Research problem and systematic search strategy for NURS6O30 Research and Evidence
VerifiedAdded on 2022/10/02
|10
|3682
|381
AI Summary
This document provides a research problem and systematic search strategy for NURS6O30 Research and Evidence. It includes PICO quantitative question, search strategy for literature, and characteristics of studies table.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

1 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
NURS6O30 Research and Evidence
ASSESSMENT 1: Research problem and systematic search strategy
Due: Week 4 Friday 30th August 24:00 hrs
Weight: 30%
NURS6O30 Research and Evidence
ASSESSMENT 1: Research problem and systematic search strategy
Due: Week 4 Friday 30th August 24:00 hrs
Weight: 30%
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
2 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
PART A: BACKGROUND SEARCH
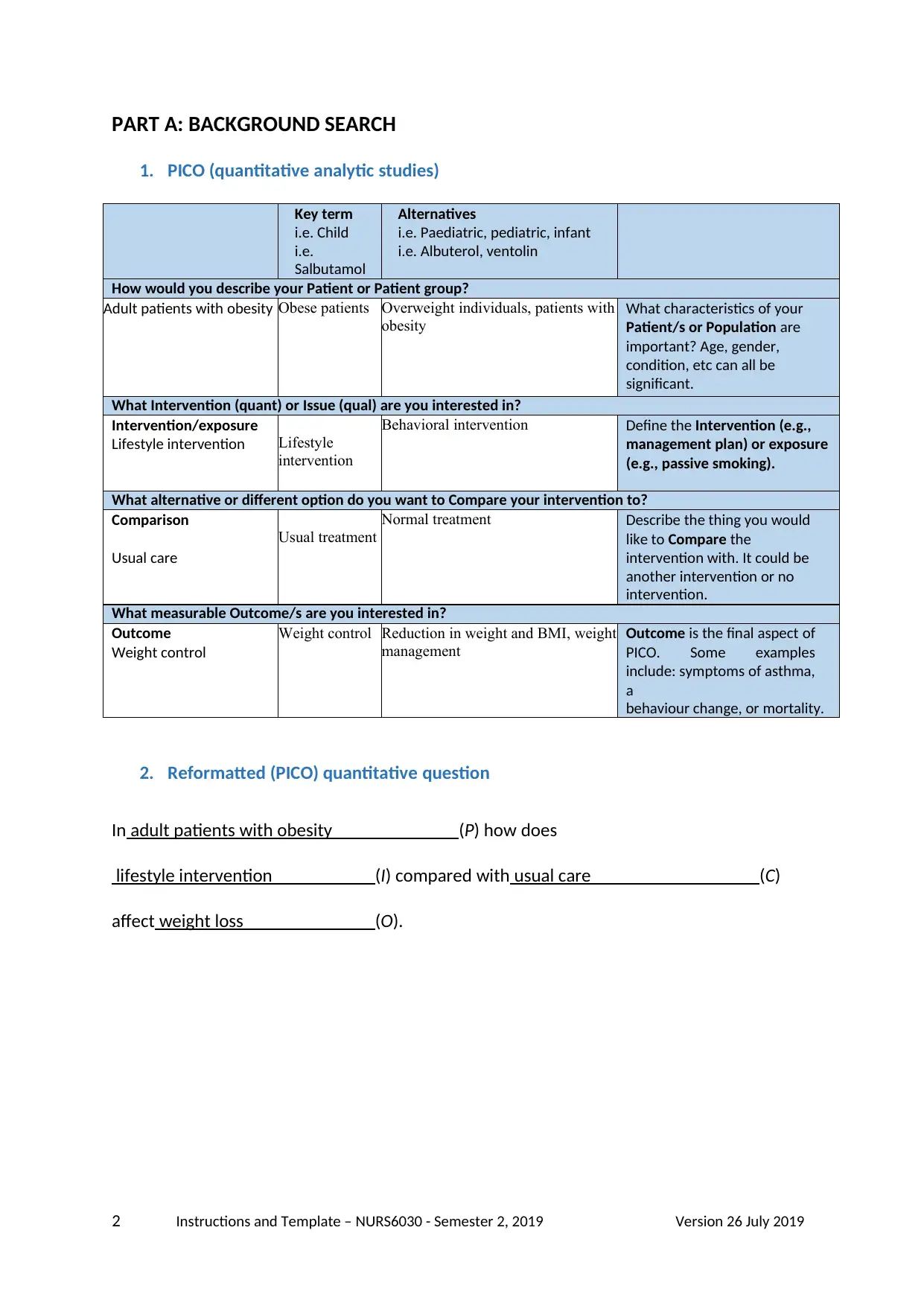
1. PICO (quantitative analytic studies)
Key term
i.e. Child
i.e.
Salbutamol
Alternatives
i.e. Paediatric, pediatric, infant
i.e. Albuterol, ventolin
How would you describe your Patient or Patient group?
Adult patients with obesity Obese patients Overweight individuals, patients with
obesity
What characteristics of your
Patient/s or Population are
important? Age, gender,
condition, etc can all be
significant.
What Intervention (quant) or Issue (qual) are you interested in?
