Production of Omega 3 Fatty Acids Using E. coli & Lignocellulosic
VerifiedAdded on 2023/04/22
|15
|3660
|228
Report
AI Summary
This report details the production of omega-3 fatty acids using E. coli and lignocellulosic hydrolysate as feedstock. It covers the metabolic pathways of E. coli, modifications at the DNA level, enzyme engineering, and immobilization techniques to enhance fatty acid production. The report also includes calculations for determining cell density in immobilized systems and discusses various strategies for improving fatty acid yields, such as overexpression of genes, regulatory mutants, and computational models. The document references relevant research to support its findings and methods. Desklib offers similar solved assignments and past papers for students.

Omega 3 fatty acids production1
PRODUCTION OF OMEGA 3 FATTY ACIDS USING E.COLI AS YOUR
ORGANISM AND LIGNOCELLULOSIC HYDROLYSATE AS YOUR FEEDSTOCK
By [Name]
Course
Professor’s Name
Institution
Location of Institution
Date
PRODUCTION OF OMEGA 3 FATTY ACIDS USING E.COLI AS YOUR
ORGANISM AND LIGNOCELLULOSIC HYDROLYSATE AS YOUR FEEDSTOCK
By [Name]
Course
Professor’s Name
Institution
Location of Institution
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Omega 3 fatty acids production 2
Introduction
Omega-3 fatty acids involve a polyunsaturated fatty acid having two terminals, methyl end
which is revealed as a tail of the series and carboxylic acid terminal which is reflected at the start
of the structure. Three classes of omega-3-fatty acids intricate in human character are alpha-
linolenic, eicosapentaenoic and docosahexaenoic acid. An omega 3-fatty acid has many double
bonds, where the critical binary link is amid the third and fourth carbon fragments from the
terminal of the carbon particle sequence (Zhang, Carothers and Keasling 2012). The short course
has a set of 18 carbon atoms or less while long chain has a series of 20 carbon atoms or more.
The above three polyunsaturates have 3, 5 or 6 double links in a carbon sequence of 18, 20, or 22
carbon atoms. As with most-generated fatty acids, all double links are in the cis-conformation
(Zhang et al. 2012).
From biotechnology perspective, FA acids are power adequate and therefore are integrated into
the intracellular. Currently, FA metabolism has got substantial attention as a path for creating
high-density movement gases. Lignocellulosic comprise primarily three polymers namely;
lignin, hemicellulose and cellulose (Kim, Ximenes, Mosier and Ladisch 2011). The
Lignocellulosic needs a powerful pretreatment to create a substrate simply hydrolysable by
feasible cellulolytic enzymes or by enzyme forming molecules, to discharge sugar needed for
fermentation (Rumbold et al. 2010). Cellulose is a central component of the biomass which is a
polymer of β-D-glucopyranose fragments linked over β-(1, 4) glycosidic links with recognised
polymorphs. (Kim et al. 2011). The hemicellulose has sideward chains including of numerous
kinds of sugars. These monosaccharides involve the uronic acid, pentose and hexoses. Lignin is
the third plentiful polymers. It is a complex, broad molecular structure cross-linked polymer of
phenolic monomers (Kim, Block and Mills 2010).
Introduction
Omega-3 fatty acids involve a polyunsaturated fatty acid having two terminals, methyl end
which is revealed as a tail of the series and carboxylic acid terminal which is reflected at the start
of the structure. Three classes of omega-3-fatty acids intricate in human character are alpha-
linolenic, eicosapentaenoic and docosahexaenoic acid. An omega 3-fatty acid has many double
bonds, where the critical binary link is amid the third and fourth carbon fragments from the
terminal of the carbon particle sequence (Zhang, Carothers and Keasling 2012). The short course
has a set of 18 carbon atoms or less while long chain has a series of 20 carbon atoms or more.
The above three polyunsaturates have 3, 5 or 6 double links in a carbon sequence of 18, 20, or 22
carbon atoms. As with most-generated fatty acids, all double links are in the cis-conformation
(Zhang et al. 2012).
From biotechnology perspective, FA acids are power adequate and therefore are integrated into
the intracellular. Currently, FA metabolism has got substantial attention as a path for creating
high-density movement gases. Lignocellulosic comprise primarily three polymers namely;
lignin, hemicellulose and cellulose (Kim, Ximenes, Mosier and Ladisch 2011). The
Lignocellulosic needs a powerful pretreatment to create a substrate simply hydrolysable by
feasible cellulolytic enzymes or by enzyme forming molecules, to discharge sugar needed for
fermentation (Rumbold et al. 2010). Cellulose is a central component of the biomass which is a
polymer of β-D-glucopyranose fragments linked over β-(1, 4) glycosidic links with recognised
polymorphs. (Kim et al. 2011). The hemicellulose has sideward chains including of numerous
kinds of sugars. These monosaccharides involve the uronic acid, pentose and hexoses. Lignin is
the third plentiful polymers. It is a complex, broad molecular structure cross-linked polymer of
phenolic monomers (Kim, Block and Mills 2010).

