Physics Sums Assignment - Thermodynamics, Mechanics, and Gases
VerifiedAdded on 2021/05/30
|10
|1079
|99
Homework Assignment
AI Summary
This physics assignment presents a series of solved problems covering key concepts in thermodynamics, mechanics, and the behavior of gases. The assignment includes detailed calculations related to specific heat capacity, latent heat, and the ideal gas law. The first section addresses heat transfer calculations, determining the specific heat capacity of brass and the latent heat of fusion of ice. Subsequent sections delve into the properties of solids, liquids, and gases, along with applications of the ideal gas law to calculate pressure. The assignment also explores concepts of torque, gear ratios, power, and angular momentum in mechanical systems, along with a discussion of braking systems in trains and cars. Finally, the document includes a comparison of different thermometers and the application of physics principles to vehicle dynamics.

Running head: PHYSICS SUMS ASSIGNMENT
Physics Sums Assignment
Name of the Student:
Student ID:
Name of the University:
Author’s note:
Physics Sums Assignment
Name of the Student:
Student ID:
Name of the University:
Author’s note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
1PHYSICS SUMS ASSIGNMENT
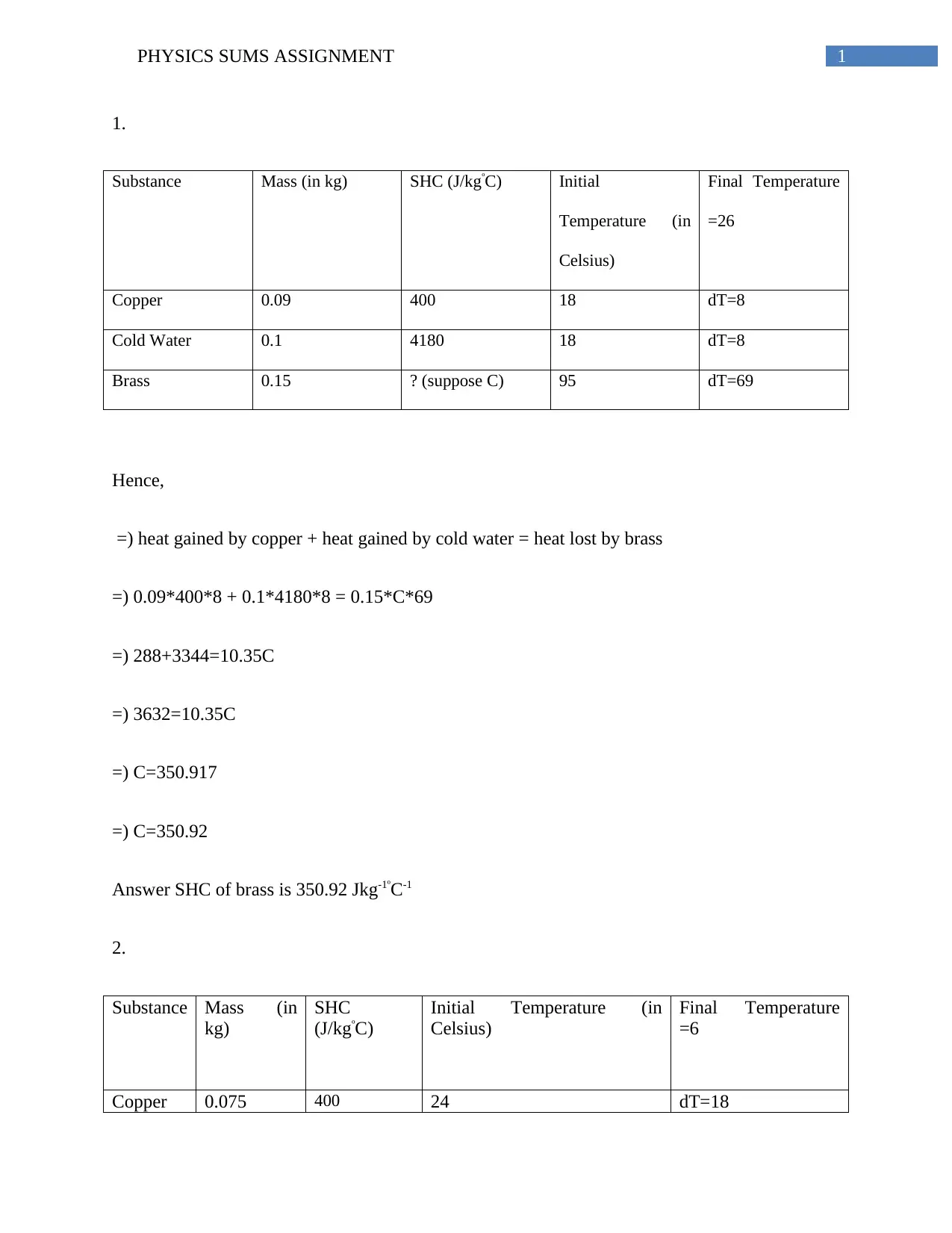
1.