Intervention/exposure
Lifestyle intervention Lifestyle
intervention
Behavioral intervention Define the Intervention (e.g.,
management plan) or exposure
(e.g., passive smoking).
What alternative or different option do you want to Compare your intervention to?
Comparison
Usual care
Usual treatment
Normal treatment Describe the thing you would
like to Compare the
intervention with. It could be
another intervention or no
intervention.
What measurable Outcome/s are you interested in?
Outcome
Weight control
Weight control Reduction in weight and BMI, weight
management
Outcome is the final aspect of
PICO. Some examples
include: symptoms of asthma,
a
behaviour change, or mortality.
2. Reformatted (PICO) quantitative question
In adult patients with obesity (P) how does
lifestyle intervention (I) compared with usual care (C)
affect weight loss (O).
PART A: BACKGROUND SEARCH
1. PICO (quantitative analytic studies)
Key term
i.e. Child
i.e.
Salbutamol
Alternatives
i.e. Paediatric, pediatric, infant
i.e. Albuterol, ventolin
How would you describe your Patient or Patient group?
Adult patients with obesity Obese patients Overweight individuals, patients with
obesity
What characteristics of your
Patient/s or Population are
important? Age, gender,
condition, etc can all be
significant.
What Intervention (quant) or Issue (qual) are you interested in?
Intervention/exposure
Lifestyle intervention Lifestyle
intervention
Behavioral intervention Define the Intervention (e.g.,
management plan) or exposure
(e.g., passive smoking).
What alternative or different option do you want to Compare your intervention to?
Comparison
Usual care
Usual treatment
Normal treatment Describe the thing you would
like to Compare the
intervention with. It could be
another intervention or no
intervention.
What measurable Outcome/s are you interested in?
Outcome
Weight control
Weight control Reduction in weight and BMI, weight
management
Outcome is the final aspect of
PICO. Some examples
include: symptoms of asthma,
a
behaviour change, or mortality.
2. Reformatted (PICO) quantitative question
In adult patients with obesity (P) how does
lifestyle intervention (I) compared with usual care (C)
affect weight loss (O).

3 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
1. Search Strategy for literature to support proposal
Fill out the search strategy for your question – add or delete lines as necessary.
Database used: CINAHL
Key terms and MESH terms:
1 Obese adults
2 Adults with obesity
3 Patients with obesity
4 Obese OR overweight patients
5 Obese individuals OR obese patients OR high BMI patients
6 Lifestyle intervention OR behavioral intervention
7 Diet AND physical activity intervention
8 Physical activity AND education intervention
9 Exercise AND behavioral therapy
10 Education AND Diet AND physical activity
11 Behavioral management OR lifestyle management
12 Usual care
13 Clinical treatment
14 Pharmacological treatment
15 Physician visit
16 Normal treatment or clinical care
17 Weight loss
18 Weight reduction
19 Obesity control
20 Reduction in BMI
21 Weight loss OR Weight reduction OR obesity control OR BMI reduction
22 Weight outcomes AND BMI; Obesity AND BMI, weight reduction AND BMI
23 Reduction in obesity issues OR decrease in obesity issues
P
I
C
O
1. Search Strategy for literature to support proposal
Fill out the search strategy for your question – add or delete lines as necessary.
Database used: CINAHL
Key terms and MESH terms:
1 Obese adults
2 Adults with obesity
3 Patients with obesity
4 Obese OR overweight patients
5 Obese individuals OR obese patients OR high BMI patients
6 Lifestyle intervention OR behavioral intervention
7 Diet AND physical activity intervention
8 Physical activity AND education intervention
9 Exercise AND behavioral therapy
10 Education AND Diet AND physical activity
11 Behavioral management OR lifestyle management
12 Usual care
13 Clinical treatment
14 Pharmacological treatment
15 Physician visit
16 Normal treatment or clinical care
17 Weight loss
18 Weight reduction
19 Obesity control
20 Reduction in BMI
21 Weight loss OR Weight reduction OR obesity control OR BMI reduction
22 Weight outcomes AND BMI; Obesity AND BMI, weight reduction AND BMI
23 Reduction in obesity issues OR decrease in obesity issues
P
I
C
O
4 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
PART B: CHARACTERISTICS OF STUDIES TABLE
2. Key papers (500 words maximum)
Select five (5) individual study papers (not reviews) that are relevant to your overall clinical question. Document the characterises of the
chosen papers using the below table. Use your own words to describe them.