Omega 3 fatty acids production 3
Many microorganisms have inherent biochemical pathways that vary biomass to produce that
equal the required fatty acids (Xu et al. 2013). However, industrial-level overproduction of fatty
acids from the above-secluded microorganism often needs genetic variation and DNA import to
fine-tune the many biological actions leading to the fatty acids. E.coli has unique gains of a
well-studied framework creature in respect to DNA expression and regulation and with a hugest
molecular technique accessible for genomic engineering (Mazumdar, Bang and Oh 2014). E. coli
strain can use a variety of carbon bases such as sugar alcohols under anaerobic and aerobic
surrounding and are best apt for many industrial goods (Kim, Block and Mills 2010).
Metabolic engineering
FA biosynthesis and deficiency are well acknowledged in E. coli where years of investigation
have been suitably outlined in the evaluation (Mazumdar, Bang and Oh 2014). In all iteration,
double carbon atoms are added from malonyl-ACP to an emerging acyl-set, and the succeeding
keto set is reduced to saturated methylene (Zhang, Agrawal and San 2012). The routine lasts
until elongated-chain acyl-ACPs are joined onto phospholipids by acyltransferase or altered to
other metabolites (Wahl, My, Dumoulin, Sturgis and Bouveret 2011). E. coli can also track FFAs
and use them as a solitary carbon base by generating acetyl-CoA over the β-oxidation trails.
Similarly, β-oxidation functions on acyl-CoA thioesters intermediates rather than acyl-ACP
thioesters. The initial phase of β-oxidation is FFAs activation to acyl-CoA by acyl-CoA
synthetase, for example, FadD (Feng and Cronan 2009). In addition, Acyl-CoA is a preliminary
stage for the development of many attractive oleochemicals yields.
Many microorganisms have inherent biochemical pathways that vary biomass to produce that
equal the required fatty acids (Xu et al. 2013). However, industrial-level overproduction of fatty
acids from the above-secluded microorganism often needs genetic variation and DNA import to
fine-tune the many biological actions leading to the fatty acids. E.coli has unique gains of a
well-studied framework creature in respect to DNA expression and regulation and with a hugest
molecular technique accessible for genomic engineering (Mazumdar, Bang and Oh 2014). E. coli
strain can use a variety of carbon bases such as sugar alcohols under anaerobic and aerobic
surrounding and are best apt for many industrial goods (Kim, Block and Mills 2010).
Metabolic engineering
FA biosynthesis and deficiency are well acknowledged in E. coli where years of investigation
have been suitably outlined in the evaluation (Mazumdar, Bang and Oh 2014). In all iteration,
double carbon atoms are added from malonyl-ACP to an emerging acyl-set, and the succeeding
keto set is reduced to saturated methylene (Zhang, Agrawal and San 2012). The routine lasts
until elongated-chain acyl-ACPs are joined onto phospholipids by acyltransferase or altered to
other metabolites (Wahl, My, Dumoulin, Sturgis and Bouveret 2011). E. coli can also track FFAs
and use them as a solitary carbon base by generating acetyl-CoA over the β-oxidation trails.
