4.38 900 43519.6
8
80 58 11.05 -4.42 960 46887.3
6
0 10 20 30 40 50 60 70
0
10000
20000
30000
40000
50000
Energy vs temp difference
Temperature difference (degree celcius)
Energy (J)
Conclusion
Independent variable would be one which can be altered by the researcher while the dependent
variable tends to be driven or determined by the value of independent variable. The dependent
variable would get altered as and when a change in introduced in the independent variable. It is
apparent that in the given case, owing to the electrical energy provided, a difference in
temperature is being produced. However, the difference in temperature is dependent on the
specific heat capacity of the aluminum cylinder used for the experiment. Hence, the independent
variable is the material used as the specific heat capacity would vary as per the same. Further, the
dependent variable would be the temperature difference produced. Based on the difference of
temperature difference produced, the energy is being determined.

PHYSICS
Discussion
Considering the broadly linear pattern of the points in the above scatter plot, it is apparent that
the experimental values tends to lend a high degree of support to the theoretical equation
represented by Q=mc ΔT
The computation of the gradient is highlighted below.
x 1=16 , y 1=14197.14
x 2=44 , y 2=34737.2
Gradient ¿ y 2− y 1
x 2−x 1
¿( 34737.2−14197.14)/( 44−16)
¿ 774.25
The above gradient represents the amount of heat which is required to increase the temperature
of the given aluminum cylinder by 1 unit.
Specific heat capacity through experiment
¿ Gradient /mass
= 774.25
996.29
¿ 0.777 J
g ° K ∨777.25 J
kg ° K
Theoretical specific heat capacity of Aluminum ¿ 904 J
kg ° K
Possible error
¿ Theoretical value−Experimental value
Experimental value ∗100
Discussion
Considering the broadly linear pattern of the points in the above scatter plot, it is apparent that
the experimental values tends to lend a high degree of support to the theoretical equation
represented by Q=mc ΔT
The computation of the gradient is highlighted below.
x 1=16 , y 1=14197.14
x 2=44 , y 2=34737.2
Gradient ¿ y 2− y 1
x 2−x 1
¿( 34737.2−14197.14)/( 44−16)
¿ 774.25
The above gradient represents the amount of heat which is required to increase the temperature
of the given aluminum cylinder by 1 unit.
Specific heat capacity through experiment
¿ Gradient /mass
= 774.25
996.29
¿ 0.777 J
g ° K ∨777.25 J
kg ° K
Theoretical specific heat capacity of Aluminum ¿ 904 J
kg ° K
Possible error
¿ Theoretical value−Experimental value
Experimental value ∗100
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PHYSICS
¿ 904−777.25
777.25 ∗100=16.31 %
It is apparent that the error is slightly above 15% and hence the given experiment and finding can
be considered as reasonably reliable as the experimental error is well within the threshold value
of 20% experimental error which serves as a benchmark for reliability (Oakes, 2012).
Percentage Uncertainty Computation
Voltmeter
Range of voltmeter reading = 0.16V
Uncertainty = 0.16/2 = 0.08V
Mean voltmeter reading = 11.025 V
Hence, percentage uncertainty = (0.08/11.025)*100 = 0.73%
Ammeter
Range of ammeter reading = 0.32 A
Uncertainty = 0.32/2 = 0.16A
Mean ammeter reading = 4.3267 A
Hence, percentage uncertainty = (0.16/4.3267)*100 = 3.70%
Since the initial temperature is held constant and also the time is measured by stop watch, the
percentage uncertainty in these is taken as zero. As a result, these would be most precise
variables as these tend to have the lowest percentage uncertainty (Morna, 2003).
Question 8
(a) Initial volume of gas V i=600 cm3 ∨0.0006 m3
Initial temperature of gas T i=20 ° C
¿ 904−777.25
777.25 ∗100=16.31 %
It is apparent that the error is slightly above 15% and hence the given experiment and finding can
be considered as reasonably reliable as the experimental error is well within the threshold value
of 20% experimental error which serves as a benchmark for reliability (Oakes, 2012).