Substance Mass (in kg) SHC (J/kgºC) Initial
Temperature (in
Celsius)
Final Temperature
=26
Copper 0.09 400 18 dT=8
Cold Water 0.1 4180 18 dT=8
Brass 0.15 ? (suppose C) 95 dT=69
Hence,
=) heat gained by copper + heat gained by cold water = heat lost by brass
=) 0.09*400*8 + 0.1*4180*8 = 0.15*C*69
=) 288+3344=10.35C
=) 3632=10.35C
=) C=350.917
=) C=350.92
Answer SHC of brass is 350.92 Jkg-1ºC-1
2.
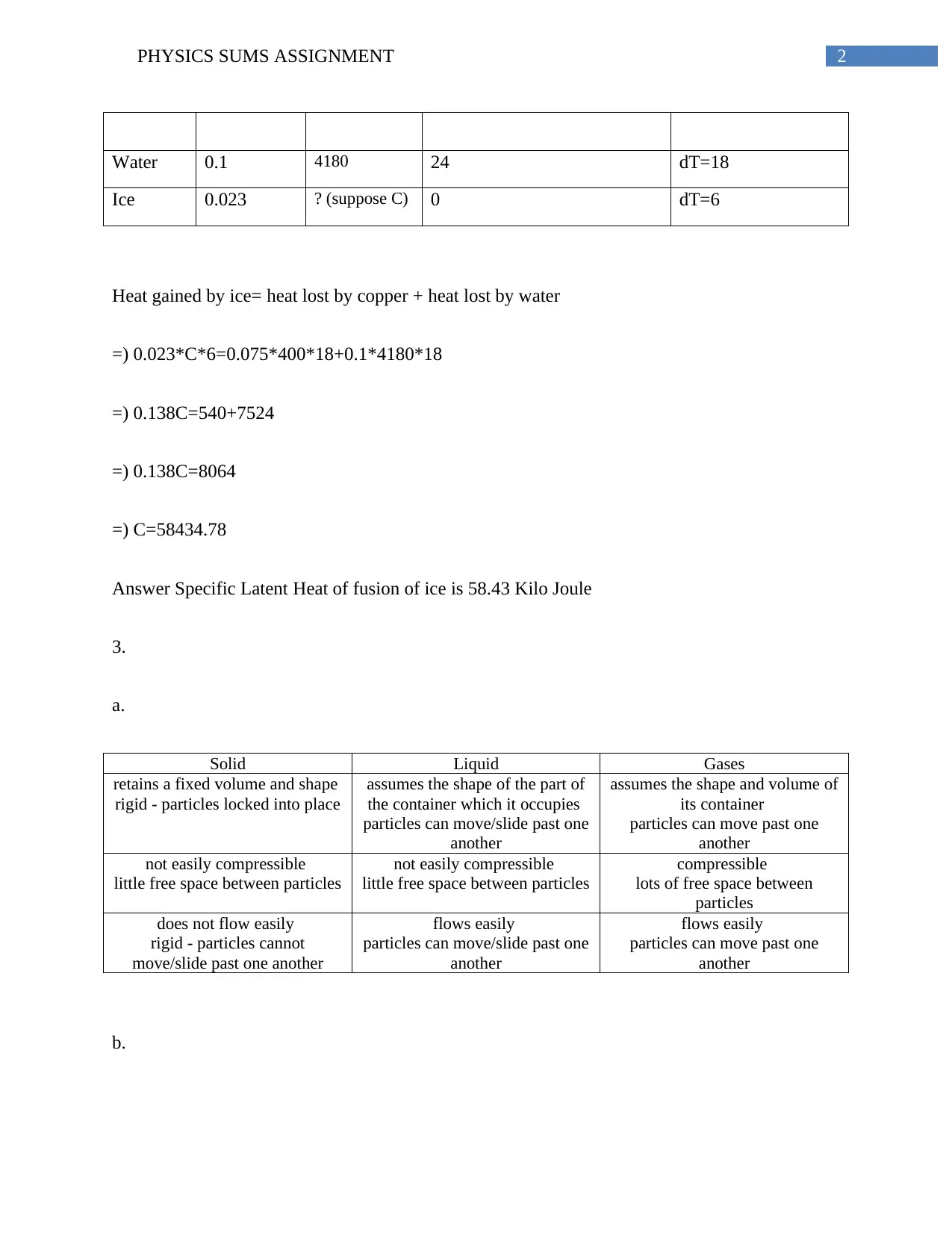
Substance Mass (in
kg)
SHC
(J/kgºC)
Initial Temperature (in
Celsius)
Final Temperature
=6
Copper 0.075 400 24 dT=18
1.