Similarly, β-oxidation functions on acyl-CoA thioesters intermediates rather than acyl-ACP
thioesters. The initial phase of β-oxidation is FFAs activation to acyl-CoA by acyl-CoA
synthetase, for example, FadD (Feng and Cronan 2009). In addition, Acyl-CoA is a preliminary
stage for the development of many attractive oleochemicals yields.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
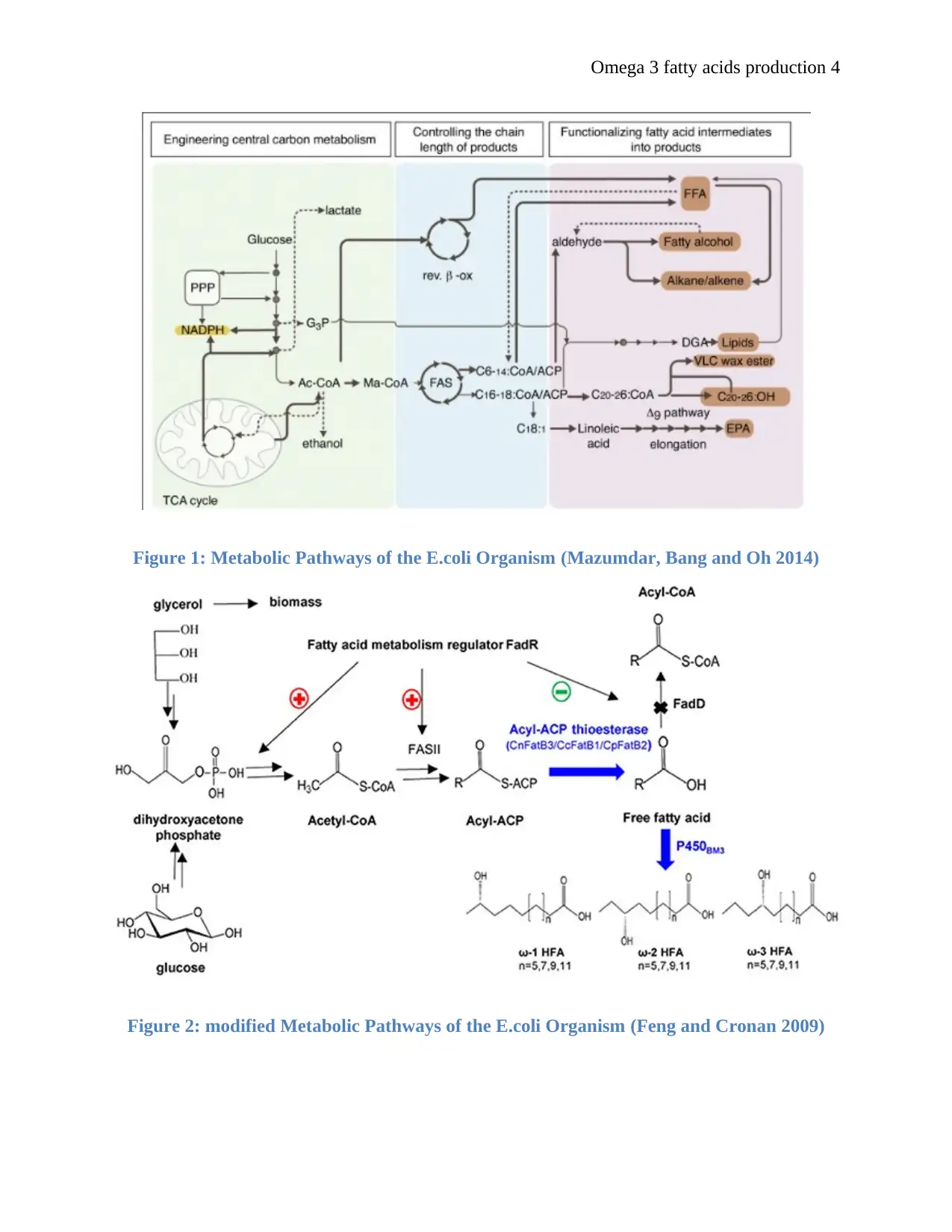
Omega 3 fatty acids production 4
Figure 1: Metabolic Pathways of the E.coli Organism (Mazumdar, Bang and Oh 2014)
Figure 2: modified Metabolic Pathways of the E.coli Organism (Feng and Cronan 2009)
Figure 1: Metabolic Pathways of the E.coli Organism (Mazumdar, Bang and Oh 2014)
Figure 2: modified Metabolic Pathways of the E.coli Organism (Feng and Cronan 2009)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Omega 3 fatty acids production 5
Modification at a DNA level
When heterologous expressed, numerous extra acyl-ACP thioesterases generate comparable
effects. In E. coli, the primary controlling indication for regulating FAB was extended sequence
acyl-ACP. The first link came from a reflection that cultured starved of glycerol showed a
reduced degree of acyl-ACP production (Wahl et al. 2011). Concurrently, flux over FAB was
established to be augmented by cytosolic overexpression of E-coli thioesterase 1, which
hydrolyses acyl-ACPs and CoA to form FFAs and subsequently reduces elongated chain acyl-
AC (My et al. 2013). In vitro researches later established that acyl-ACP directly constrain
acetyl-CoA carboxylase and to a slighter extent b-ketoacyl-ACP synthase III (Fabh) and enoyl-
ACP reductase (FabI) (My et al. 2013).
Pinpointing the rate-imitating phase in FAB would be worth to manufacturing strains for FFA
assembly (Laluce, Schenberg, Gallardo, Coradello and Pombeiro-Sponchiado 2012). Unluckily,
small kinetic factors have been established as an effect to the masses of acyl-ACP intermediates
and the hardship in measuring the protein-bound products and substrates. Moreover, the
complete range of acyl-ACP-mediated restriction of all enzymes in FAB has yet to be established
(Nawabi, Bauer, Kyrpides and Lykidis 2011).
