The Role of HbS Binding to GP1bα in Platelet Activation in SCD
VerifiedAdded on 2023/06/11
|22
|6294
|174
Thesis and Dissertation
AI Summary
This thesis provides a comprehensive overview of sickle cell disease (SCD), focusing on the mechanism by which sickle hemoglobin (HbS) binding to glycoprotein 1bα (GP1bα) activates platelets. It begins with an introduction to SCD, its historical discovery, and genetic inheritance patterns. The thesis then delves into the structure of sickle hemoglobin, highlighting the amino acid substitution that leads to its altered conformation and polymerization. The epidemiology of SCD, including its prevalence and mortality rates across different regions, is discussed. The pathophysiology of SCD, including vaso-occlusion, hemolytic anemia, and platelet activation and aggregation, are explored in detail. Special emphasis is placed on the role of HbS binding to GP1bα in triggering platelet activation, which contributes to the complications of SCD. The thesis also mentions methods for measuring platelet activation and other relevant parameters. Desklib offers students access to this thesis and a wealth of other study resources.

Running Head: SICKLE CELL DISEASE 0
HbS binding to GP1bα Activates Platelets in Sickle Cell disease
Student Name
[Pick the date]
HbS binding to GP1bα Activates Platelets in Sickle Cell disease
Student Name
[Pick the date]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SICKLE CELL DISEASE 1
Table of Contents
Introduction....................................................................................................................................1
Structure of Sickle haemoglobin...................................................................................................3
Epidemiology of sickle cells disease.............................................................................................5
Genetic inheritances.......................................................................................................................7
Pathophysiology..........................................................................................................................10
Vaso Occlusion........................................................................................................................10
Hemolytic Anemia...................................................................................................................11
Platelet activation and aggregation..............................................................................................12
References....................................................................................................................................15
Table of Contents
Introduction....................................................................................................................................1
Structure of Sickle haemoglobin...................................................................................................3
Epidemiology of sickle cells disease.............................................................................................5
Genetic inheritances.......................................................................................................................7
Pathophysiology..........................................................................................................................10
Vaso Occlusion........................................................................................................................10
Hemolytic Anemia...................................................................................................................11
Platelet activation and aggregation..............................................................................................12
References....................................................................................................................................15

SICKLE CELL DISEASE 2
Introduction
Sickle cells disease is not a new concept. It has been affecting people for many years. It was
discovered in 1910 for the first time in the United States in a patient from West Indies by a
cardiologist named Herrick. In 1951 a Nobel Prize winner chemist Pauling with his team found
that the oxygen-transporting proteins named hemoglobin had an altered structure in a patient
with SCD. Ingram and Hunt in 1956 discovered the replacement of glutamic acid by valine on
6th position. In 1970 the relation between the abnormal structure of hemoglobin and RBCs has
been discovered. The cure of this disease has been reported by bone marrow transplantation
which was applied on a child. The preventive treatment for this disease is proposed in 1995 and
to stop the complications of SCD, Hydroxyurea was found to be a proven drug (Information
Centre for Sickle Cell and Thalassemia Disorder, 2002). Some of the studies suggested that the
history of sickle cell disease is thousands of years old in Africa and known by various different
names in different languages (Serjeant, 2013).
Sickle cells disease is a genetic disorder inherited from parents to the children’s. It is associated
with defect or abnormality in red blood cells (World Health Organisation, 2006). The normal
red blood cells are disc-shaped but the changes occurred in hemoglobin causes the sickle shape
of erythrocytes (Brent Sickle Cell & Thalassemia Centre, 2015). These cells with altered shape
are rigid and may be burst when transported via blood vessels. This may become the reason for
the decreased amount of red blood cells inside the body and causes anemia. When
abnormalities or inheritance occur in oxygen-carrying proteins hemoglobin S also called sickle
hemoglobin, it leads to the sickle cell anemia (a sickle cell disease). Inheritance word itself
indicated that the disease is this disease is transfer to the next generation by passing abnormal
haemoglobin genes. Some of the diseases associated to sickle cell disease are: haemoglobin SC
disease, sickle cell anaemia and haemoglobin Sβ thalassemia, haemoglobin SS disease,
Introduction
Sickle cells disease is not a new concept. It has been affecting people for many years. It was
discovered in 1910 for the first time in the United States in a patient from West Indies by a
cardiologist named Herrick. In 1951 a Nobel Prize winner chemist Pauling with his team found
that the oxygen-transporting proteins named hemoglobin had an altered structure in a patient
with SCD. Ingram and Hunt in 1956 discovered the replacement of glutamic acid by valine on
6th position. In 1970 the relation between the abnormal structure of hemoglobin and RBCs has
been discovered. The cure of this disease has been reported by bone marrow transplantation
which was applied on a child. The preventive treatment for this disease is proposed in 1995 and
to stop the complications of SCD, Hydroxyurea was found to be a proven drug (Information
Centre for Sickle Cell and Thalassemia Disorder, 2002). Some of the studies suggested that the
history of sickle cell disease is thousands of years old in Africa and known by various different
names in different languages (Serjeant, 2013).