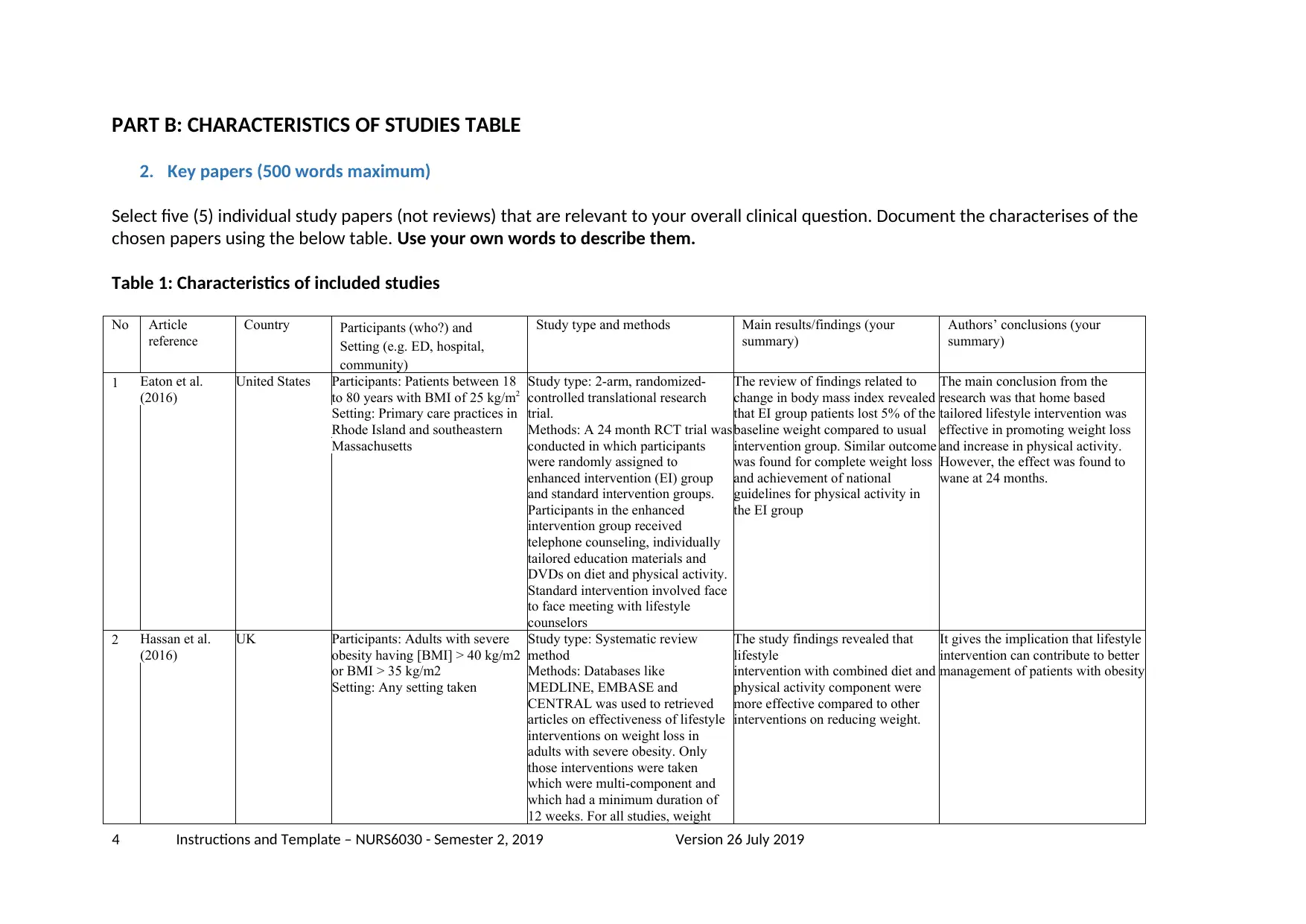
Table 1: Characteristics of included studies
No Article
reference
Country Participants (who?) and
Setting (e.g. ED, hospital,
community)
Study type and methods Main results/findings (your
summary)
Authors’ conclusions (your
summary)
1 Eaton et al.
(2016)
United States Participants: Patients between 18
to 80 years with BMI of 25 kg/m2
Setting: Primary care practices in
Rhode Island and southeastern
Massachusetts
Study type: 2-arm, randomized-
controlled translational research
trial.
Methods: A 24 month RCT trial was
conducted in which participants
were randomly assigned to
enhanced intervention (EI) group
and standard intervention groups.
Participants in the enhanced
intervention group received
telephone counseling, individually
tailored education materials and
DVDs on diet and physical activity.
Standard intervention involved face
to face meeting with lifestyle
counselors
The review of findings related to
change in body mass index revealed
that EI group patients lost 5% of the
baseline weight compared to usual
intervention group. Similar outcome
was found for complete weight loss
and achievement of national
guidelines for physical activity in
the EI group
The main conclusion from the
research was that home based
tailored lifestyle intervention was
effective in promoting weight loss
and increase in physical activity.
However, the effect was found to
wane at 24 months.
2 Hassan et al.
(2016)
UK Participants: Adults with severe
obesity having [BMI] > 40 kg/m2
or BMI > 35 kg/m2
Setting: Any setting taken
Study type: Systematic review
method
Methods: Databases like
MEDLINE, EMBASE and
CENTRAL was used to retrieved
articles on effectiveness of lifestyle
interventions on weight loss in
adults with severe obesity. Only
those interventions were taken
which were multi-component and
which had a minimum duration of
12 weeks. For all studies, weight
The study findings revealed that
lifestyle
intervention with combined diet and
physical activity component were
more effective compared to other
interventions on reducing weight.