The translational and transcriptional parameter of FAB is not fully comprehended (My et al.
2013). Binary transcriptional controllers, FadR and FabR, are intricate in regulating unsaturated
B-oxidation and FAB; however, a transcriptional aspect has not been linked with controlling
genes expression coding all FAB enzymes (Feng and Cronan 2012). It is in disparity where other
microbes such as Streptococcus pneumonia and Bacillus subtilis where transcription elements
have complete regulator over the FAB genes appearance.
Modification at a DNA level
When heterologous expressed, numerous extra acyl-ACP thioesterases generate comparable
effects. In E. coli, the primary controlling indication for regulating FAB was extended sequence
acyl-ACP. The first link came from a reflection that cultured starved of glycerol showed a
reduced degree of acyl-ACP production (Wahl et al. 2011). Concurrently, flux over FAB was
established to be augmented by cytosolic overexpression of E-coli thioesterase 1, which
hydrolyses acyl-ACPs and CoA to form FFAs and subsequently reduces elongated chain acyl-
AC (My et al. 2013). In vitro researches later established that acyl-ACP directly constrain
acetyl-CoA carboxylase and to a slighter extent b-ketoacyl-ACP synthase III (Fabh) and enoyl-
ACP reductase (FabI) (My et al. 2013).
Pinpointing the rate-imitating phase in FAB would be worth to manufacturing strains for FFA
assembly (Laluce, Schenberg, Gallardo, Coradello and Pombeiro-Sponchiado 2012). Unluckily,
small kinetic factors have been established as an effect to the masses of acyl-ACP intermediates
and the hardship in measuring the protein-bound products and substrates. Moreover, the
complete range of acyl-ACP-mediated restriction of all enzymes in FAB has yet to be established
(Nawabi, Bauer, Kyrpides and Lykidis 2011).
The translational and transcriptional parameter of FAB is not fully comprehended (My et al.
2013). Binary transcriptional controllers, FadR and FabR, are intricate in regulating unsaturated
B-oxidation and FAB; however, a transcriptional aspect has not been linked with controlling
genes expression coding all FAB enzymes (Feng and Cronan 2012). It is in disparity where other
microbes such as Streptococcus pneumonia and Bacillus subtilis where transcription elements
have complete regulator over the FAB genes appearance.

Omega 3 fatty acids production 6
All the biofuels resultants from E. coli are derivatives from the central carbon catabolism
variation, and the routine comprises the transformation of pentose or hexose sugar molecules to
C2 for the microbial production of biofuels (Kim, Block and Mills 2010).
In E.coli, the genetic factor coding ACC are positioned in three distal operons (accD, accBC,
accA) (Sabri, Nielsen and Vickers 2012). Planes of all fours ACC subdivision transcriptions
have been established to compare with development proportion, identical to the general speed of
fab (Bokinsky et al. 2011). It is recognised that AccB auto-controls transcript of accBC, most
probably by DNA attachment in its developer zone. Moreover, accD and accA translation is
auto-controlled by linking of RNA to the AccAD compound, thus offering response regulation of
translation (My et al. 2013). But, past these networks, the control of ACC is ill understood.
Assumed the primary function of malonyl-CoA creation, comprehending the regulation of ACC,
and in what way it coordinates with the FAB remnants, safeguard further examination (Zhang,
Agrawal and San 2012).
Enzyme engineering
A favourable adding to the overexpression of genetic factor that is complex in fatty acid
biosynthesis offers the regulatory mutants use (Wang et al. 2013). A possible target to improve
the FFA yields is the carbon-storage regulator, which encompasses of the CsrA protein and the
non-coding RNAs CsrB and CsrC (Ogasawara, Shinohara, Yamamoto and Ishihama 2012). A
more obvious challenger to alter the controlling scheme is the fatty acid deprivation repressor,
FadR. In an E. coli strain with a fadE removal and with tesA overexpression, coexpression of
FadR occasioned in a more than 7-fold improved FFA creation. As a result to fabA and fabB, the
All the biofuels resultants from E. coli are derivatives from the central carbon catabolism
variation, and the routine comprises the transformation of pentose or hexose sugar molecules to
C2 for the microbial production of biofuels (Kim, Block and Mills 2010).
In E.coli, the genetic factor coding ACC are positioned in three distal operons (accD, accBC,
accA) (Sabri, Nielsen and Vickers 2012). Planes of all fours ACC subdivision transcriptions
have been established to compare with development proportion, identical to the general speed of
fab (Bokinsky et al. 2011). It is recognised that AccB auto-controls transcript of accBC, most
probably by DNA attachment in its developer zone. Moreover, accD and accA translation is
auto-controlled by linking of RNA to the AccAD compound, thus offering response regulation of
translation (My et al. 2013). But, past these networks, the control of ACC is ill understood.