Sickle cells disease is a genetic disorder inherited from parents to the children’s. It is associated
with defect or abnormality in red blood cells (World Health Organisation, 2006). The normal
red blood cells are disc-shaped but the changes occurred in hemoglobin causes the sickle shape
of erythrocytes (Brent Sickle Cell & Thalassemia Centre, 2015). These cells with altered shape
are rigid and may be burst when transported via blood vessels. This may become the reason for
the decreased amount of red blood cells inside the body and causes anemia. When
abnormalities or inheritance occur in oxygen-carrying proteins hemoglobin S also called sickle
hemoglobin, it leads to the sickle cell anemia (a sickle cell disease). Inheritance word itself
indicated that the disease is this disease is transfer to the next generation by passing abnormal
haemoglobin genes. Some of the diseases associated to sickle cell disease are: haemoglobin SC
disease, sickle cell anaemia and haemoglobin Sβ thalassemia, haemoglobin SS disease,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

SICKLE CELL DISEASE 3
haemoglobin SC, haemoglobin SB 0 or β0 thalassemia, haemoglobin SD, SE and SO
(Healthline, 2017). This thesis also involves the discussion of hemoglobin and glycoprotein role
in platelet activation and aggregation. Other molecules and processes associated with sickle cell
disease also will be discussed. Hemoglobin is the molecule that maintains the highly conserved
state in different species within erythrocytes. The main role of hemoglobin is to carry oxygen to
various tissues form lungs. Persons with sickle cell disease Hb changes to stiff rods inside the
RBCs. Hb the depletion of nitric acid by Hb produces hydroxyl radicals which trigger
peroxidation of lipid membrane which results in cellular damage. Increased level of Hb in
plasma associated with vascular and organ abnormality like hemolysis. Release of Hb during
hemolysis produces ROS, which results in platelet activation (Helms et al. 2013). Various
studies suggested that when these Hb binds to GP1bα, the platelet activation has been triggered.
This interaction of hemoglobin to GP1bα stimulates some events like change in platelet shape,
secretion of granules and signalling process which leads to activate the function of integrin’s.
These pathways involved in activation of platelets mediated by GP1bα. This glycoprotein is a
surface membrane of platelet is consists of heterodimers where one alpha chain and one beta
chain linked to each other via a disulfide bond. This glycoprotein works as a receptor for VWF
(Madabhushi, Zhang, Kelkar, Dayananda, and Neelamegham, 2014). It is also initiated events
of intracellular signaling like the increased level of calcium in the cytoplasm, protein kinase
pathway activation which leads to activation of platelets. The initiation of GP1b alpha by Hb is
responsible for platelet apoptosis (Annarapu, Singhal, Sheetal, Ojha and Guchhait, 2016).
Some methods like measurement of platelet activation markers by using flow cytometry,
extracellular hemoglobin measurement by using ELISA, Coagulation essay, VWF: RCO assay
and platelet activation will be discussed in this dissertation to understand the activation of
platelets by binding of Hb to GP1bα. The epidemiological aspects, genetical aspects involved in
this study will also be discussed to provide a better understanding of this topic.
haemoglobin SC, haemoglobin SB 0 or β0 thalassemia, haemoglobin SD, SE and SO
(Healthline, 2017). This thesis also involves the discussion of hemoglobin and glycoprotein role
in platelet activation and aggregation. Other molecules and processes associated with sickle cell
disease also will be discussed. Hemoglobin is the molecule that maintains the highly conserved
state in different species within erythrocytes. The main role of hemoglobin is to carry oxygen to
various tissues form lungs. Persons with sickle cell disease Hb changes to stiff rods inside the
RBCs. Hb the depletion of nitric acid by Hb produces hydroxyl radicals which trigger
peroxidation of lipid membrane which results in cellular damage. Increased level of Hb in
plasma associated with vascular and organ abnormality like hemolysis. Release of Hb during
hemolysis produces ROS, which results in platelet activation (Helms et al. 2013). Various
studies suggested that when these Hb binds to GP1bα, the platelet activation has been triggered.
This interaction of hemoglobin to GP1bα stimulates some events like change in platelet shape,
secretion of granules and signalling process which leads to activate the function of integrin’s.
These pathways involved in activation of platelets mediated by GP1bα. This glycoprotein is a
surface membrane of platelet is consists of heterodimers where one alpha chain and one beta
chain linked to each other via a disulfide bond. This glycoprotein works as a receptor for VWF
(Madabhushi, Zhang, Kelkar, Dayananda, and Neelamegham, 2014). It is also initiated events
of intracellular signaling like the increased level of calcium in the cytoplasm, protein kinase
pathway activation which leads to activation of platelets. The initiation of GP1b alpha by Hb is
responsible for platelet apoptosis (Annarapu, Singhal, Sheetal, Ojha and Guchhait, 2016).