It gives the implication that lifestyle
intervention can contribute to better
management of patients with obesity
PART B: CHARACTERISTICS OF STUDIES TABLE
2. Key papers (500 words maximum)
Select five (5) individual study papers (not reviews) that are relevant to your overall clinical question. Document the characterises of the
chosen papers using the below table. Use your own words to describe them.
Table 1: Characteristics of included studies
No Article
reference
Country Participants (who?) and
Setting (e.g. ED, hospital,
community)
Study type and methods Main results/findings (your
summary)
Authors’ conclusions (your
summary)
1 Eaton et al.
(2016)
United States Participants: Patients between 18
to 80 years with BMI of 25 kg/m2
Setting: Primary care practices in
Rhode Island and southeastern
Massachusetts
Study type: 2-arm, randomized-
controlled translational research
trial.
Methods: A 24 month RCT trial was
conducted in which participants
were randomly assigned to
enhanced intervention (EI) group
and standard intervention groups.
Participants in the enhanced
intervention group received
telephone counseling, individually
tailored education materials and
DVDs on diet and physical activity.
Standard intervention involved face
to face meeting with lifestyle
counselors
The review of findings related to
change in body mass index revealed
that EI group patients lost 5% of the
baseline weight compared to usual
intervention group. Similar outcome
was found for complete weight loss
and achievement of national
guidelines for physical activity in
the EI group
The main conclusion from the
research was that home based
tailored lifestyle intervention was
effective in promoting weight loss
and increase in physical activity.
However, the effect was found to
wane at 24 months.
2 Hassan et al.
(2016)
UK Participants: Adults with severe
obesity having [BMI] > 40 kg/m2
or BMI > 35 kg/m2
Setting: Any setting taken
Study type: Systematic review
method
Methods: Databases like
MEDLINE, EMBASE and
CENTRAL was used to retrieved
articles on effectiveness of lifestyle
interventions on weight loss in
adults with severe obesity. Only
those interventions were taken
which were multi-component and
which had a minimum duration of
12 weeks. For all studies, weight
The study findings revealed that
lifestyle
intervention with combined diet and
physical activity component were
more effective compared to other
interventions on reducing weight.