Assumed the primary function of malonyl-CoA creation, comprehending the regulation of ACC,
and in what way it coordinates with the FAB remnants, safeguard further examination (Zhang,
Agrawal and San 2012).
Enzyme engineering
A favourable adding to the overexpression of genetic factor that is complex in fatty acid
biosynthesis offers the regulatory mutants use (Wang et al. 2013). A possible target to improve
the FFA yields is the carbon-storage regulator, which encompasses of the CsrA protein and the
non-coding RNAs CsrB and CsrC (Ogasawara, Shinohara, Yamamoto and Ishihama 2012). A
more obvious challenger to alter the controlling scheme is the fatty acid deprivation repressor,
FadR. In an E. coli strain with a fadE removal and with tesA overexpression, coexpression of
FadR occasioned in a more than 7-fold improved FFA creation. As a result to fabA and fabB, the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Omega 3 fatty acids production 7
FadR coexpression causes to an upsurge of the unsaturated fatty acid content ranging from 13 to
43% in the strain creation (Feng and Cronan 2009).
To circumvent product ruin, numerous inquiries have been performed in a strain that was
suppressed in fatty acid b-oxidation (Laluce et al. 2012). Though many investigators recognised
that FFA concentrations enriched upon restricted obliteration of the B-oxidation paths or did not
control the triumph of this elimination, Liu et al. (2012) did not notice an optimistic influence
when thioesterase overexpression was mixed with the destruction of fadL, fadD and fade. In the
exceeding investigation, it was projected that the B-oxidation pathways cannot cope with the
large FFA production (Xu et al. 2013).
For progression in omega 3 FFA productions on a complete scale, computational models of the
E. coli metabolism has been used, and abundant eliminations in the glycolysis or Tricarboxylic
acid sequence has been inspected together with DNA of overexpression of FA biosynthesis.
Gene's destruction responsible for acetate formation has been tried to progress malonyl-CoA or
FFA production. This tactic reduced acetate growth. However, in the two latter scholars of
acetate generation decrease did not advance the FFA yield. In its place, Zhang et al. (2012) state
that acetate creation is already reduced in active FFA makers. Wide-ranging research in what
method to progress the malonyl-CoA strengths in the cytosol has been completed by Zhang,
Agrawal and San (2012).
Immobilisation
The approaches of hold using absorbent biomass support particles (BSPs), which is gorgeous due
to its easiness and suitability, depends on the intrinsic capability of adhesive cells, as a result of
their development, to build a sheath round the support substance or the capability of flocculent
FadR coexpression causes to an upsurge of the unsaturated fatty acid content ranging from 13 to
43% in the strain creation (Feng and Cronan 2009).
To circumvent product ruin, numerous inquiries have been performed in a strain that was
suppressed in fatty acid b-oxidation (Laluce et al. 2012). Though many investigators recognised
that FFA concentrations enriched upon restricted obliteration of the B-oxidation paths or did not
control the triumph of this elimination, Liu et al. (2012) did not notice an optimistic influence
when thioesterase overexpression was mixed with the destruction of fadL, fadD and fade. In the
exceeding investigation, it was projected that the B-oxidation pathways cannot cope with the
large FFA production (Xu et al. 2013).
For progression in omega 3 FFA productions on a complete scale, computational models of the
E. coli metabolism has been used, and abundant eliminations in the glycolysis or Tricarboxylic
acid sequence has been inspected together with DNA of overexpression of FA biosynthesis.
Gene's destruction responsible for acetate formation has been tried to progress malonyl-CoA or
FFA production. This tactic reduced acetate growth. However, in the two latter scholars of
acetate generation decrease did not advance the FFA yield. In its place, Zhang et al. (2012) state
that acetate creation is already reduced in active FFA makers. Wide-ranging research in what
method to progress the malonyl-CoA strengths in the cytosol has been completed by Zhang,
Agrawal and San (2012).
Immobilisation
The approaches of hold using absorbent biomass support particles (BSPs), which is gorgeous due
to its easiness and suitability, depends on the intrinsic capability of adhesive cells, as a result of
their development, to build a sheath round the support substance or the capability of flocculent
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Omega 3 fatty acids production 8
cells to make flocs inside the permeable assembly. Several studies have investigated the E.coli
immobilisation using BSPs in shake-flask culture (Feng and Cronan 2012). As the E.coli are not
effectively recollected in reticulated polyvinyl formal (PVF) resin BSPs with mediums of
comparatively microscopic stomas such as pore width of 60microm, covering the BSP surface
with many polymers are scrutinised as a method of stimulating cell embedment. When a
positively charged polyamino acids such as poly-L-arginine, poly-L-lysine and poly-L-ornithine
were adsorbed onto the particle surface, they were established to upsurge the immobilised cell
density, while negatively charged and neutral polyamino acids such as poly-L-glutamic and
poly-L-asparagine are not sufficient (Zhang et al. 2012).