Some methods like measurement of platelet activation markers by using flow cytometry,
extracellular hemoglobin measurement by using ELISA, Coagulation essay, VWF: RCO assay
and platelet activation will be discussed in this dissertation to understand the activation of
platelets by binding of Hb to GP1bα. The epidemiological aspects, genetical aspects involved in
this study will also be discussed to provide a better understanding of this topic.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SICKLE CELL DISEASE 4
Structure of Sickle haemoglobin
The Normal haemoglobin is composed of total four protein chains and about four short non
protein molecules called heme. There are two alpha globin chains and two beta globin chains in
single haemoglobin. Each globin chains associated with heme prosthetic group, it is the site for
binding and release of oxygen. During binding and release of an oxygen compound a αβ dimer
moves (Weatherall and Clegg 2001). Haemoglobin S which is the altered form of normal
haemoglobin is a tetramer contains four subunits, where two subunits are of α and other two are
beta globin. The structure of two alpha subunits is same as in normal haemoglobin but beta
subunits are different. These subunits are the site for valine residues substitution to glutamic
acid. Alpha subunit contains 141 amino acids and on the other hand primary structure of beta
subunit has 146 amino acid (Marengo-Rowe, 2006). There are single heme binding sites for all
four subunits; these sites are responsible for oxygen transport. The secondary structure of
haemoglobin s contains complete alpha helicase which is separated and joint by random coils.
In secondary structure there is no beta sheet. Both alpha and beta subunits made consists of
eight alpha helicase. The binding between these four subunits is strongly bounded with salt
bridges and hydrogen bonds (Mackey, no date). The tertiary structure is similar as secondary. In
quaternary structure there are two confirmations called T and R protein states are same as wild-
type hemoglobin A R confirmation which is open and in the mobile state responsible for
keeping home binding pocket open. These pockets also unhindered by salt bridges and
hydrogen bonds. At the distal end of an alpha chain between heme binding pockets the T state
has been constricted and stabilize by salt bridges. In a normal haemoglobin A, this T
confirmation accounts for decreasing oxygen affinity. It is also responsible for promoting
polymerisation of haemoglobin S in haemoglobin S both the subunits are divided as valine
acceptors and donors. The polymerisation occurred in Hb S is a primary pathogenic event that
occurs in SCD (Steinberg, 2018).
Structure of Sickle haemoglobin
The Normal haemoglobin is composed of total four protein chains and about four short non
protein molecules called heme. There are two alpha globin chains and two beta globin chains in
single haemoglobin. Each globin chains associated with heme prosthetic group, it is the site for
binding and release of oxygen. During binding and release of an oxygen compound a αβ dimer
moves (Weatherall and Clegg 2001). Haemoglobin S which is the altered form of normal
haemoglobin is a tetramer contains four subunits, where two subunits are of α and other two are
beta globin. The structure of two alpha subunits is same as in normal haemoglobin but beta
subunits are different. These subunits are the site for valine residues substitution to glutamic
acid. Alpha subunit contains 141 amino acids and on the other hand primary structure of beta
subunit has 146 amino acid (Marengo-Rowe, 2006). There are single heme binding sites for all
four subunits; these sites are responsible for oxygen transport. The secondary structure of
haemoglobin s contains complete alpha helicase which is separated and joint by random coils.
In secondary structure there is no beta sheet. Both alpha and beta subunits made consists of
eight alpha helicase. The binding between these four subunits is strongly bounded with salt
bridges and hydrogen bonds (Mackey, no date). The tertiary structure is similar as secondary. In
quaternary structure there are two confirmations called T and R protein states are same as wild-
type hemoglobin A R confirmation which is open and in the mobile state responsible for
keeping home binding pocket open. These pockets also unhindered by salt bridges and
hydrogen bonds. At the distal end of an alpha chain between heme binding pockets the T state
has been constricted and stabilize by salt bridges. In a normal haemoglobin A, this T
confirmation accounts for decreasing oxygen affinity. It is also responsible for promoting
polymerisation of haemoglobin S in haemoglobin S both the subunits are divided as valine
acceptors and donors. The polymerisation occurred in Hb S is a primary pathogenic event that
occurs in SCD (Steinberg, 2018).
