Physics Report: Li4Ti5O12 Battery Electrode Material Analysis
VerifiedAdded on 2022/11/25
|5
|792
|229
Report
AI Summary
This report delves into the physics of lithium-ion batteries, with a specific focus on the spinel titanate family, particularly Li4Ti5O12, as an electrode material. It explores the significance of high power-density, high energy density, and long lifespan in Li-ion batteries, especially for electric vehicles. The report investigates the role of work function and contact potential in influencing the performance of Li4Ti5O12, including its chemical stability in electrolytes and its impact on electron transfer. It also examines the effects of metal additives and particle size reduction on battery performance, discussing the potential for crystal reconstruction and the introduction of defects. The report references key studies to support its analysis of Li-ion battery physics.

PHYSICS/ELECTROCHEM - BATTERY PHYSICS
By Name
Course
Instructor
Institution
Location
Date
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Introduction
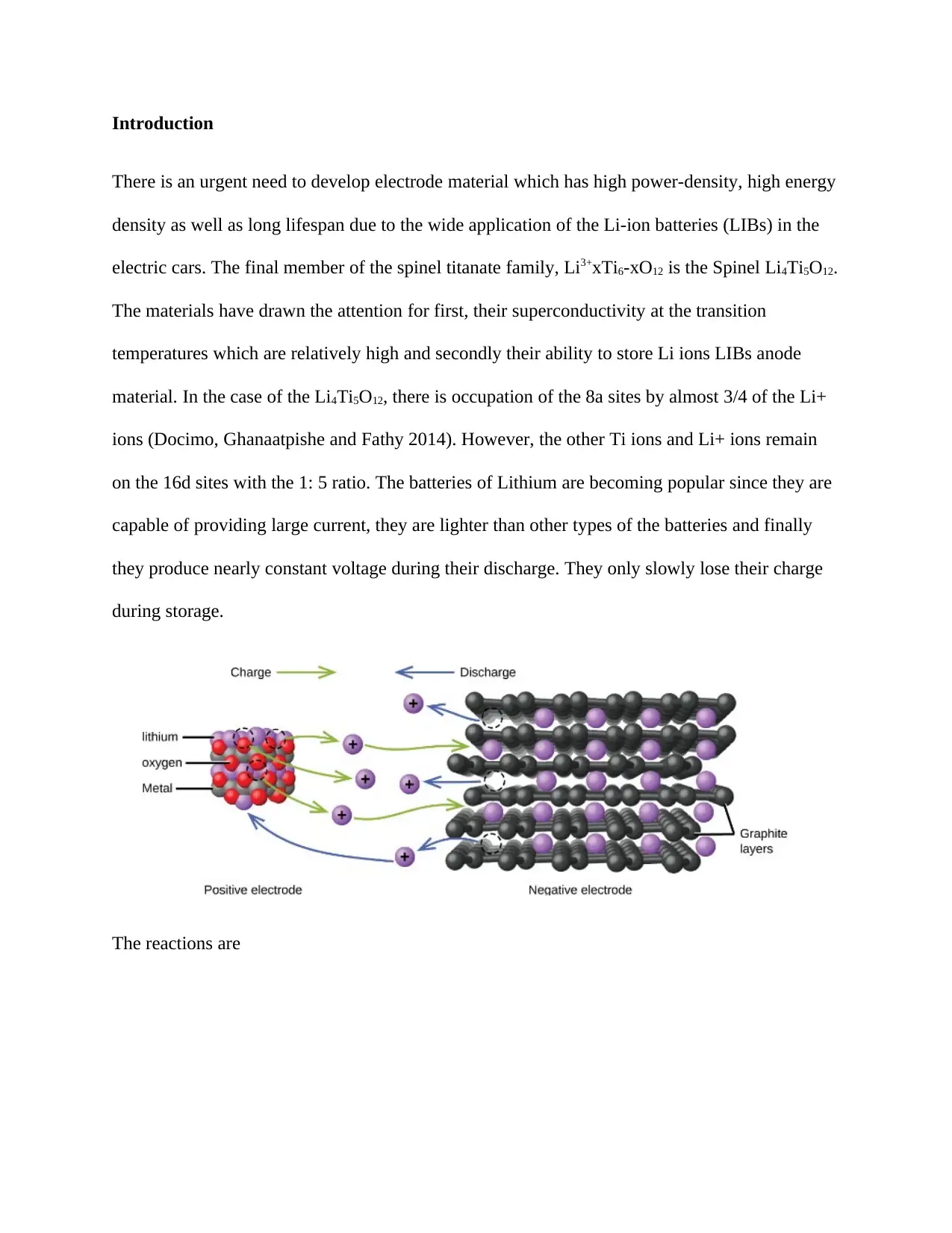
There is an urgent need to develop electrode material which has high power-density, high energy
density as well as long lifespan due to the wide application of the Li-ion batteries (LIBs) in the
electric cars. The final member of the spinel titanate family, Li3+xTi6-xO12 is the Spinel Li4Ti5O12.