The primary constraint observed with the above technique is the limitation during the product
and substrates diffusion through the cell wall of E.coli, trailed by weak holding bulk of the
permeable biomass. Irrespective of the engineering limitations of retaining, the effects show that
E.coli cells are actively immobilised with the resin BSPs by the electrostatic linkage amongst
charged polymers on the surface of BSPs and the negatively charged ions in the E.coli (Feng and
Cronan 2012).
Calculation
The preliminary phase in creating the transformation from concentration to the amount of ligand
particles per bead is to scrutinise the number of beads per millilitre of crammed resin. The
method is to compute the mean bead size and then use sphere-packing concept as the
fundamental for approximating the number of beads in one millilitre of resin (Valera, Morales,
Vanmaercke, Morfa, Cortés and Casañas 2015).
For example, beads of agarose with a width of 45 and 165 um
cells to make flocs inside the permeable assembly. Several studies have investigated the E.coli
immobilisation using BSPs in shake-flask culture (Feng and Cronan 2012). As the E.coli are not
effectively recollected in reticulated polyvinyl formal (PVF) resin BSPs with mediums of
comparatively microscopic stomas such as pore width of 60microm, covering the BSP surface
with many polymers are scrutinised as a method of stimulating cell embedment. When a
positively charged polyamino acids such as poly-L-arginine, poly-L-lysine and poly-L-ornithine
were adsorbed onto the particle surface, they were established to upsurge the immobilised cell
density, while negatively charged and neutral polyamino acids such as poly-L-glutamic and
poly-L-asparagine are not sufficient (Zhang et al. 2012).
The primary constraint observed with the above technique is the limitation during the product
and substrates diffusion through the cell wall of E.coli, trailed by weak holding bulk of the
permeable biomass. Irrespective of the engineering limitations of retaining, the effects show that
E.coli cells are actively immobilised with the resin BSPs by the electrostatic linkage amongst
charged polymers on the surface of BSPs and the negatively charged ions in the E.coli (Feng and
Cronan 2012).
Calculation
The preliminary phase in creating the transformation from concentration to the amount of ligand
particles per bead is to scrutinise the number of beads per millilitre of crammed resin. The
method is to compute the mean bead size and then use sphere-packing concept as the
fundamental for approximating the number of beads in one millilitre of resin (Valera, Morales,
Vanmaercke, Morfa, Cortés and Casañas 2015).
For example, beads of agarose with a width of 45 and 165 um

Omega 3 fatty acids production 9
Thus, the dimension of a solitary pellet at a thickness of 45um
The radium=0.0225
The sphere volume formula=4/3* π *r3 (Valera et al. 2015).
4/3* π *0.02253
For 0.00225 cm, the distinct bead capacity =4.77*10-7mL
For indeed miniature pipette, the bulk is 0.477nL!
For 165um diameter size
Radium 82.5=0.0825
Sphere volume=4/3*π*r3
4/3* π *0.08253
=2.352*10-3 ml
=2352.07nL!
For the whole bead population, the discrete bead capacity range from 0.477nL!-2352.07nL!
Utilising the sphere-packing concept which designates that spheres packs with 65-74% efficacy,
beaded agarose is a bead size, and they are neither firm nor impeccably spherical. And so, the
sphere-packing model might give only an estimated true value for the element numbers in a
precise volume (Conway and Sloane 2013).
Thus, the dimension of a solitary pellet at a thickness of 45um
The radium=0.0225
The sphere volume formula=4/3* π *r3 (Valera et al. 2015).
4/3* π *0.02253
For 0.00225 cm, the distinct bead capacity =4.77*10-7mL
For indeed miniature pipette, the bulk is 0.477nL!
For 165um diameter size
Radium 82.5=0.0825
Sphere volume=4/3*π*r3
4/3* π *0.08253
=2.352*10-3 ml
=2352.07nL!
For the whole bead population, the discrete bead capacity range from 0.477nL!-2352.07nL!
Utilising the sphere-packing concept which designates that spheres packs with 65-74% efficacy,
beaded agarose is a bead size, and they are neither firm nor impeccably spherical. And so, the
sphere-packing model might give only an estimated true value for the element numbers in a
precise volume (Conway and Sloane 2013).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Omega 3 fatty acids production 10
Supposing 75% packing effectiveness, 0.75 mL of real bead capacity will resolve to produce
closely 1mL of bed size (Conway and Sloane 2013).