Substance Mass (in kg) SHC (J/kgºC) Initial
Temperature (in
Celsius)
Final Temperature
=26
Copper 0.09 400 18 dT=8
Cold Water 0.1 4180 18 dT=8
Brass 0.15 ? (suppose C) 95 dT=69
Hence,
=) heat gained by copper + heat gained by cold water = heat lost by brass
=) 0.09*400*8 + 0.1*4180*8 = 0.15*C*69
=) 288+3344=10.35C
=) 3632=10.35C
=) C=350.917
=) C=350.92
Answer SHC of brass is 350.92 Jkg-1ºC-1
2.
Substance Mass (in
kg)
SHC
(J/kgºC)
Initial Temperature (in
Celsius)
Final Temperature
=6
Copper 0.075 400 24 dT=18
2PHYSICS SUMS ASSIGNMENT
Water 0.1 4180 24 dT=18
Ice 0.023 ? (suppose C) 0 dT=6
Heat gained by ice= heat lost by copper + heat lost by water
=) 0.023*C*6=0.075*400*18+0.1*4180*18
=) 0.138C=540+7524
=) 0.138C=8064
=) C=58434.78
Answer Specific Latent Heat of fusion of ice is 58.43 Kilo Joule
3.
a.
Solid Liquid Gases
retains a fixed volume and shape
rigid - particles locked into place
assumes the shape of the part of
the container which it occupies
particles can move/slide past one
another
assumes the shape and volume of
its container
particles can move past one
another
not easily compressible
little free space between particles
not easily compressible
little free space between particles
compressible
lots of free space between
particles
does not flow easily
rigid - particles cannot
move/slide past one another
flows easily
particles can move/slide past one
another
flows easily
particles can move past one
another
b.
Water 0.1 4180 24 dT=18
Ice 0.023 ? (suppose C) 0 dT=6
Heat gained by ice= heat lost by copper + heat lost by water
=) 0.023*C*6=0.075*400*18+0.1*4180*18
=) 0.138C=540+7524
=) 0.138C=8064
=) C=58434.78
Answer Specific Latent Heat of fusion of ice is 58.43 Kilo Joule
3.
a.
Solid Liquid Gases
retains a fixed volume and shape
rigid - particles locked into place
assumes the shape of the part of
the container which it occupies
particles can move/slide past one
another
assumes the shape and volume of
its container
particles can move past one
another
not easily compressible
little free space between particles
not easily compressible
little free space between particles
compressible
lots of free space between
particles
does not flow easily
rigid - particles cannot
move/slide past one another
flows easily
particles can move/slide past one
another
flows easily
particles can move past one
another
b.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3PHYSICS SUMS ASSIGNMENT
The ice cube would consume the heat from the atmosphere and the molecular forces binding the
ice cube in the solid structure would be decreased causing the ice to melt in liquid state.
c.
The Specific Latent Heat of fusion of ice is 58.43 Kilo Joule and the specific heat capacity of
water is 4180 Joule. Hence, ice and water consumes lot of energy in compare to other
substances.
d.
The temperature of the gas can be defined as the measurement of the mean kinetic energy present
in the gas. The pressure can be defined as the force acting on the unit area of the gas.
e.
PV=nRT (where R=8.314J/mol K)
V=0.12^3 = 0.001728= 1728*10-6
n=4.0*1026/1.5*1023=2.67*103
T=336.15
P=2.67*103*8.314*336.15/1728*10-6 =4.313*109
Pressure on each wall is 4.313*109 Pa
f.
