Analysis of Structure and Properties of Sodium and its Compounds
VerifiedAdded on 2020/12/29
|8
|1133
|493
Report
AI Summary
This report provides a comprehensive overview of sodium, a group one metal, and its compounds. It begins with an introduction to sodium's significance, followed by an examination of its occurrence, including its presence in the Earth's crust and common compounds like sodium chloride and sodium carbonate, detailing their uses. The report then delves into the atomic structure of sodium, illustrating how it forms ionic bonds by giving away its valence electron, and presents its physical properties, such as boiling point, electronegativity, and conductivity. It explains the formation of sodium compounds, highlighting the concept of ionic bonding with examples like NaCl. The report further elucidates the differences between sodium and its compounds, focusing on their structural formations and reactivity. The conclusion emphasizes the importance of understanding the properties of sodium and its compounds for safe and effective utilization. The report includes references to books, journals, and online resources.

STRUCTURE AND PROPERTIES OF A
GROUP ONE METAL AND ITS
COMPOUND
GROUP ONE METAL AND ITS
COMPOUND
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

TABLE OF CONTENTS
INTRODUCTION...........................................................................................................................1
MAIN BODY..................................................................................................................................1
Occurrence of Sodium with its uses............................................................................................1
Its atomic structure and physical properties................................................................................1
Formation of Sodium compounds...............................................................................................3
Using examples explain and illustrate this statement..................................................................3
Differences between Sodium and its compounds........................................................................4
CONCLUSION................................................................................................................................5
REFERENCES................................................................................................................................6
INTRODUCTION...........................................................................................................................1
MAIN BODY..................................................................................................................................1
Occurrence of Sodium with its uses............................................................................................1
Its atomic structure and physical properties................................................................................1
Formation of Sodium compounds...............................................................................................3
Using examples explain and illustrate this statement..................................................................3
Differences between Sodium and its compounds........................................................................4
CONCLUSION................................................................................................................................5
REFERENCES................................................................................................................................6

INTRODUCTION
This report is going to emphasis on the brief introduction on significance of Sodium,
which is a member of the metal alkali group which has atomic number 11 on the s-block of
periodic table. It is the 6th most common element.
MAIN BODY
Occurrence of Sodium with its uses
It is found abundantly on the crust of earth, approximately 2.6% in both the forms, that is
element form (NA+) and compound as well. It can never be found as a free element in
nature.
The most common compound is Sodium Chloride (NaCl), popularly known as common
salt and is primarily used in food industry and functioning of metabolic activities in
humans, animals and plants.
Another compound is Sodium Carbonate (Na2CO3) used as water softener for various
purposes like washing, laundering etc.
Sodium bicarbonate (Na2CO3), also called as can be used in food and beverages industry.
It helps to maintain the transmission of nutrients to all neurons, tissues and blood in order
to regulate the water levels.
It uses as chemical reagent as the manufacturing of paper, soaps, glass etc. and also acts
as heat exchanger for nuclear reactors.
Its atomic structure and physical properties
The atomic structure of Sodium forms ionic bonds as it gives away its valence electrons
easily to non-metallic or other metallic atom to attain constancy of stable noble gas
configuration. Its electronic configuration is (2,8,1). In the below representation, the neutral atom
of sodium, Na, loses an electron because there is only one valence electron in the orbital. This
will make its outer orbital, i.e. 2nd energy level with 8 electrons giving it the stability, thus
resulting as overall charge of 1+, with cation.
1
This report is going to emphasis on the brief introduction on significance of Sodium,
which is a member of the metal alkali group which has atomic number 11 on the s-block of
periodic table. It is the 6th most common element.
MAIN BODY
Occurrence of Sodium with its uses
It is found abundantly on the crust of earth, approximately 2.6% in both the forms, that is
element form (NA+) and compound as well. It can never be found as a free element in
nature.
The most common compound is Sodium Chloride (NaCl), popularly known as common
salt and is primarily used in food industry and functioning of metabolic activities in
humans, animals and plants.
Another compound is Sodium Carbonate (Na2CO3) used as water softener for various
purposes like washing, laundering etc.
Sodium bicarbonate (Na2CO3), also called as can be used in food and beverages industry.
It helps to maintain the transmission of nutrients to all neurons, tissues and blood in order
to regulate the water levels.
It uses as chemical reagent as the manufacturing of paper, soaps, glass etc. and also acts
as heat exchanger for nuclear reactors.
Its atomic structure and physical properties
The atomic structure of Sodium forms ionic bonds as it gives away its valence electrons
easily to non-metallic or other metallic atom to attain constancy of stable noble gas
configuration. Its electronic configuration is (2,8,1). In the below representation, the neutral atom
of sodium, Na, loses an electron because there is only one valence electron in the orbital. This
will make its outer orbital, i.e. 2nd energy level with 8 electrons giving it the stability, thus
resulting as overall charge of 1+, with cation.