Number of beads per mL in 45 um
=0.75(1bead/4.77*10-7mL)
=1.572*106 beads/mL of bed
=about 1.6 million beads per millilitre of resin
Number of beads per mL in 165 um diameter
=0.75(1bead/2.352*10-3 ml)
=318.876 beads/mL of bed
=approximately 319 beads per millilitre of resin
For that reason, the trivial length of the carrier will give a superior level of immobilised cells
than great diameter since the permeability will be low.
Supposing 75% packing effectiveness, 0.75 mL of real bead capacity will resolve to produce
closely 1mL of bed size (Conway and Sloane 2013).
Number of beads per mL in 45 um
=0.75(1bead/4.77*10-7mL)
=1.572*106 beads/mL of bed
=about 1.6 million beads per millilitre of resin
Number of beads per mL in 165 um diameter
=0.75(1bead/2.352*10-3 ml)
=318.876 beads/mL of bed
=approximately 319 beads per millilitre of resin
For that reason, the trivial length of the carrier will give a superior level of immobilised cells
than great diameter since the permeability will be low.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Omega 3 fatty acids production 11
References
Bokinsky, G., Peralta-Yahya, P.P., George, A., Holmes, B.M., Steen, E.J., Dietrich, J., Lee, T.S.,
Tullman-Ercek, D., Voigt, C.A., Simmons, B.A. and Keasling, J.D., 2011. Synthesis of three
advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia
coli. Proceedings of the National Academy of Sciences, 108(50), pp.19949-19954. [Online].
Retrieved at: http://www.pnas.org/content/108/50/19949.short , [Accessed on 11 December
2018].
Conway, J.H. and Sloane, N.J.A., 2013. Sphere packings, lattices and groups (Vol. 290).
Springer Science & Business Media. [Online]. Retrieved at: https://books.google.com/books?
hl=en&lr=&id=hoTjBwAAQBAJ&oi=fnd&pg=PR5&dq=the+sphere-
packing+theory+&ots=_zhHhPh_wL&sig=6GOtwUHfHJL2xEPgMl57OfPMQbg, [Accessed on
11 December 2018].
Feng, Y. and Cronan, J.E., 2009. A new member of the Escherichia coli fad regulon:
transcriptional regulation of fadM (ybaW). Journal of bacteriology, 191(20), pp.6320-6328.
[Online]. Retrieved at: http://jb.asm.org/content/191/20/6320.short, [Accessed on 11 December
2018].
Feng, Y. and Cronan, J.E., 2012. Crosstalk of Escherichia coli FadR with global regulators in
expression of fatty acid transport genes. PloS one, 7(9), p.e46275. [Online]. Retrieved at:
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0046275, [Accessed on 11
December 2018].
Kim, J.H., Block, D.E. and Mills, D.A., 2010. Simultaneous consumption of pentose and hexose
sugars: an optimal microbial phenotype for efficient fermentation of lignocellulosic
References
Bokinsky, G., Peralta-Yahya, P.P., George, A., Holmes, B.M., Steen, E.J., Dietrich, J., Lee, T.S.,
Tullman-Ercek, D., Voigt, C.A., Simmons, B.A. and Keasling, J.D., 2011. Synthesis of three
advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia
coli. Proceedings of the National Academy of Sciences, 108(50), pp.19949-19954. [Online].
Retrieved at: http://www.pnas.org/content/108/50/19949.short , [Accessed on 11 December
2018].
Conway, J.H. and Sloane, N.J.A., 2013. Sphere packings, lattices and groups (Vol. 290).
Springer Science & Business Media. [Online]. Retrieved at: https://books.google.com/books?
hl=en&lr=&id=hoTjBwAAQBAJ&oi=fnd&pg=PR5&dq=the+sphere-
packing+theory+&ots=_zhHhPh_wL&sig=6GOtwUHfHJL2xEPgMl57OfPMQbg, [Accessed on
11 December 2018].
Feng, Y. and Cronan, J.E., 2009. A new member of the Escherichia coli fad regulon:
transcriptional regulation of fadM (ybaW). Journal of bacteriology, 191(20), pp.6320-6328.
[Online]. Retrieved at: http://jb.asm.org/content/191/20/6320.short, [Accessed on 11 December
2018].
Feng, Y. and Cronan, J.E., 2012. Crosstalk of Escherichia coli FadR with global regulators in
expression of fatty acid transport genes. PloS one, 7(9), p.e46275. [Online]. Retrieved at:
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0046275, [Accessed on 11
December 2018].