Percentage Uncertainty Computation
Voltmeter
Range of voltmeter reading = 0.16V
Uncertainty = 0.16/2 = 0.08V
Mean voltmeter reading = 11.025 V
Hence, percentage uncertainty = (0.08/11.025)*100 = 0.73%
Ammeter
Range of ammeter reading = 0.32 A
Uncertainty = 0.32/2 = 0.16A
Mean ammeter reading = 4.3267 A
Hence, percentage uncertainty = (0.16/4.3267)*100 = 3.70%
Since the initial temperature is held constant and also the time is measured by stop watch, the
percentage uncertainty in these is taken as zero. As a result, these would be most precise
variables as these tend to have the lowest percentage uncertainty (Morna, 2003).
Question 8
(a) Initial volume of gas V i=600 cm3 ∨0.0006 m3
Initial temperature of gas T i=20 ° C

PHYSICS
Final temperature of gas T f =−4 ° C
Final (new) volume of gas V f =?
Type of process
It is apparent that pressure of the process is not changing and thus, this is termed as adiabatic
process.
Temperature in Kelvin unit
Initial temperature of gas T i=20+273.15=293.15 K
Final temperature of gas T f =−4 +273.15=269.15 K
As per Charles law, the final volume for an adiabatic process is computed as shown below:
V i
T i
= V f
T f
Or
V f = V i∗T f
T i
¿ 0.0006∗( 269.15
293.15 )
¿ 0.000551 m3
Therefore, the new volume of the xenon gas would be 0.000551 m3 .
(b) Initial diameter of basketball = 30cm
Initial pressure = 1atm or 105Pa
Final volume = ½ of initial volume
Final temperature of gas T f =−4 ° C
Final (new) volume of gas V f =?
Type of process
It is apparent that pressure of the process is not changing and thus, this is termed as adiabatic
process.
Temperature in Kelvin unit
Initial temperature of gas T i=20+273.15=293.15 K
Final temperature of gas T f =−4 +273.15=269.15 K
As per Charles law, the final volume for an adiabatic process is computed as shown below:
V i
T i
= V f
T f
Or
V f = V i∗T f
T i
¿ 0.0006∗( 269.15
293.15 )
¿ 0.000551 m3
Therefore, the new volume of the xenon gas would be 0.000551 m3 .
(b) Initial diameter of basketball = 30cm
Initial pressure = 1atm or 105Pa
Final volume = ½ of initial volume

PHYSICS
Final volume, initial volume, final pressure =?
Type of process
It is apparent that temperature of the process is not changing and thus, this is termed as
isothermal process.
Initial volume of basketball ¿ 4
3 π r 3= 4
3 π (30)3 =113142.9 cm3
1 cm3=10−6 m3
V i=113142.9∗10−6 =0.11314 m3
Final volume ¿ ½∗0.11314=0.05657 m3
As per Boyle’s law
PV =constant
Pi V i=Pf V f
105∗0.11314=Pf ∗0.05657
Pf =2∗105 Pa
Therefore, the initial and final volume of basketball is 0.11314 m3 and 0.05657 m3 respectively.
Also, the final pressure comes out to be 2∗105 Pa.
(c) Initial temperature T i=20 ° C
Initial pressure Pi=140 mmHgor 18665.13 Pa
Final temperature T f =30° C
Final pressure Pf =?
Type of process
Final volume, initial volume, final pressure =?
Type of process
It is apparent that temperature of the process is not changing and thus, this is termed as
isothermal process.
Initial volume of basketball ¿ 4
3 π r 3= 4
3 π (30)3 =113142.9 cm3
1 cm3=10−6 m3
V i=113142.9∗10−6 =0.11314 m3
Final volume ¿ ½∗0.11314=0.05657 m3
As per Boyle’s law
PV =constant
Pi V i=Pf V f
105∗0.11314=Pf ∗0.05657
Pf =2∗105 Pa
Therefore, the initial and final volume of basketball is 0.11314 m3 and 0.05657 m3 respectively.
Also, the final pressure comes out to be 2∗105 Pa.
(c) Initial temperature T i=20 ° C
Initial pressure Pi=140 mmHgor 18665.13 Pa
Final temperature T f =30° C
Final pressure Pf =?