It gives the implication that lifestyle
intervention can contribute to better
management of patients with obesity
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
4 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
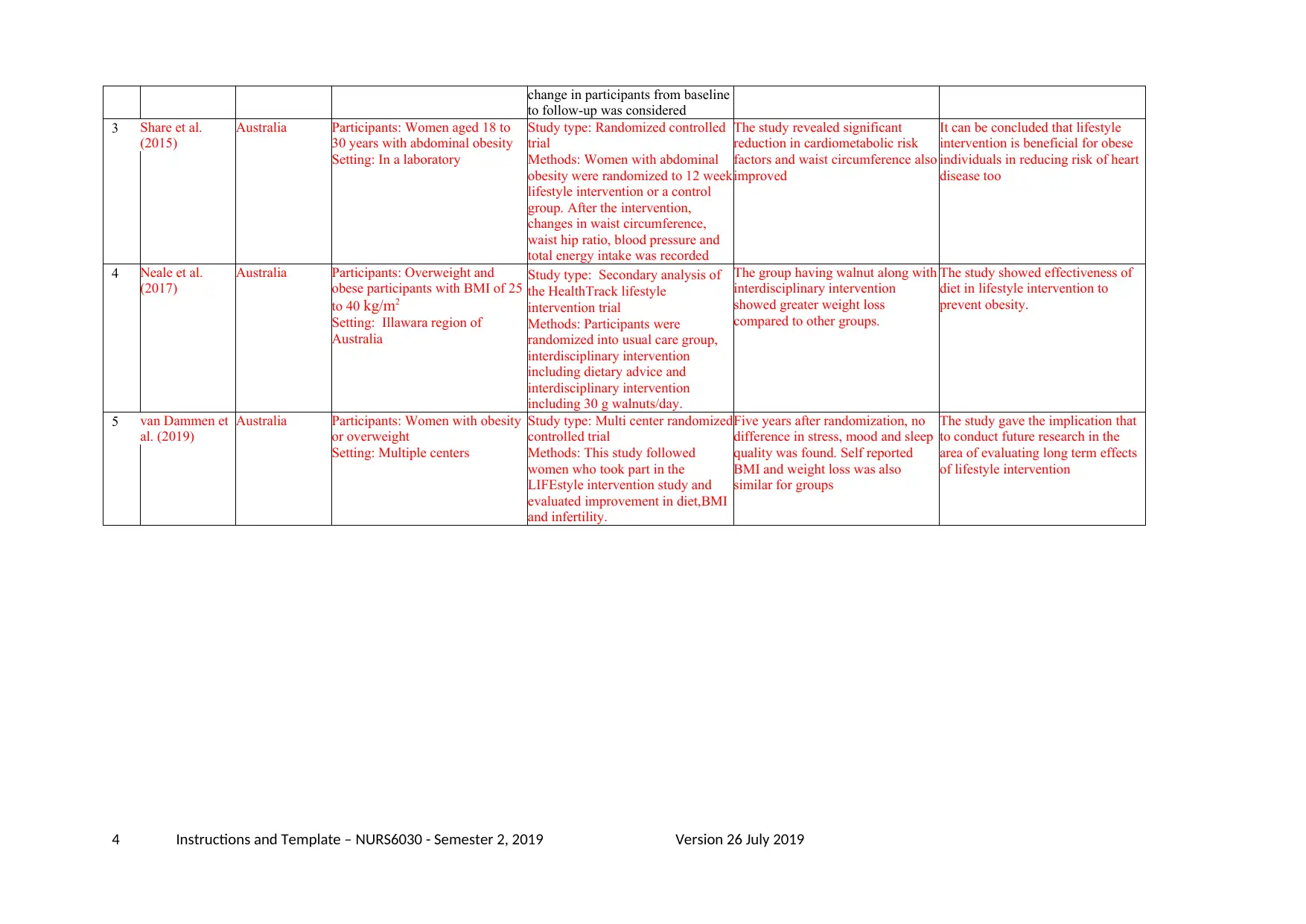
change in participants from baseline
to follow-up was considered
3 Share et al.
(2015)
Australia Participants: Women aged 18 to
30 years with abdominal obesity
Setting: In a laboratory
Study type: Randomized controlled
trial
Methods: Women with abdominal
obesity were randomized to 12 week
lifestyle intervention or a control
group. After the intervention,
changes in waist circumference,
waist hip ratio, blood pressure and
total energy intake was recorded
The study revealed significant
reduction in cardiometabolic risk
factors and waist circumference also
improved
It can be concluded that lifestyle
intervention is beneficial for obese
individuals in reducing risk of heart
disease too
4 Neale et al.
(2017)
Australia Participants: Overweight and
obese participants with BMI of 25
to 40 kg/m2
Setting: Illawara region of
Australia
Study type: Secondary analysis of
the HealthTrack lifestyle
intervention trial
Methods: Participants were
randomized into usual care group,
interdisciplinary intervention
including dietary advice and
interdisciplinary intervention
including 30 g walnuts/day.
The group having walnut along with
interdisciplinary intervention
showed greater weight loss
compared to other groups.
The study showed effectiveness of
diet in lifestyle intervention to
prevent obesity.
5 van Dammen et
al. (2019)
Australia Participants: Women with obesity
or overweight
Setting: Multiple centers
Study type: Multi center randomized
controlled trial
Methods: This study followed
women who took part in the
LIFEstyle intervention study and
evaluated improvement in diet,BMI
and infertility.
Five years after randomization, no
difference in stress, mood and sleep
quality was found. Self reported
BMI and weight loss was also
similar for groups
The study gave the implication that
to conduct future research in the
area of evaluating long term effects
of lifestyle intervention
change in participants from baseline
to follow-up was considered
3 Share et al.
(2015)
Australia Participants: Women aged 18 to
30 years with abdominal obesity
Setting: In a laboratory
Study type: Randomized controlled
trial
Methods: Women with abdominal
obesity were randomized to 12 week
lifestyle intervention or a control
group. After the intervention,
changes in waist circumference,
waist hip ratio, blood pressure and
total energy intake was recorded
The study revealed significant
reduction in cardiometabolic risk
factors and waist circumference also
improved
It can be concluded that lifestyle
intervention is beneficial for obese
individuals in reducing risk of heart
disease too
4 Neale et al.
(2017)
Australia Participants: Overweight and
obese participants with BMI of 25
to 40 kg/m2
Setting: Illawara region of
Australia
Study type: Secondary analysis of
the HealthTrack lifestyle
intervention trial
Methods: Participants were
randomized into usual care group,
interdisciplinary intervention
including dietary advice and
interdisciplinary intervention
including 30 g walnuts/day.
The group having walnut along with
interdisciplinary intervention
showed greater weight loss
compared to other groups.
The study showed effectiveness of
diet in lifestyle intervention to
prevent obesity.
5 van Dammen et
al. (2019)
Australia Participants: Women with obesity
or overweight
Setting: Multiple centers
Study type: Multi center randomized
controlled trial
Methods: This study followed
women who took part in the
LIFEstyle intervention study and
evaluated improvement in diet,BMI
and infertility.
Five years after randomization, no
difference in stress, mood and sleep
quality was found. Self reported
BMI and weight loss was also
similar for groups
The study gave the implication that
to conduct future research in the
area of evaluating long term effects
of lifestyle intervention

5 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
PART C: APPRAISAL
3. Appraisal of a single paper completed using the appropriate CASP tool (500 words
maximum for the answers entered into the tool)
Choose a single quantitative study. Choose an RCT, cohort or case control study.
Once you have chosen your paper, select the correct CASP assessment tool from the
templates provided and paste the tool into this section. Templates are provided on
Canvas.
Appraise the paper using the questions and hints provided.
Justify your answer to each section using the handy hints in the tool itself.