Kim, J.H., Block, D.E. and Mills, D.A., 2010. Simultaneous consumption of pentose and hexose
sugars: an optimal microbial phenotype for efficient fermentation of lignocellulosic

Omega 3 fatty acids production 12
biomass. Applied microbiology and biotechnology, 88(5), pp.1077-1085. [Online]. Retrieved at:
https://link.springer.com/article/10.1007/s00253-010-2839-1, [Accessed on 11 December 2018].
Kim, Y., Ximenes, E., Mosier, N.S. and Ladisch, M.R., 2011. Soluble inhibitors/deactivators of
cellulase enzymes from lignocellulosic biomass. Enzyme and Microbial Technology, 48(4-5),
pp.408-415. [Online]. Retrieved at:
https://www.sciencedirect.com/science/article/pii/S0141022911000251, [Accessed on 11
December 2018].
Laluce, C., Schenberg, A.C.G., Gallardo, J.C.M., Coradello, L.F.C. and Pombeiro-Sponchiado,
S.R., 2012. Advances and developments in strategies to improve strains of Saccharomyces
cerevisiae and processes to obtain the lignocellulosic ethanol− a review. Applied biochemistry
and biotechnology, 166(8), pp.1908-1926. [Online]. Retrieved at:
https://link.springer.com/article/10.1007/s12010-012-9619-6, [Accessed on 11 December 2018].
Liu, H., Yu, C., Feng, D., Cheng, T., Meng, X., Liu, W., Zou, H. and Xian, M., 2012. Production
of extracellular fatty acid using engineered Escherichia coli. Microbial cell factories, 11(1), p.41.
[Online]. Retrieved at: https://microbialcellfactories.biomedcentral.com/articles/10.1186/1475-
2859-11-41, [Accessed on 11 December 2018].
Mazumdar, S., Bang, J. and Oh, M.K., 2014. L-Lactate production from seaweed hydrolysate of
Laminaria japonica using metabolically engineered Escherichia coli. Applied biochemistry and
biotechnology, 172(4), pp.1938-1952. [Online]. Retrieved at:
https://link.springer.com/article/10.1007/s12010-013-0653-9, [Accessed on 11 December 2018].
My, L., Rekoske, B., Lemke, J.J., Viala, J.P., Gourse, R.L. and Bouveret, E., 2013. Transcription
of the Escherichia coli fatty acid synthesis operon fabHDG is directly activated by FadR and
biomass. Applied microbiology and biotechnology, 88(5), pp.1077-1085. [Online]. Retrieved at:
https://link.springer.com/article/10.1007/s00253-010-2839-1, [Accessed on 11 December 2018].
Kim, Y., Ximenes, E., Mosier, N.S. and Ladisch, M.R., 2011. Soluble inhibitors/deactivators of
cellulase enzymes from lignocellulosic biomass. Enzyme and Microbial Technology, 48(4-5),
pp.408-415. [Online]. Retrieved at:
https://www.sciencedirect.com/science/article/pii/S0141022911000251, [Accessed on 11
December 2018].
Laluce, C., Schenberg, A.C.G., Gallardo, J.C.M., Coradello, L.F.C. and Pombeiro-Sponchiado,
S.R., 2012. Advances and developments in strategies to improve strains of Saccharomyces
cerevisiae and processes to obtain the lignocellulosic ethanol− a review. Applied biochemistry
and biotechnology, 166(8), pp.1908-1926. [Online]. Retrieved at:
https://link.springer.com/article/10.1007/s12010-012-9619-6, [Accessed on 11 December 2018].
Liu, H., Yu, C., Feng, D., Cheng, T., Meng, X., Liu, W., Zou, H. and Xian, M., 2012. Production
of extracellular fatty acid using engineered Escherichia coli. Microbial cell factories, 11(1), p.41.
[Online]. Retrieved at: https://microbialcellfactories.biomedcentral.com/articles/10.1186/1475-
2859-11-41, [Accessed on 11 December 2018].
Mazumdar, S., Bang, J. and Oh, M.K., 2014. L-Lactate production from seaweed hydrolysate of
Laminaria japonica using metabolically engineered Escherichia coli. Applied biochemistry and
biotechnology, 172(4), pp.1938-1952. [Online]. Retrieved at:
https://link.springer.com/article/10.1007/s12010-013-0653-9, [Accessed on 11 December 2018].
My, L., Rekoske, B., Lemke, J.J., Viala, J.P., Gourse, R.L. and Bouveret, E., 2013. Transcription
of the Escherichia coli fatty acid synthesis operon fabHDG is directly activated by FadR and
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 15
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