Type of process
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

PHYSICS
It is apparent that volume of the process is not changing and thus, this is termed as isochoric
process.
Temperature in Kelvin unit
Initial temperature of gas T i=20+273.15=293.15 K
Final temperature of gas T f =30+273.15=303.15 K
As per pressure law (Amontons’s law)
P ∝T
P
T =Constant
Pi
Ti
= Pf
T f
Now,
Pf = Pi
Ti
∗T f
¿ 18665.13∗303.15
293.15
¿ 19301.84 Pa
(d) (i) Pump cylinder volume ¿ 2.6 ×10−4 m3
Tire pressure ¿ 1.11× 10−3 atm
Tire volume ¿ 10 ×10−4 m3
Pressure of gas before pumping ¿ 1 atm
Temperature (would not change) =15 ° C
Moles of gas which are in the pump before it is being compressed
It is apparent that volume of the process is not changing and thus, this is termed as isochoric
process.
Temperature in Kelvin unit
Initial temperature of gas T i=20+273.15=293.15 K
Final temperature of gas T f =30+273.15=303.15 K
As per pressure law (Amontons’s law)
P ∝T
P
T =Constant
Pi
Ti
= Pf
T f
Now,
Pf = Pi
Ti
∗T f
¿ 18665.13∗303.15
293.15
¿ 19301.84 Pa
(d) (i) Pump cylinder volume ¿ 2.6 ×10−4 m3
Tire pressure ¿ 1.11× 10−3 atm
Tire volume ¿ 10 ×10−4 m3
Pressure of gas before pumping ¿ 1 atm
Temperature (would not change) =15 ° C
Moles of gas which are in the pump before it is being compressed

PHYSICS
PV =nRT
P=1 atm
R=0.08205 L atm
mol K
T =15 ° C=288.15 K
V =2.6× 10−4 m3=0.26 L
Now ,
PV =nRT
1 atm ×0.26 L=n× 0.08205 L atm
mol K ×288.15 K
n=0.010997 mol
Hence, the moles of gas which are in the pump before it is being compressed is 0.010997 mol .
Number of moles of gas which are there in the tyre before the additional air is added
PV =nRT
P=1.11 ×10−3 atm
R=0.08205 L atm
mol K
T =15 ° C=288.15 K
V =10 × 10−4 m3=1 L
Now ,
PV =nRT
1.11 ×10−3 atm ×1 L=n × 0.08205 Latm
mol K × 288.15 K
PV =nRT
P=1 atm
R=0.08205 L atm
mol K
T =15 ° C=288.15 K
V =2.6× 10−4 m3=0.26 L
Now ,
PV =nRT
1 atm ×0.26 L=n× 0.08205 L atm
mol K ×288.15 K
n=0.010997 mol
Hence, the moles of gas which are in the pump before it is being compressed is 0.010997 mol .
Number of moles of gas which are there in the tyre before the additional air is added
PV =nRT
P=1.11 ×10−3 atm
R=0.08205 L atm
mol K
T =15 ° C=288.15 K
V =10 × 10−4 m3=1 L
Now ,
PV =nRT
1.11 ×10−3 atm ×1 L=n × 0.08205 Latm
mol K × 288.15 K

PHYSICS
n=4.694 × 10−5 mol
Hence, the moles of gas which are there in the tyre before the additional air is added is
4.694 ×10−5 mol .
Number of moles which are there in total in the tyres once the pump is empty
Final pressure needs to be determined before computing the number of moles in this case.
p 1 v 1=p 2 v 2
( 1.11 ×10−3 atm ) × ( 0.26 L )= ( p 2× 10 ×10− 4 )
p 2=0.2886 atm
Now,
PV =nRT
0.2886 atm ×1 L=n× 0.08205 L atm
mol K ×288.15 K
n=0.0122067 moles
Hence, Number of moles which are there in total in the tyres once the pump is empty is
0.0122067 moles .
(ii) Final pressure inside the tyre would be computed as p 1 v 1=p 2 v 2
( 1.11 ×10−3 atm ) × ( 0.26 L )= ( p 2× 10 ×10− 4 )
p 2=0.2886 atm
Question 9
(a) First law of Thermodynamics
n=4.694 × 10−5 mol
Hence, the moles of gas which are there in the tyre before the additional air is added is
4.694 ×10−5 mol .