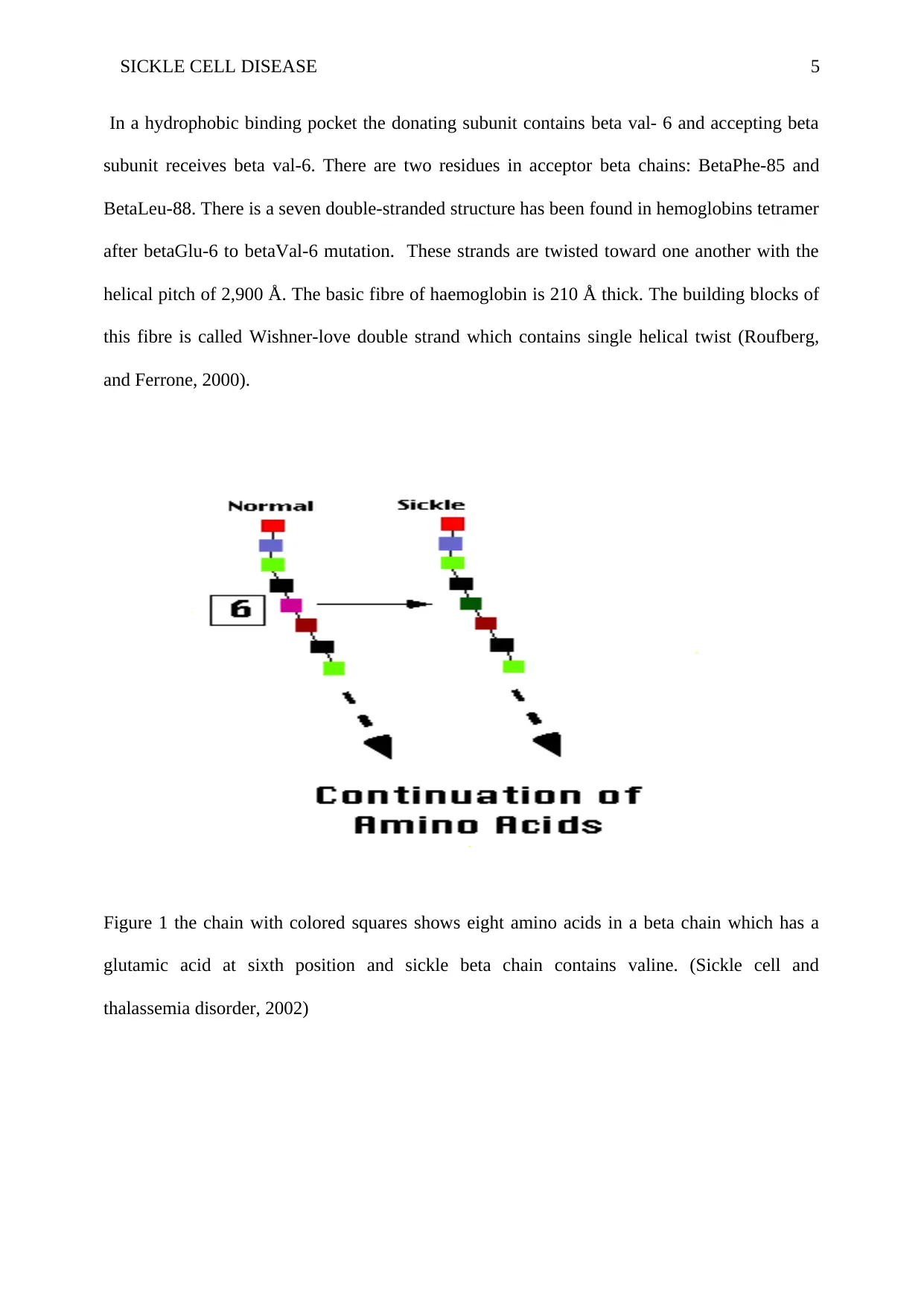
SICKLE CELL DISEASE 5
In a hydrophobic binding pocket the donating subunit contains beta val- 6 and accepting beta
subunit receives beta val-6. There are two residues in acceptor beta chains: BetaPhe-85 and
BetaLeu-88. There is a seven double-stranded structure has been found in hemoglobins tetramer
after betaGlu-6 to betaVal-6 mutation. These strands are twisted toward one another with the
helical pitch of 2,900 Å. The basic fibre of haemoglobin is 210 Å thick. The building blocks of
this fibre is called Wishner-love double strand which contains single helical twist (Roufberg,
and Ferrone, 2000).
Figure 1 the chain with colored squares shows eight amino acids in a beta chain which has a
glutamic acid at sixth position and sickle beta chain contains valine. (Sickle cell and
thalassemia disorder, 2002)
In a hydrophobic binding pocket the donating subunit contains beta val- 6 and accepting beta
subunit receives beta val-6. There are two residues in acceptor beta chains: BetaPhe-85 and
BetaLeu-88. There is a seven double-stranded structure has been found in hemoglobins tetramer
after betaGlu-6 to betaVal-6 mutation. These strands are twisted toward one another with the
helical pitch of 2,900 Å. The basic fibre of haemoglobin is 210 Å thick. The building blocks of
this fibre is called Wishner-love double strand which contains single helical twist (Roufberg,
and Ferrone, 2000).