1
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Figure 1. Pictorial representation of neutral sodium atom (loses e- to form a cation Na+)
(Source: Lithium atomic Structure Diagram Fresh 2 6 Molecular and Ionic Pounds, 2013)
Below table giving the number of free electrons present in orbitals, due to Pauli
Exclusion Principle so that Sodium’s electronic configuration [1s22s22p63s1] can be done.
SL
No.
Physical Properties Description
1 Boiling Point 883 °C; it changes properties at elevated pressure
2 Electronegativity Low value of 0.93; Its outermost orbital has one free
electron that can be easily removed
3 Melting Point Low M.P. and can
4 Color White-silver
5 Softness It can easily can using a knife
6 Conductivity Good movement of electrons, thus good conductor
of electricity and heat
7 Luster Shining
8 Ductility Can be stretched into thin wires
2
Number of Energy
levels
3
First Energy level 2
Second Energy level 8
Third Energy level 1
(Source: Lithium atomic Structure Diagram Fresh 2 6 Molecular and Ionic Pounds, 2013)
Below table giving the number of free electrons present in orbitals, due to Pauli
Exclusion Principle so that Sodium’s electronic configuration [1s22s22p63s1] can be done.
SL
No.
Physical Properties Description
1 Boiling Point 883 °C; it changes properties at elevated pressure
2 Electronegativity Low value of 0.93; Its outermost orbital has one free
electron that can be easily removed
3 Melting Point Low M.P. and can
4 Color White-silver
5 Softness It can easily can using a knife
6 Conductivity Good movement of electrons, thus good conductor
of electricity and heat
7 Luster Shining
8 Ductility Can be stretched into thin wires
2
Number of Energy
levels
3
First Energy level 2
Second Energy level 8
Third Energy level 1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
9 Malleability Ability to be bent or dis-shape
10 Density 0.971 g/cc; can easily water float.
Formation of Sodium compounds
This element does not found naturally in free form. Its reactivity is so high that its existence is
only in compound form and due to this, mostly kept in kerosene to avoid any accident. It easily
gets reacted explosively with oxygen at room temperature only. In regard to this, as already
mentioned that it has one electron in its outermost orbit which easily gets attracted by losing and
making an ionic or covalent bond to form a crystal lattice. All ionic compounds take place by
complete transfer of electrons between cation and anion. For e.g. NaCl, where Na+ cation has a
strong electrostatic attraction towards Cl- anion.
Using examples explain and illustrate this statement
With reference to the above statement of Group one metals bond ironically with non-
metals, the below illustration must be refereed.
The electronic configuration of Chlorine is 2,8,7 and on the other hand, Sodium’s is
2,8,1. This clearly indicated that cl- needs one extra electron to reach its stable noble gas
configuration in order to complete its octet. This transfer helps in achieving the stable
configurations.
Here, Na+ donates one electron that approaches Cl-, which is gained by it to form a
balanced bond.
Na → Na+ + e-
Cl + e- → Cl-
Symbol of
Element
Atomic
Number
Mass
Number
Number of
Neutrons
Number of
Protons
Number of
Electrons
Na 11 23 12 11 12
Cl 17 35 18 17 17
3
10 Density 0.971 g/cc; can easily water float.
Formation of Sodium compounds
This element does not found naturally in free form. Its reactivity is so high that its existence is
only in compound form and due to this, mostly kept in kerosene to avoid any accident. It easily
gets reacted explosively with oxygen at room temperature only. In regard to this, as already
mentioned that it has one electron in its outermost orbit which easily gets attracted by losing and
making an ionic or covalent bond to form a crystal lattice. All ionic compounds take place by
complete transfer of electrons between cation and anion. For e.g. NaCl, where Na+ cation has a
strong electrostatic attraction towards Cl- anion.
Using examples explain and illustrate this statement
With reference to the above statement of Group one metals bond ironically with non-
metals, the below illustration must be refereed.
The electronic configuration of Chlorine is 2,8,7 and on the other hand, Sodium’s is
2,8,1. This clearly indicated that cl- needs one extra electron to reach its stable noble gas
configuration in order to complete its octet. This transfer helps in achieving the stable
configurations.
Here, Na+ donates one electron that approaches Cl-, which is gained by it to form a
balanced bond.
