EGH463 Plant and Process Design Report: Energy Balance Calculations
VerifiedAdded on 2023/01/06
|5
|532
|75
Report
AI Summary
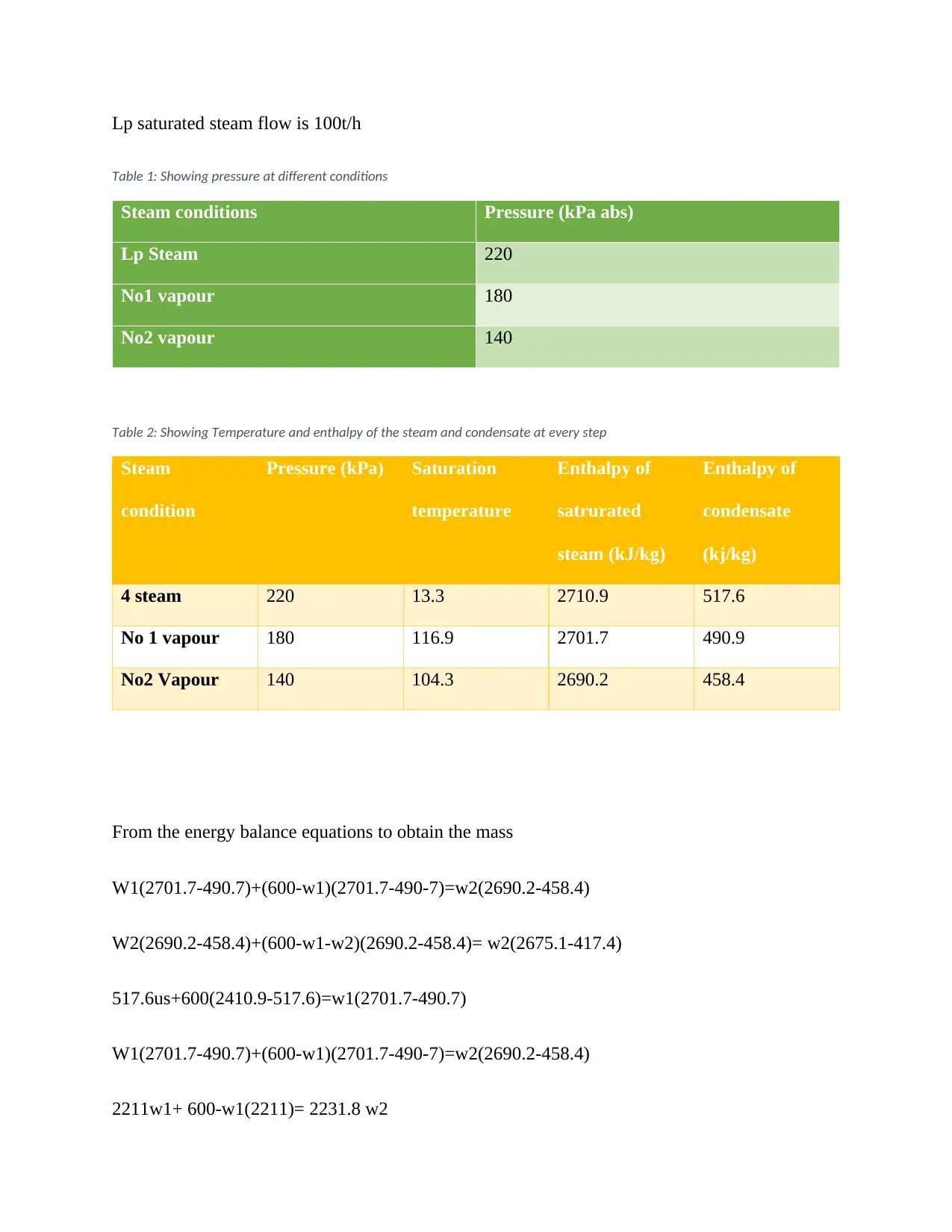
This report presents an analysis of plant and process design, focusing on energy balance calculations and water evaporation. It includes detailed calculations for steam conditions, enthalpy, and mass flow rates at various stages. The report provides insights into the quantity of water evaporated per hour and the minimum cooling water required. Furthermore, it calculates the quantity of total fresh water produced. The report also references relevant standards for process design and control, and includes a P&ID diagram for an integrated gasification combined cycle (IGCC) schematic, demonstrating the application of thermodynamics and heat transfer principles in plant design. The assignment solution contributes to the understanding of power cycles and industrial processes.
1 out of 5






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)