Figure 1 the chain with colored squares shows eight amino acids in a beta chain which has a
glutamic acid at sixth position and sickle beta chain contains valine. (Sickle cell and
thalassemia disorder, 2002)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

SICKLE CELL DISEASE 6
Figure 2 polymerized sickle hemoglobin (polymers of hemoglobin the sphere represents each
hemoglobin molecule. The twist occurs in α helical bundle consists of fourteen HbS chains
(Sickle cell and thalassemia disorder, 2002).
Epidemiology of sickle cells disease
Sickle cell disorder has been found to be the most common monogenic disorder which has
affects many peoples every year in all over the world. About 5- 7 % of the total population of
the world affected by the abnormality in hemoglobin gene and sickle cell disease is found to be
most common. In Africa and Asian region, this disease impacts with the greatest burden and the
prevalence are ranges from 10 to 45 % in various areas of Africa. Particularly in Nigeria Carrier
prevalence is approximately 20 to 30 %. Sickle cell disease affecting 2 to 3 % of the total
population of Nigeria which is more than 160 million.
https://www.hindawi.com/journals/anemia/2015/791498/. According to a study conducted by
Jastaniah (2011) this severe disorder affected around 72,000 people in the United States, more
than 200000 infants in Africa are born every year with sickle cell disease. Around 250,000
children are born yearly with sickle cell anemia in all over the globe. Particularly in Brazil 2500
children’s are born annually with SCD. Among the non-white population which is 44.66 % by
2000, of Brazil around 1 to 6 % found to have Hb S gene (Lervolino, Baldin, Picado, Calil, Viel
Figure 2 polymerized sickle hemoglobin (polymers of hemoglobin the sphere represents each
hemoglobin molecule. The twist occurs in α helical bundle consists of fourteen HbS chains
(Sickle cell and thalassemia disorder, 2002).
Epidemiology of sickle cells disease
Sickle cell disorder has been found to be the most common monogenic disorder which has
affects many peoples every year in all over the world. About 5- 7 % of the total population of
the world affected by the abnormality in hemoglobin gene and sickle cell disease is found to be
most common. In Africa and Asian region, this disease impacts with the greatest burden and the
prevalence are ranges from 10 to 45 % in various areas of Africa. Particularly in Nigeria Carrier
prevalence is approximately 20 to 30 %. Sickle cell disease affecting 2 to 3 % of the total
population of Nigeria which is more than 160 million.
https://www.hindawi.com/journals/anemia/2015/791498/. According to a study conducted by
Jastaniah (2011) this severe disorder affected around 72,000 people in the United States, more
than 200000 infants in Africa are born every year with sickle cell disease. Around 250,000
children are born yearly with sickle cell anemia in all over the globe. Particularly in Brazil 2500
children’s are born annually with SCD. Among the non-white population which is 44.66 % by
2000, of Brazil around 1 to 6 % found to have Hb S gene (Lervolino, Baldin, Picado, Calil, Viel
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SICKLE CELL DISEASE 7
and Campos, 2011). This is expected that by 2050 the number of affected children will be
reached to 400000 and the number of this disease cases will be increased nearly 30 % in all
over the world. Mostly it will affect sub-Saharan Africa (DeBaun and Galadanci, 2018).
It was also estimated that SCD affects 100000 people approximately. It has been studied that
prevalence of affected infants is 2.55 per 1000. Mostly the children with the traits of this
disease born in high-income countries and survives but in low-income countries the children
with this disorder die earlier the developed countries facilitate diagnosis and care for the patient
which becomes the reason of survival for them but on the other hand in poor countries, the
children were not diagnosed. (Before 5 years of age). Now talking about mortality this disorder
accounts for 3.4 % of mortality among 5 years aged children’s globally and 6.4 % particularly
in Africa (Piel et al. 2013). It was also estimated that 10 to 40 % population carries the gene of
sickle cell and results in 2 % SCD prevalence. Hemoglobin disorders were affecting 75 % of
birth but now it is common in 89 % of births in 71 % of countries. A significant variant of this
disorder has been carried by around 5.2 % of the total population and around 1.1 percent
couples are at risk of giving birth with hemoglobin disorders worldwide. In western Pacific
region among all the population of 1761 million 2 % of births are recorded under 5 % mortality
and in South East Asia it is 1.6 %.
In urban areas of Britain, sickle cell disease is common and they estimated that in London
nearly 2000 patients are affected by this disorder. Nearly 14000 people in UK living with sickle
cell disease which is equivalent to 1 in 4600 person. Screening of infants indicated that 1 in
every 2000 screened with positive SCD. Another study published in journal of clinical
pathology shows reported that SCD is became common disease in England. It was also reported
that 1.47 % of all infants in England born with sickle cell gene. Geographical frequency has
been highlighted and it was found that in London and nearby urban area babies are affected
more. With Highest prevalence of the traits of sickle cell particularly in Africa occurs between
and Campos, 2011). This is expected that by 2050 the number of affected children will be
reached to 400000 and the number of this disease cases will be increased nearly 30 % in all
over the world. Mostly it will affect sub-Saharan Africa (DeBaun and Galadanci, 2018).