F=P*A = 4.313*109 *0.0144 = 62.10*106 N/m2
The ice cube would consume the heat from the atmosphere and the molecular forces binding the
ice cube in the solid structure would be decreased causing the ice to melt in liquid state.
c.
The Specific Latent Heat of fusion of ice is 58.43 Kilo Joule and the specific heat capacity of
water is 4180 Joule. Hence, ice and water consumes lot of energy in compare to other
substances.
d.
The temperature of the gas can be defined as the measurement of the mean kinetic energy present
in the gas. The pressure can be defined as the force acting on the unit area of the gas.
e.
PV=nRT (where R=8.314J/mol K)
V=0.12^3 = 0.001728= 1728*10-6
n=4.0*1026/1.5*1023=2.67*103
T=336.15
P=2.67*103*8.314*336.15/1728*10-6 =4.313*109
Pressure on each wall is 4.313*109 Pa
f.
F=P*A = 4.313*109 *0.0144 = 62.10*106 N/m2
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4PHYSICS SUMS ASSIGNMENT
g.
Using Ideal gas Equation,
1 mole of any gas occupies 2.24 m3 at stp (standard temperature and pressure, taken as 273K and
101325 Pa
P1V1/n1T1=P2V2/n2T2
Therefore,
n2= P2V2 T1/P1V1 T2 (since n1=1)
= 4.313*109*1728*10-6*273/101325*2.24*336.15
= 26.67
Answer 26.67 moles
4.
a.
Different thermometers are used for measuring the temperature of bodies (living and non living)
based on the range of temperature and method of measuring. For example, the probe
thermometer is used for calculating the temperature of the food, liquids, and semi solids with the
help of an antenna attached to it. The K-type thermocouples are helpful for measuring extreme
temperatures and used in laboratories and industrial sectors.
b.
g.
Using Ideal gas Equation,
1 mole of any gas occupies 2.24 m3 at stp (standard temperature and pressure, taken as 273K and
101325 Pa
P1V1/n1T1=P2V2/n2T2
Therefore,
n2= P2V2 T1/P1V1 T2 (since n1=1)
= 4.313*109*1728*10-6*273/101325*2.24*336.15
= 26.67
Answer 26.67 moles
4.
a.
Different thermometers are used for measuring the temperature of bodies (living and non living)
based on the range of temperature and method of measuring. For example, the probe