Number of moles which are there in total in the tyres once the pump is empty
Final pressure needs to be determined before computing the number of moles in this case.
p 1 v 1=p 2 v 2
( 1.11 ×10−3 atm ) × ( 0.26 L )= ( p 2× 10 ×10− 4 )
p 2=0.2886 atm
Now,
PV =nRT
0.2886 atm ×1 L=n× 0.08205 L atm
mol K ×288.15 K
n=0.0122067 moles
Hence, Number of moles which are there in total in the tyres once the pump is empty is
0.0122067 moles .
(ii) Final pressure inside the tyre would be computed as p 1 v 1=p 2 v 2
( 1.11 ×10−3 atm ) × ( 0.26 L )= ( p 2× 10 ×10− 4 )
p 2=0.2886 atm
Question 9
(a) First law of Thermodynamics
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
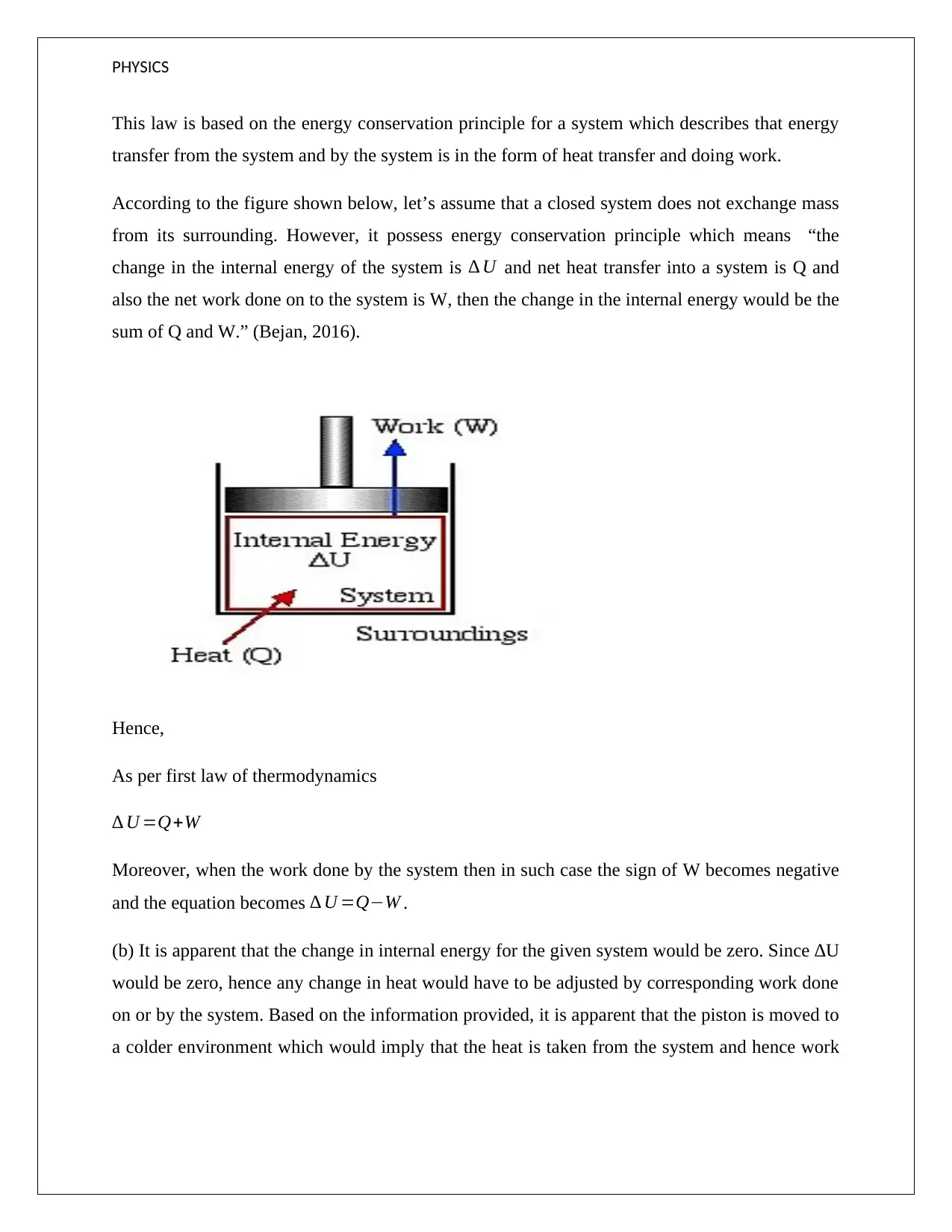
PHYSICS
This law is based on the energy conservation principle for a system which describes that energy
transfer from the system and by the system is in the form of heat transfer and doing work.