It was also estimated that SCD affects 100000 people approximately. It has been studied that
prevalence of affected infants is 2.55 per 1000. Mostly the children with the traits of this
disease born in high-income countries and survives but in low-income countries the children
with this disorder die earlier the developed countries facilitate diagnosis and care for the patient
which becomes the reason of survival for them but on the other hand in poor countries, the
children were not diagnosed. (Before 5 years of age). Now talking about mortality this disorder
accounts for 3.4 % of mortality among 5 years aged children’s globally and 6.4 % particularly
in Africa (Piel et al. 2013). It was also estimated that 10 to 40 % population carries the gene of
sickle cell and results in 2 % SCD prevalence. Hemoglobin disorders were affecting 75 % of
birth but now it is common in 89 % of births in 71 % of countries. A significant variant of this
disorder has been carried by around 5.2 % of the total population and around 1.1 percent
couples are at risk of giving birth with hemoglobin disorders worldwide. In western Pacific
region among all the population of 1761 million 2 % of births are recorded under 5 % mortality
and in South East Asia it is 1.6 %.
In urban areas of Britain, sickle cell disease is common and they estimated that in London
nearly 2000 patients are affected by this disorder. Nearly 14000 people in UK living with sickle
cell disease which is equivalent to 1 in 4600 person. Screening of infants indicated that 1 in
every 2000 screened with positive SCD. Another study published in journal of clinical
pathology shows reported that SCD is became common disease in England. It was also reported
that 1.47 % of all infants in England born with sickle cell gene. Geographical frequency has
been highlighted and it was found that in London and nearby urban area babies are affected
more. With Highest prevalence of the traits of sickle cell particularly in Africa occurs between

SICKLE CELL DISEASE 8
a latitudes of 15 degree north and 20 degree south with prevalence rate of 10 % to 40 %. A
Study conducted by Agasa et al. (2010) stated that around 19 % neonates found to have
defected haemoglobin level. In that it was also reported after studying 2000 infants in a hospital
of Tanzania that the haemoglobin levels altered in the bases of geographical regions.
The parents from coastal areas had 35.6 % incidence rate among their new-borns and 6.7 % in
northern areas (Mulumba and Wilson, 2015). As the awareness and knowledge has been raised
in various countries about this disease results in increasing the number of survivals, specifically
in Africa around 40% population is now urbanised and the healthcare facilities are improved in
past few years. Low cost and simple mechanisms to diagnose adult and children’s are there
now. World health organisation indicated that around 50 % of participated states will be having
SCD control program in coming few years. Prevalence of positive tests found particularly in
England was 1:2000. Among 5.2 percent patient with sickle cell disease suffers severe pain 3 to
ten times in a year (Yale, Nagib, and Guthrie, 2000). It was estimated that prevalence of this-
this disease carriers in twenty-five states of Europe is nearly 1/150 (Orphanet, 2018).
Genetic inheritances
Sickle cell disease is inherited in the pattern of autosomal recessive which indicates the two
copies of gene in every cell develop mutation (Genetic Home Reference, 2018). Autosomal
word refers to that mutation is not only occur in X and Y chromosome therefore it affects both
male and females. The recessive word indicates that the mutation have to be there in both the
parents if it going to occur in new-born. When both male and female carries asymptomatic
genetic disease, each infant has probability of 25 % to receive defective genes, 50% chances of
having one abnormal gene and 25 % chances of acquiring unaffected genes. The infants may
have a trait of sickle cell if particularly one parent is affected with the disease. The children do
not have side effects if only one gene is mutated (Smith, 2015). . The genes that is responsible
a latitudes of 15 degree north and 20 degree south with prevalence rate of 10 % to 40 %. A
Study conducted by Agasa et al. (2010) stated that around 19 % neonates found to have
defected haemoglobin level. In that it was also reported after studying 2000 infants in a hospital
of Tanzania that the haemoglobin levels altered in the bases of geographical regions.
The parents from coastal areas had 35.6 % incidence rate among their new-borns and 6.7 % in
northern areas (Mulumba and Wilson, 2015). As the awareness and knowledge has been raised
in various countries about this disease results in increasing the number of survivals, specifically
in Africa around 40% population is now urbanised and the healthcare facilities are improved in
past few years. Low cost and simple mechanisms to diagnose adult and children’s are there
now. World health organisation indicated that around 50 % of participated states will be having
SCD control program in coming few years. Prevalence of positive tests found particularly in
England was 1:2000. Among 5.2 percent patient with sickle cell disease suffers severe pain 3 to
ten times in a year (Yale, Nagib, and Guthrie, 2000). It was estimated that prevalence of this-
this disease carriers in twenty-five states of Europe is nearly 1/150 (Orphanet, 2018).