thermometer is used for calculating the temperature of the food, liquids, and semi solids with the
help of an antenna attached to it. The K-type thermocouples are helpful for measuring extreme
temperatures and used in laboratories and industrial sectors.
b.
5PHYSICS SUMS ASSIGNMENT
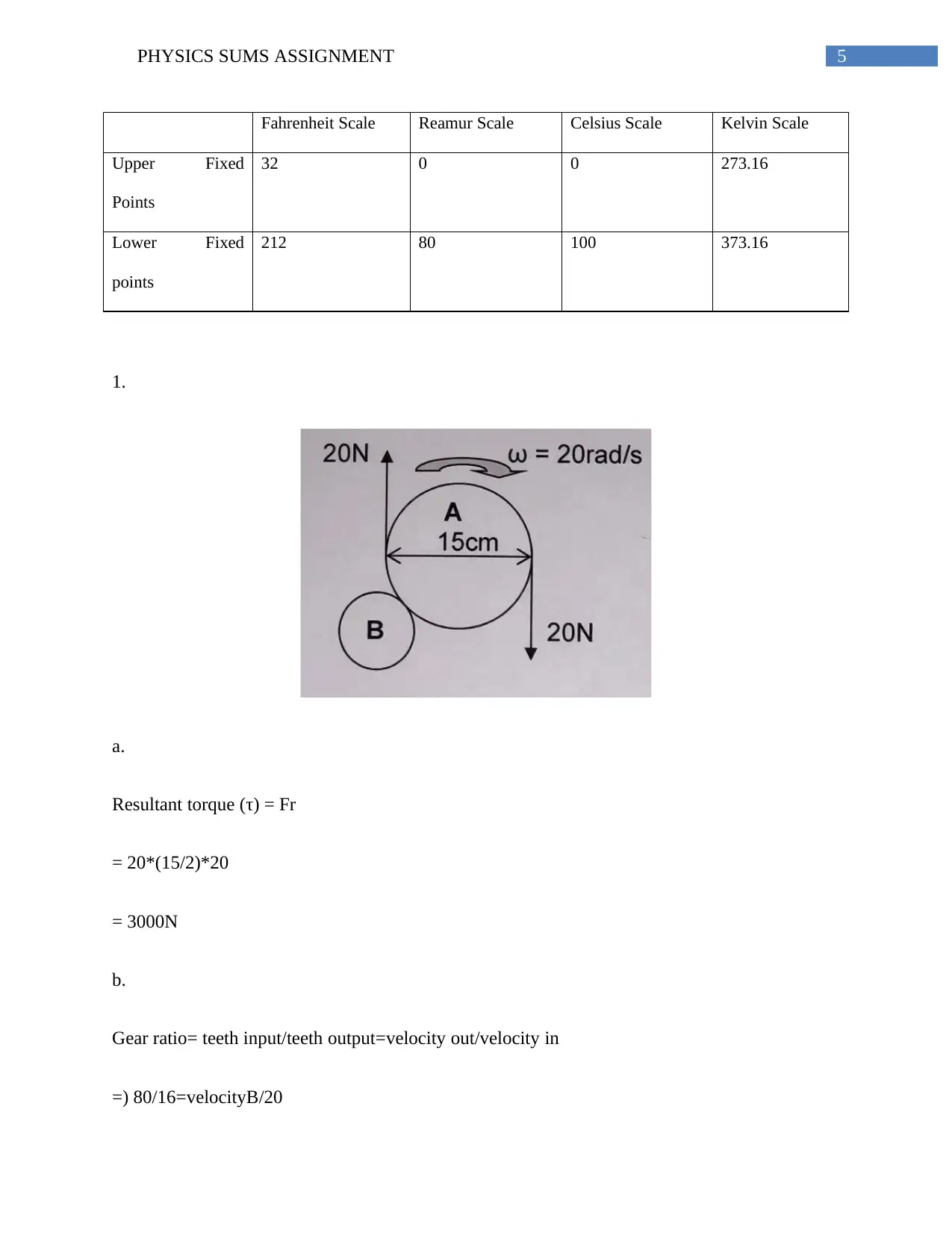
Fahrenheit Scale Reamur Scale Celsius Scale Kelvin Scale
Upper Fixed
Points
32 0 0 273.16
Lower Fixed
points
212 80 100 373.16
1.
a.
Resultant torque (τ) = Fr
= 20*(15/2)*20
= 3000N
b.
Gear ratio= teeth input/teeth output=velocity out/velocity in
=) 80/16=velocityB/20
Fahrenheit Scale Reamur Scale Celsius Scale Kelvin Scale
Upper Fixed
Points
32 0 0 273.16
Lower Fixed
points
212 80 100 373.16
1.
a.
Resultant torque (τ) = Fr
= 20*(15/2)*20
= 3000N
b.
Gear ratio= teeth input/teeth output=velocity out/velocity in
=) 80/16=velocityB/20
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6PHYSICS SUMS ASSIGNMENT
=) velocity=100
Answer Velocity of B gear= 100rad/s
c.
Resultant torque (τ) = Fr
r can be calculated by using 1:5 ratio between A and B gear
r=15/2*5 =1.5
Resultant torque (τ) =20*1.5*100
= 3000N
d.
Power=torgue.2pi.velocity
=3000*2*3.14*20
= 376991.12
e.
Power=torgue.2pi.velocity
=3000*2*3.14*100
=1884955.59
f.
=) velocity=100
Answer Velocity of B gear= 100rad/s
c.
Resultant torque (τ) = Fr
r can be calculated by using 1:5 ratio between A and B gear
r=15/2*5 =1.5
Resultant torque (τ) =20*1.5*100
= 3000N
d.
Power=torgue.2pi.velocity
=3000*2*3.14*20
= 376991.12
e.
Power=torgue.2pi.velocity
=3000*2*3.14*100
=1884955.59
f.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7PHYSICS SUMS ASSIGNMENT
KE=I*v2/2
=0.20*202/2
=40kg/m2s2
Angular Momentum= inertia*angular speed
=0.20*20
=4kg/m2s
2.
a.
Advantages of using triple valve brakes: The triple valve breaks helps in charging air into an air
tank that is ready to be used, applying the brakes, and releasing them. It is used in Trains mostly
b.
In modern cars, the application of the brakes allows the energy transfer of electrical to chemical
to mechanical. The battery of the car helps in combustion of the petrol which in turn would force
the car to stop.
c.