Article (provide full citation and doi)
Eaton, C. B., Hartman, S. J., Perzanowski, E., Pan, G., Roberts, M. B., Risica, P. M., ... & Marcus, B. H. (2016). A
randomized clinical trial of a tailored lifestyle intervention for obese, sedentary, primary care patients. The Annals of
Family Medicine, 14(4), 311-319. doi: 10.1370/afm.1952.
CASP Checklist: 11 questions to help you make sense of a Randomised Controlled Trial
Section A: Are the results of the study valid?
Screening Questions
1. Did the trial address a clearly focused issue?
__Yes __No __Can’t tell
Justify your answer:
The trial has a clear and focused issue which is evidenced by the research aim which clearly defines the population (obese primary care
patients, intervention (tailored lifestyle intervention), comparison (face-to-face meeting with patient and outcome (weight loss and
increase in physical activity) of interest for the study.
HINT: An issue can be ‘focused’ In terms of
The population studied
The intervention given
The comparator given
The outcomes considered
2. Was the assignment of patients to treatments randomised?
__Yes __No __Can’t tell
Justify your answer:
PART C: APPRAISAL
3. Appraisal of a single paper completed using the appropriate CASP tool (500 words
maximum for the answers entered into the tool)
Choose a single quantitative study. Choose an RCT, cohort or case control study.
Once you have chosen your paper, select the correct CASP assessment tool from the
templates provided and paste the tool into this section. Templates are provided on
Canvas.
Appraise the paper using the questions and hints provided.
Justify your answer to each section using the handy hints in the tool itself.
Article (provide full citation and doi)
Eaton, C. B., Hartman, S. J., Perzanowski, E., Pan, G., Roberts, M. B., Risica, P. M., ... & Marcus, B. H. (2016). A
randomized clinical trial of a tailored lifestyle intervention for obese, sedentary, primary care patients. The Annals of
Family Medicine, 14(4), 311-319. doi: 10.1370/afm.1952.
CASP Checklist: 11 questions to help you make sense of a Randomised Controlled Trial
Section A: Are the results of the study valid?
Screening Questions
1. Did the trial address a clearly focused issue?
__Yes __No __Can’t tell
Justify your answer:
The trial has a clear and focused issue which is evidenced by the research aim which clearly defines the population (obese primary care
patients, intervention (tailored lifestyle intervention), comparison (face-to-face meeting with patient and outcome (weight loss and
increase in physical activity) of interest for the study.
HINT: An issue can be ‘focused’ In terms of
The population studied
The intervention given
The comparator given
The outcomes considered
2. Was the assignment of patients to treatments randomised?
__Yes __No __Can’t tell
Justify your answer:

5 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
The process of randomization was completed by block randomization method using a random number generator. In accordance with
requirement for a RCT study, the author considered allocation concealment strategy and several staffs like research assistant, data
entry staffs, primary care providers and study staffs were blinded to the allocation.
HINT: Consider
How was this carried out?
Was the allocation sequence concealed from
3. Were all of the patients who entered the trial properly accounted for at its conclusion?
__Yes __No __Can’t tell
Justify your answer:
The author completely accounted for patients till the end of the study. This is evidence by data on number of participants after follow-
up and those who left the treatment midway. The study gave detailed figures regarding number of participants in the enhanced
intervention and standard care group who left the study after 6 months visit, 12 months visit, 18 months visit and 24 months visit.
HINT: Consider
Was the trial stopped early?
Were patients analysed in the groups to which they were randomised?
Detailed questions
4. Were patients, health workers and study personnel ‘blind’ to treatment?
__Yes __No __Can’t tell
Justify your answer:
Only health workers and study personnel were blinded to the treatment
HINT: Think about
Patients?
Health workers?
Study personnel?
5. Were the groups similar at the start of the trial?
__Yes __No __Can’t tell
Justify your answer:
The similarity of the group at the start of the trial is understood from the inclusion criteria as participants both in the intervention and
control group were selected based on those criterion. This included participants between 18 to 80 years of age and those with BMI of
25 kg/m2. This was also considered after randomization process as four participants were excluded as they became
pregnant after randomization process.
HINT: Look at
Other factors that might affect the outcome such as age,
sex, social class
researchers and patients?
6. Aside from the experimental intervention were the groups treated equally?
__Yes __No __Can’t tell
Justify your answer:
Is it worth continuing?
The process of randomization was completed by block randomization method using a random number generator. In accordance with
requirement for a RCT study, the author considered allocation concealment strategy and several staffs like research assistant, data
entry staffs, primary care providers and study staffs were blinded to the allocation.
HINT: Consider
How was this carried out?
Was the allocation sequence concealed from
3. Were all of the patients who entered the trial properly accounted for at its conclusion?
__Yes __No __Can’t tell
Justify your answer:
The author completely accounted for patients till the end of the study. This is evidence by data on number of participants after follow-
up and those who left the treatment midway. The study gave detailed figures regarding number of participants in the enhanced
intervention and standard care group who left the study after 6 months visit, 12 months visit, 18 months visit and 24 months visit.
HINT: Consider
Was the trial stopped early?
Were patients analysed in the groups to which they were randomised?
Detailed questions
4. Were patients, health workers and study personnel ‘blind’ to treatment?
__Yes __No __Can’t tell
Justify your answer:
Only health workers and study personnel were blinded to the treatment
HINT: Think about
Patients?