According to the figure shown below, let’s assume that a closed system does not exchange mass
from its surrounding. However, it possess energy conservation principle which means “the
change in the internal energy of the system is ∆ U and net heat transfer into a system is Q and
also the net work done on to the system is W, then the change in the internal energy would be the
sum of Q and W.” (Bejan, 2016).
Hence,
As per first law of thermodynamics
∆ U =Q+W
Moreover, when the work done by the system then in such case the sign of W becomes negative
and the equation becomes ∆ U =Q−W .
(b) It is apparent that the change in internal energy for the given system would be zero. Since ∆U
would be zero, hence any change in heat would have to be adjusted by corresponding work done
on or by the system. Based on the information provided, it is apparent that the piston is moved to
a colder environment which would imply that the heat is taken from the system and hence work
This law is based on the energy conservation principle for a system which describes that energy
transfer from the system and by the system is in the form of heat transfer and doing work.
According to the figure shown below, let’s assume that a closed system does not exchange mass
from its surrounding. However, it possess energy conservation principle which means “the
change in the internal energy of the system is ∆ U and net heat transfer into a system is Q and
also the net work done on to the system is W, then the change in the internal energy would be the
sum of Q and W.” (Bejan, 2016).
Hence,
As per first law of thermodynamics
∆ U =Q+W
Moreover, when the work done by the system then in such case the sign of W becomes negative
and the equation becomes ∆ U =Q−W .
(b) It is apparent that the change in internal energy for the given system would be zero. Since ∆U
would be zero, hence any change in heat would have to be adjusted by corresponding work done
on or by the system. Based on the information provided, it is apparent that the piston is moved to
a colder environment which would imply that the heat is taken from the system and hence work

PHYSICS
would be done on the system and not by the system. As a result, the movement of the piston
would be in downward direction due to the movement to a cold environment (Lewis, 2007).
References
Bejan, A. (2016) Advanced Engineering Thermodynamics. 4th edn. Sydney: John Wiley & Sons.
Kutz, M(2005) Heat Transfer Calculation. 7th edn. New York: McGraw Hill Professional.
Lewis, T.J. (2007) Heat and Thermodynamics: A historical Perspective.5th edn. California:
Greenwood Press.
Oakes, H. E. (2012) Heat and Thermodynamics. 2nd edn. New York: Infobase Learning.
Morna, J. M. (2003) Introduction to thermal systems engineering: thermodynamics, fluid
mechanics, and heat transfer, Volume 1. 6th edn. London; Wiley Publishers.
would be done on the system and not by the system. As a result, the movement of the piston
would be in downward direction due to the movement to a cold environment (Lewis, 2007).
References
Bejan, A. (2016) Advanced Engineering Thermodynamics. 4th edn. Sydney: John Wiley & Sons.
Kutz, M(2005) Heat Transfer Calculation. 7th edn. New York: McGraw Hill Professional.
Lewis, T.J. (2007) Heat and Thermodynamics: A historical Perspective.5th edn. California:
Greenwood Press.
Oakes, H. E. (2012) Heat and Thermodynamics. 2nd edn. New York: Infobase Learning.
Morna, J. M. (2003) Introduction to thermal systems engineering: thermodynamics, fluid
mechanics, and heat transfer, Volume 1. 6th edn. London; Wiley Publishers.
1 out of 21
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