The materials have drawn the attention for first, their superconductivity at the transition
temperatures which are relatively high and secondly their ability to store Li ions LIBs anode
material. In the case of the Li4Ti5O12, there is occupation of the 8a sites by almost 3/4 of the Li+
ions (Docimo, Ghanaatpishe and Fathy 2014). However, the other Ti ions and Li+ ions remain
on the 16d sites with the 1: 5 ratio. The batteries of Lithium are becoming popular since they are
capable of providing large current, they are lighter than other types of the batteries and finally
they produce nearly constant voltage during their discharge. They only slowly lose their charge
during storage.
The reactions are
There is an urgent need to develop electrode material which has high power-density, high energy
density as well as long lifespan due to the wide application of the Li-ion batteries (LIBs) in the
electric cars. The final member of the spinel titanate family, Li3+xTi6-xO12 is the Spinel Li4Ti5O12.
The materials have drawn the attention for first, their superconductivity at the transition
temperatures which are relatively high and secondly their ability to store Li ions LIBs anode
material. In the case of the Li4Ti5O12, there is occupation of the 8a sites by almost 3/4 of the Li+
ions (Docimo, Ghanaatpishe and Fathy 2014). However, the other Ti ions and Li+ ions remain
on the 16d sites with the 1: 5 ratio. The batteries of Lithium are becoming popular since they are
capable of providing large current, they are lighter than other types of the batteries and finally
they produce nearly constant voltage during their discharge. They only slowly lose their charge
during storage.
The reactions are

When the coefficient is representing moles, the value of X is no more than 0.5moles. The voltage
of the battery is usually about 3.7 V.
Work Function
In order to understand the field emission properties of electrodes as cathode as anode in the
LIBs, workfunction is very critical. Workfunction is defined as the minimum energy needed to
take away one electron from the position described as Fermi level, to the vacuum level. This
makes it potentially capable in the expression of the electron loss capability from the surface of
Li4Ti5O12. . Work function therefore affects the chemical stability of Li4Ti5O12 which has been
immersed in the electrolyte (Do et al.2018). This is experienced in the detailed redox reaction
which takes place between the electrolyte and Li4Ti5O12 of Lithium ion batteries. The transfer of
the ions in the LIBs will therefore only be possible once the last value or the threshold value of
the workfunction is met since it will directly influence the electron dislodge from the cathode to
the anode (Seaman, Dao and McPhee 2014).
Contact Potential
The contact potential affects the operating density of the current per unit area. It will also
improve the anode/cathode rate of the reaction with the given rated current. This is one of the
ways of ameliorating the transfer of charges through polarization (Do et al.2018). Reducing the
size of the particles in the order of nanometers magnitude is a technique of increasing the limit of
of the battery is usually about 3.7 V.
Work Function
In order to understand the field emission properties of electrodes as cathode as anode in the
LIBs, workfunction is very critical. Workfunction is defined as the minimum energy needed to
take away one electron from the position described as Fermi level, to the vacuum level. This
makes it potentially capable in the expression of the electron loss capability from the surface of
Li4Ti5O12. . Work function therefore affects the chemical stability of Li4Ti5O12 which has been
immersed in the electrolyte (Do et al.2018). This is experienced in the detailed redox reaction
which takes place between the electrolyte and Li4Ti5O12 of Lithium ion batteries. The transfer of
the ions in the LIBs will therefore only be possible once the last value or the threshold value of
the workfunction is met since it will directly influence the electron dislodge from the cathode to
the anode (Seaman, Dao and McPhee 2014).