Health workers?
Study personnel?
5. Were the groups similar at the start of the trial?
__Yes __No __Can’t tell
Justify your answer:
The similarity of the group at the start of the trial is understood from the inclusion criteria as participants both in the intervention and
control group were selected based on those criterion. This included participants between 18 to 80 years of age and those with BMI of
25 kg/m2. This was also considered after randomization process as four participants were excluded as they became
pregnant after randomization process.
HINT: Look at
Other factors that might affect the outcome such as age,
sex, social class
researchers and patients?
6. Aside from the experimental intervention were the groups treated equally?
__Yes __No __Can’t tell
Justify your answer:
Is it worth continuing?
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
Equal treatment of groups in RCT means keeping all the others conditions apart from the intervention same for both
intervention and control group till the end of the trial (Spieth et al., 2016). This is seen in the study by proper follow up
of all participants from baseline till the end of the study and the collection of data regarding physical activity, waist
circumference, height, weight and resting heart rate for all participants.
Section B: What are the results?
7. How large was the treatment effect?
Justify your answer:
The treatment effect is RCT is estimated by means of proper measurement of outcome and the statistical difference in outcomes for
both groups. The key outcomes that were measured in the study includes demographic data, obesity related data such as height, waist,
waist circumference, resting heart rate and blood pressure and physical activity. However, the primary outcome has not been clearly
specified. The treatment effect is considered by the researcher evidenced by the definition regarding clinically significant weight loss.
Weight loss of 5% was considered clinically significant. In EI group, 5% weight loss was achieved. However, for the outcome of physical
activity, the intervention group achieved clinically significant levels of physical activity after 6 months of trial.
HINT: Consider
What outcomes were measured?
Is the primary outcome clearly specified?
What results were found for each outcome?
8. How precise was the estimate of the treatment effect?
Justify your answer:
The preciseness of the treatment effect is understood from the review of p value for each outcomes. As the p value for each outcomes
was recorded, it shows that the preciseness was considered. Statistically significant difference was found for participants in the
intervention group at 6 months and 12 months.
HINT: Consider
What are the confidence limits?
Section C: Will the results help locally?
9. Can the results be applied in your context? (or to the local population?)
__Yes __No __Can’t tell
Justify your answer:
The study was done with obese patients in primary care. However, the number of patients visiting primary care does not represent the
whole population with obesity. Hence, the study findings cannot be applied in local population and taking participants direct from
community would have helped to get participants groups who are representative of the target population.
HINT: Consider whether
Do you think that the patients covered by the trial
are similar enough to the patients to whom you will
apply this?, if not how to they differ?
10. Were all clinically important outcomes considered?
__Yes __No __Can’t tell
Justify your answer:
Yes, all clinically important outcomes were considered. The aim of the study was to mainly evaluate effect of the intervention on weight
loss and physical activity. Considering the aim of the study, the study considered all parameters related to weight loss by collecting data
on weight, height and waist circumference. In addition, the time for moderate to vigorous physical activity was considered too.
Equal treatment of groups in RCT means keeping all the others conditions apart from the intervention same for both
intervention and control group till the end of the trial (Spieth et al., 2016). This is seen in the study by proper follow up
of all participants from baseline till the end of the study and the collection of data regarding physical activity, waist
circumference, height, weight and resting heart rate for all participants.
Section B: What are the results?
7. How large was the treatment effect?
Justify your answer:
The treatment effect is RCT is estimated by means of proper measurement of outcome and the statistical difference in outcomes for
both groups. The key outcomes that were measured in the study includes demographic data, obesity related data such as height, waist,
waist circumference, resting heart rate and blood pressure and physical activity. However, the primary outcome has not been clearly
specified. The treatment effect is considered by the researcher evidenced by the definition regarding clinically significant weight loss.
Weight loss of 5% was considered clinically significant. In EI group, 5% weight loss was achieved. However, for the outcome of physical
activity, the intervention group achieved clinically significant levels of physical activity after 6 months of trial.
HINT: Consider
What outcomes were measured?
Is the primary outcome clearly specified?
What results were found for each outcome?
8. How precise was the estimate of the treatment effect?
Justify your answer:
The preciseness of the treatment effect is understood from the review of p value for each outcomes. As the p value for each outcomes
was recorded, it shows that the preciseness was considered. Statistically significant difference was found for participants in the
intervention group at 6 months and 12 months.
HINT: Consider
What are the confidence limits?
Section C: Will the results help locally?
9. Can the results be applied in your context? (or to the local population?)
__Yes __No __Can’t tell
Justify your answer:
The study was done with obese patients in primary care. However, the number of patients visiting primary care does not represent the
whole population with obesity. Hence, the study findings cannot be applied in local population and taking participants direct from
community would have helped to get participants groups who are representative of the target population.
HINT: Consider whether
Do you think that the patients covered by the trial
are similar enough to the patients to whom you will
apply this?, if not how to they differ?