Element Mass (in kg) SHC (J/kgºC) Initial Kinetic
Energy
Final Kinetic
Energy=30%
Car 1700 2500 6.659*106 dt= 4.661*106
KE=I*v2/2
=0.20*202/2
=40kg/m2s2
Angular Momentum= inertia*angular speed
=0.20*20
=4kg/m2s
2.
a.
Advantages of using triple valve brakes: The triple valve breaks helps in charging air into an air
tank that is ready to be used, applying the brakes, and releasing them. It is used in Trains mostly
b.
In modern cars, the application of the brakes allows the energy transfer of electrical to chemical
to mechanical. The battery of the car helps in combustion of the petrol which in turn would force
the car to stop.
c.
Element Mass (in kg) SHC (J/kgºC) Initial Kinetic
Energy
Final Kinetic
Energy=30%
Car 1700 2500 6.659*106 dt= 4.661*106

8PHYSICS SUMS ASSIGNMENT
Initial velocity= 88.51
Final velocity=0
Initial temp= 305K
dE=mCdT
4.661*106=2.7*2500*dT
=) dT=4.661*106/(2.7*2500)
=) dT= 690.52
Hence, the final temperature could be 305+690.52
= 995.52K
= 722.52ºC
3.
a. Since while turning the bike can bend for getting better angular frequency and hence managing
the sharp turns easily with static Inertia and gaining acceleration for high speed. Hence, bike is
better for sharp turns and high speed in compare to the trucks.
b. The driving steady, smoothly and safely across rugged terrain would be easily managed by the
trucks as the trucks have bigger mass and hence they can gain better Inertia while driving on
rugged terrain.
Initial velocity= 88.51
Final velocity=0
Initial temp= 305K
dE=mCdT
4.661*106=2.7*2500*dT
=) dT=4.661*106/(2.7*2500)
=) dT= 690.52
Hence, the final temperature could be 305+690.52
= 995.52K
= 722.52ºC
3.
a. Since while turning the bike can bend for getting better angular frequency and hence managing
the sharp turns easily with static Inertia and gaining acceleration for high speed. Hence, bike is
better for sharp turns and high speed in compare to the trucks.
b. The driving steady, smoothly and safely across rugged terrain would be easily managed by the
trucks as the trucks have bigger mass and hence they can gain better Inertia while driving on
rugged terrain.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9PHYSICS SUMS ASSIGNMENT
Bibliography
Bejan, A., 2016. Advanced engineering thermodynamics. John Wiley & Sons.
Brandao, F., Horodecki, M., Ng, N., Oppenheim, J. and Wehner, S., 2015. The second laws of
quantum thermodynamics. Proceedings of the National Academy of Sciences, 112(11), pp.3275-
3279.
D'Alessio, L., Kafri, Y., Polkovnikov, A. and Rigol, M., 2016. From quantum chaos and
eigenstate thermalization to statistical mechanics and thermodynamics. Advances in Physics,
65(3), pp.239-362.
Kondepudi, D. and Prigogine, I., 2014. Modern thermodynamics: from heat engines to
dissipative structures. John Wiley & Sons.
Parrondo, J.M., Horowitz, J.M. and Sagawa, T., 2015. Thermodynamics of information. Nature
physics, 11(2), p.131.
Sandler, S.I., 2017. Chemical, biochemical, and engineering thermodynamics. John Wiley &
Sons.
Bibliography
Bejan, A., 2016. Advanced engineering thermodynamics. John Wiley & Sons.
Brandao, F., Horodecki, M., Ng, N., Oppenheim, J. and Wehner, S., 2015. The second laws of
quantum thermodynamics. Proceedings of the National Academy of Sciences, 112(11), pp.3275-
3279.
D'Alessio, L., Kafri, Y., Polkovnikov, A. and Rigol, M., 2016. From quantum chaos and
eigenstate thermalization to statistical mechanics and thermodynamics. Advances in Physics,
65(3), pp.239-362.
Kondepudi, D. and Prigogine, I., 2014. Modern thermodynamics: from heat engines to
dissipative structures. John Wiley & Sons.
Parrondo, J.M., Horowitz, J.M. and Sagawa, T., 2015. Thermodynamics of information. Nature
physics, 11(2), p.131.
Sandler, S.I., 2017. Chemical, biochemical, and engineering thermodynamics. John Wiley &
Sons.
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.