Genetic inheritances
Sickle cell disease is inherited in the pattern of autosomal recessive which indicates the two
copies of gene in every cell develop mutation (Genetic Home Reference, 2018). Autosomal
word refers to that mutation is not only occur in X and Y chromosome therefore it affects both
male and females. The recessive word indicates that the mutation have to be there in both the
parents if it going to occur in new-born. When both male and female carries asymptomatic
genetic disease, each infant has probability of 25 % to receive defective genes, 50% chances of
having one abnormal gene and 25 % chances of acquiring unaffected genes. The infants may
have a trait of sickle cell if particularly one parent is affected with the disease. The children do
not have side effects if only one gene is mutated (Smith, 2015). . The genes that is responsible
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

SICKLE CELL DISEASE 9
for controlling the production of protein in RBCs, (red blood cells) known as haemoglobin.
Haemoglobin attached O2 in lungs and transfers it to peripheral tissues like liver and muscle.
Some of the person has one normal and one sickle haemoglobin gene. This is known as sickle
cell trait. A person with this trait has less probability of developing sickle cell disease with
increasing age. On conception time People may receive a gene of sickle cell or not. That is why
sickle cell trait and sickle cell disease cannot contract. If an infant is born with the traits of
sickle cells will be having this trait throughout his or her life. The same will happened if the
baby is born with sickle cell genes. Illness is not occurred in the case of sickle cell traits. With
the time the sickle cell severity can be changed which is not because of change in genes of
sickle cell (Frenette and Atwah, 2007). People with inherited sickle hemoglobin genes develop
sickle cell disease.
Figure 3 shows inheritance of sickle cell disease (Agbabiaka, 2015)
for controlling the production of protein in RBCs, (red blood cells) known as haemoglobin.
Haemoglobin attached O2 in lungs and transfers it to peripheral tissues like liver and muscle.
Some of the person has one normal and one sickle haemoglobin gene. This is known as sickle
cell trait. A person with this trait has less probability of developing sickle cell disease with
increasing age. On conception time People may receive a gene of sickle cell or not. That is why
sickle cell trait and sickle cell disease cannot contract. If an infant is born with the traits of
sickle cells will be having this trait throughout his or her life. The same will happened if the
baby is born with sickle cell genes. Illness is not occurred in the case of sickle cell traits. With
the time the sickle cell severity can be changed which is not because of change in genes of
sickle cell (Frenette and Atwah, 2007). People with inherited sickle hemoglobin genes develop
sickle cell disease.
Figure 3 shows inheritance of sickle cell disease (Agbabiaka, 2015)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SICKLE CELL DISEASE 10
The above diagram shows the inheritance pattern of sickle cell disease from parents to their
children it also shows the ratio and probability of occurrence of this disorder in next generation.
According to an article published in Genesis international by Agbabiaka (2015), a person
inherits a single set of genes from mother or father to their children. It has been reported that
either two normal HbA genes can be inherited in a person, one unaffected gene and one
abnormal gene (HbS), or two mutated genes, which depends on parent genes composition.
When a single HbS and one HbA gene carried by a person, the normal gene may overcome the
effects of abnormal hemoglobin gene, to stop the occurrence of symptoms of SCD. This type of
person has sickle cell traits and acts as a carrier for these traits and may transfer it to their
babies. When a baby inherits two affected HbS genes from both the parents, they develop sickle
cell disease. They carry only affected genes to transfer to their children.
As mentioned above hemoglobin consist four subunits of protein. HBB gene provides messages
for making beta globin. The mutations occur in this gene causes different versions of beta
globin. Single specific HBB gene mutation results in an affected beta globin called Hemoglobin
S. Various other mutation occurs in this gene causes a decreased level of beta globin, this
abnormality is known as beta thalassemia. In a person with SCD one beta globin is replaced by
hemoglobin S in hemoglobin subunits but on the other hand in sickle cell anemia both beta
globins has been changed with hemoglobin S.in other disease related to sickle cell single
subunit is replaced with HbS in hemoglobin. Other subunits have been replaced with various
effected variants like hemoglobin C. People with HbS thalassemia disorder have mutation
where hemoglobin S and beta thalassemia occur together. The defected versions of this beta
globin cause red blood cells to become sickle-shaped. The death of these immature RBCs
results in anemia (Genetic Home References, 2018)
The above diagram shows the inheritance pattern of sickle cell disease from parents to their
children it also shows the ratio and probability of occurrence of this disorder in next generation.
According to an article published in Genesis international by Agbabiaka (2015), a person
inherits a single set of genes from mother or father to their children. It has been reported that
either two normal HbA genes can be inherited in a person, one unaffected gene and one
abnormal gene (HbS), or two mutated genes, which depends on parent genes composition.
When a single HbS and one HbA gene carried by a person, the normal gene may overcome the
effects of abnormal hemoglobin gene, to stop the occurrence of symptoms of SCD. This type of
person has sickle cell traits and acts as a carrier for these traits and may transfer it to their
babies. When a baby inherits two affected HbS genes from both the parents, they develop sickle
cell disease. They carry only affected genes to transfer to their children.