10. Were all clinically important outcomes considered?
__Yes __No __Can’t tell
Justify your answer:
Yes, all clinically important outcomes were considered. The aim of the study was to mainly evaluate effect of the intervention on weight
loss and physical activity. Considering the aim of the study, the study considered all parameters related to weight loss by collecting data
on weight, height and waist circumference. In addition, the time for moderate to vigorous physical activity was considered too.

5 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
HINT: Consider
Is there other information you would like to have seen?
If not, does this affect the decision?
11. What are the implications of this study for practice? Are the benefits worth the harms and
costs?
__Yes __No __Can’t tell
Justify your answer:
The study is worth the harm and benefits as it gives the implication that primary care physician have a critical role in supporting weight
loss. It gave the insight that use of tailored lifestyle intervention with minimal face-to-face contact is more effective in controlling
obesity compared to other interventions.
HINT: Consider
Ways the research may be used
HINT: Consider
Is there other information you would like to have seen?
If not, does this affect the decision?
11. What are the implications of this study for practice? Are the benefits worth the harms and
costs?
__Yes __No __Can’t tell
Justify your answer:
The study is worth the harm and benefits as it gives the implication that primary care physician have a critical role in supporting weight
loss. It gave the insight that use of tailored lifestyle intervention with minimal face-to-face contact is more effective in controlling
obesity compared to other interventions.
HINT: Consider
Ways the research may be used

5 Instructions and Template – NURS6030 - Semester 2, 2019 Version 26 July 2019
References:
Eaton, C. B., Hartman, S. J., Perzanowski, E., Pan, G., Roberts, M. B., Risica, P. M., ... & Marcus, B. H. (2016). A
randomized clinical trial of a tailored lifestyle intervention for obese, sedentary, primary care patients. The
Annals of Family Medicine, 14(4), 311-319.
Hassan, Y., Head, V., Jacob, D., Bachmann, M. O., Diu, S., & Ford, J. (2016). Lifestyle interventions for weight loss in
adults with severe obesity: a systematic review. Clinical obesity, 6(6), 395-403.
Neale, E. P., Tapsell, L. C., Martin, A., Batterham, M. J., Wibisono, C., & Probst, Y. C. (2017). Impact of providing
walnut samples in a lifestyle intervention for weight loss: a secondary analysis of the HealthTrack trial. Food &
nutrition research, 61(1), 1344522. doi:10.1080/16546628.2017.1344522
Share, B. L., Naughton, G. A., Obert, P., Peat, J. K., Aumand, E. A., & Kemp, J. G. (2015). Effects of a multi-disciplinary
lifestyle intervention on cardiometabolic risk factors in young women with abdominal obesity: a randomised
controlled trial. PLoS One, 10(6), e0130270.
Spieth, P. M., Kubasch, A. S., Penzlin, A. I., Illigens, B. M. W., Barlinn, K., & Siepmann, T. (2016). Randomized
controlled trials–a matter of design. Neuropsychiatric Disease and Treatment, 12, 1341.
van Dammen, L., Wekker, V., de Rooij, S. R., Mol, B. W. J., Groen, H., Hoek, A., & Roseboom, T. J. (2019). The effects
of a pre-conception lifestyle intervention in women with obesity and infertility on perceived stress, mood
symptoms, sleep and quality of life. PloS one, 14(2), e0212914.
References:
Eaton, C. B., Hartman, S. J., Perzanowski, E., Pan, G., Roberts, M. B., Risica, P. M., ... & Marcus, B. H. (2016). A
randomized clinical trial of a tailored lifestyle intervention for obese, sedentary, primary care patients. The
Annals of Family Medicine, 14(4), 311-319.
Hassan, Y., Head, V., Jacob, D., Bachmann, M. O., Diu, S., & Ford, J. (2016). Lifestyle interventions for weight loss in
adults with severe obesity: a systematic review. Clinical obesity, 6(6), 395-403.
Neale, E. P., Tapsell, L. C., Martin, A., Batterham, M. J., Wibisono, C., & Probst, Y. C. (2017). Impact of providing
walnut samples in a lifestyle intervention for weight loss: a secondary analysis of the HealthTrack trial. Food &
nutrition research, 61(1), 1344522. doi:10.1080/16546628.2017.1344522
Share, B. L., Naughton, G. A., Obert, P., Peat, J. K., Aumand, E. A., & Kemp, J. G. (2015). Effects of a multi-disciplinary
lifestyle intervention on cardiometabolic risk factors in young women with abdominal obesity: a randomised
controlled trial. PLoS One, 10(6), e0130270.
Spieth, P. M., Kubasch, A. S., Penzlin, A. I., Illigens, B. M. W., Barlinn, K., & Siepmann, T. (2016). Randomized
controlled trials–a matter of design. Neuropsychiatric Disease and Treatment, 12, 1341.
van Dammen, L., Wekker, V., de Rooij, S. R., Mol, B. W. J., Groen, H., Hoek, A., & Roseboom, T. J. (2019). The effects
of a pre-conception lifestyle intervention in women with obesity and infertility on perceived stress, mood
symptoms, sleep and quality of life. PloS one, 14(2), e0212914.
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