Contact Potential
The contact potential affects the operating density of the current per unit area. It will also
improve the anode/cathode rate of the reaction with the given rated current. This is one of the
ways of ameliorating the transfer of charges through polarization (Do et al.2018). Reducing the
size of the particles in the order of nanometers magnitude is a technique of increasing the limit of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

the solid solution. Similarly, there is simultaneous introduction of more defects like interstitials
or vacancies to improve the intrinsic mobility of the Li ions.
Metal additive/combination impacts on the performance
There will be occurrence of the contact problem between conductive additive materials like
metals and the active particles. In addition, where there is a decrease in the size of the particles in
the magnitude order of nanometer, there is likelihood of the occurrence of other unknown effects
(Bukun et al.2013). For example, the reconstruction of the crystals of the surface as well as the
structures of the electronics as a result of the nanocrystallization will affect the performance of
the LIBs cells. It is important to note that some of these additives increases resistance to the
moving electrons that have already been dislodged from cathode as they move to the anode.
or vacancies to improve the intrinsic mobility of the Li ions.
Metal additive/combination impacts on the performance
There will be occurrence of the contact problem between conductive additive materials like
metals and the active particles. In addition, where there is a decrease in the size of the particles in
the magnitude order of nanometer, there is likelihood of the occurrence of other unknown effects
(Bukun et al.2013). For example, the reconstruction of the crystals of the surface as well as the
structures of the electronics as a result of the nanocrystallization will affect the performance of
the LIBs cells. It is important to note that some of these additives increases resistance to the
moving electrons that have already been dislodged from cathode as they move to the anode.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

REFERENCES
Bukun, N.G., Grafov, B.M. and Dobrovol’skii, Y.A., 2013. XI Meeting “Fundamental Problems
of Solid State Ionics”. Russian Journal of Electrochemistry, 49(7), pp.599-599.
Do, J.Y., Son, N., Park, N.K., Kwak, B.S., Baek, J.I., Ryu, H.J. and Kang, M., 2018. Reliable
oxygen transfer in MgAl2O4 spinel through the reversible formation of oxygen vacancies by
Cu2+/Fe3+ anchoring. Applied energy, 219, pp.138-150.
Docimo, D., Ghanaatpishe, M. and Fathy, H.K., 2014, December. Development and
experimental parameterization of a physics-based second-order lithium-ion battery model.
In ASME 2014 dynamic systems and control conference. American Society of Mechanical
Engineers Digital Collection.
Seaman, A., Dao, T.S. and McPhee, J., 2014. A survey of mathematics-based equivalent-circuit
and electrochemical battery models for hybrid and electric vehicle simulation. Journal of Power
Sources, 256, pp.410-423.
Bukun, N.G., Grafov, B.M. and Dobrovol’skii, Y.A., 2013. XI Meeting “Fundamental Problems
of Solid State Ionics”. Russian Journal of Electrochemistry, 49(7), pp.599-599.
Do, J.Y., Son, N., Park, N.K., Kwak, B.S., Baek, J.I., Ryu, H.J. and Kang, M., 2018. Reliable
oxygen transfer in MgAl2O4 spinel through the reversible formation of oxygen vacancies by
Cu2+/Fe3+ anchoring. Applied energy, 219, pp.138-150.
Docimo, D., Ghanaatpishe, M. and Fathy, H.K., 2014, December. Development and
experimental parameterization of a physics-based second-order lithium-ion battery model.
In ASME 2014 dynamic systems and control conference. American Society of Mechanical
Engineers Digital Collection.
Seaman, A., Dao, T.S. and McPhee, J., 2014. A survey of mathematics-based equivalent-circuit
and electrochemical battery models for hybrid and electric vehicle simulation. Journal of Power
Sources, 256, pp.410-423.
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