As mentioned above hemoglobin consist four subunits of protein. HBB gene provides messages
for making beta globin. The mutations occur in this gene causes different versions of beta
globin. Single specific HBB gene mutation results in an affected beta globin called Hemoglobin
S. Various other mutation occurs in this gene causes a decreased level of beta globin, this
abnormality is known as beta thalassemia. In a person with SCD one beta globin is replaced by
hemoglobin S in hemoglobin subunits but on the other hand in sickle cell anemia both beta
globins has been changed with hemoglobin S.in other disease related to sickle cell single
subunit is replaced with HbS in hemoglobin. Other subunits have been replaced with various
effected variants like hemoglobin C. People with HbS thalassemia disorder have mutation
where hemoglobin S and beta thalassemia occur together. The defected versions of this beta
globin cause red blood cells to become sickle-shaped. The death of these immature RBCs
results in anemia (Genetic Home References, 2018)

SICKLE CELL DISEASE 11
Pathophysiology
An abnormal hemoglobin is produced because of point mutation occurred in Beta-globin gene.
The mutation occurred in the beta-globin gene where the 17th nucleotide is exchanged from
thymine to adenine and instead of 6th amino acid becoming glutamic acid it changes to valine.
A hydrophilic motif has been produced in an HbS tetramer and leads to binding between beta 1
and beta 2 chains of 2 molecules of hemoglobin (Rees, Williams and Gladwin, 2010). A
polymer has been produced by this crystallization, which grows and ensures the filling of
erythrocyte; it also disrupts the structure and flexibility and leads to dehydration in a cell. The
extension and rate of polymerization in sickle hemoglobin is same as to the extension and
timespan of deoxygenation in hemoglobin, in erythrocyte the fetal hemoglobin is present which
results in the reduction in HbS concentration (Vekilov, 2007). The determination of severe SCD
is mainly depended on the rate and extent of polymerization in HbS. Similarly by inhibiting the
transport of cation channels stops dehydration of erythrocyte and reduced concentration of HbS,
hemolysis reduction; hemoglobin concentration is increased by hydroxycarbamide, hemolysis
reduction, and acute vaso-occlusion prevention. The pathophysiological process is involved to
trigger these manifestations: one is vaso occlusion and another one is hemolytic anemia.
Vaso Occlusion
Vaso occlusions are found to be the hallmark of SCD. VOC involved in pain occur in various
parts of the body such as legs, back, knees, chest, and stomach. The acute vaso occlusion has
occurred when erythrocyte and leucocyte entrapped in microcirculation, which results in
vascular obstruction and ischemia of tissues. HbS polymerisation is required for this process to
be occurred. Among the hemolytic anemias, the vasculopathy of SCD makes it different. Vaso
occlusion is depended upon features of erythrocyte such as the content of the polymer, cellular
damage which interacts with factors associated with the environment of the cell, endothelial
Pathophysiology
An abnormal hemoglobin is produced because of point mutation occurred in Beta-globin gene.
The mutation occurred in the beta-globin gene where the 17th nucleotide is exchanged from
thymine to adenine and instead of 6th amino acid becoming glutamic acid it changes to valine.
A hydrophilic motif has been produced in an HbS tetramer and leads to binding between beta 1
and beta 2 chains of 2 molecules of hemoglobin (Rees, Williams and Gladwin, 2010). A
polymer has been produced by this crystallization, which grows and ensures the filling of
erythrocyte; it also disrupts the structure and flexibility and leads to dehydration in a cell. The
extension and rate of polymerization in sickle hemoglobin is same as to the extension and
timespan of deoxygenation in hemoglobin, in erythrocyte the fetal hemoglobin is present which
results in the reduction in HbS concentration (Vekilov, 2007). The determination of severe SCD
is mainly depended on the rate and extent of polymerization in HbS. Similarly by inhibiting the
transport of cation channels stops dehydration of erythrocyte and reduced concentration of HbS,
hemolysis reduction; hemoglobin concentration is increased by hydroxycarbamide, hemolysis
reduction, and acute vaso-occlusion prevention. The pathophysiological process is involved to
trigger these manifestations: one is vaso occlusion and another one is hemolytic anemia.
Vaso Occlusion
Vaso occlusions are found to be the hallmark of SCD. VOC involved in pain occur in various
parts of the body such as legs, back, knees, chest, and stomach. The acute vaso occlusion has
occurred when erythrocyte and leucocyte entrapped in microcirculation, which results in
vascular obstruction and ischemia of tissues. HbS polymerisation is required for this process to
be occurred. Among the hemolytic anemias, the vasculopathy of SCD makes it different. Vaso
occlusion is depended upon features of erythrocyte such as the content of the polymer, cellular
damage which interacts with factors associated with the environment of the cell, endothelial
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 22
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