Na → Na+ + e-
Cl + e- → Cl-
Symbol of
Element
Atomic
Number
Mass
Number
Number of
Neutrons
Number of
Protons
Number of
Electrons
Na 11 23 12 11 12
Cl 17 35 18 17 17
3
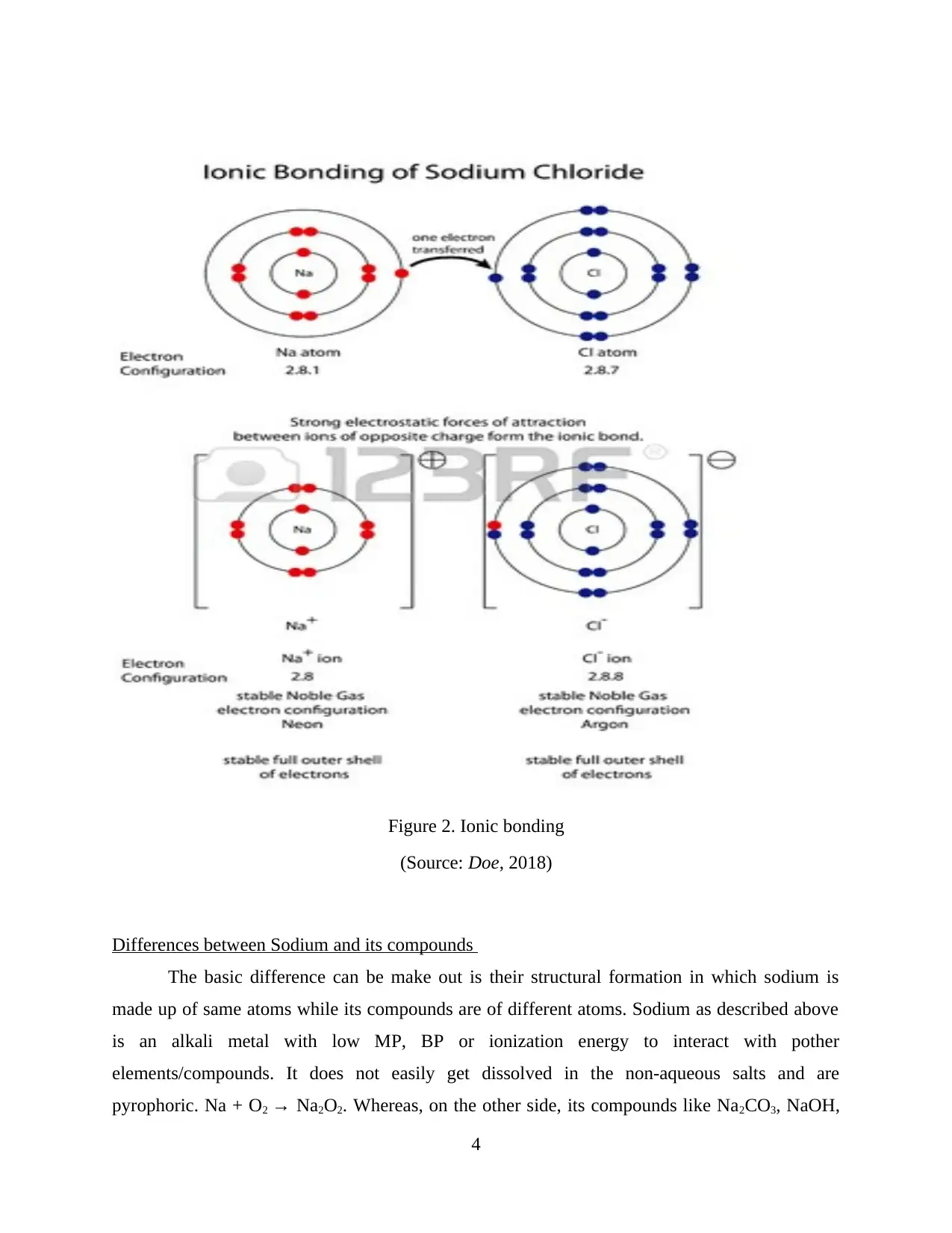
Figure 2. Ionic bonding
(Source: Doe, 2018)
Differences between Sodium and its compounds
The basic difference can be make out is their structural formation in which sodium is
made up of same atoms while its compounds are of different atoms. Sodium as described above
is an alkali metal with low MP, BP or ionization energy to interact with pother
elements/compounds. It does not easily get dissolved in the non-aqueous salts and are
pyrophoric. Na + O2 → Na2O2. Whereas, on the other side, its compounds like Na2CO3, NaOH,
4
(Source: Doe, 2018)
Differences between Sodium and its compounds
The basic difference can be make out is their structural formation in which sodium is
made up of same atoms while its compounds are of different atoms. Sodium as described above
is an alkali metal with low MP, BP or ionization energy to interact with pother
elements/compounds. It does not easily get dissolved in the non-aqueous salts and are
pyrophoric. Na + O2 → Na2O2. Whereas, on the other side, its compounds like Na2CO3, NaOH,
4
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Na2SO4 etc. are directly can react with binary compounds or water to form hydroxides, salts or
oxides. These are in swift reactive state as these are non-pyrophoric. E.g. Na2CO3 Heat→ Na2CO3
+ H2O +CO2.
CONCLUSION
It is evident that the compounds would have stronger properties that its actual element,
thus this element with its compounds utilization must be done with proper guidance and
complete awareness.
5
oxides. These are in swift reactive state as these are non-pyrophoric. E.g. Na2CO3 Heat→ Na2CO3
+ H2O +CO2.
CONCLUSION
It is evident that the compounds would have stronger properties that its actual element,
thus this element with its compounds utilization must be done with proper guidance and
complete awareness.
5
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

REFERENCES
Books and Journals
Sridharan, R., 2017. Chemistry of sodium coolant for fast reactor applications. In Proceedings of
the thirteenth DAE-BRNS nuclear and radiochemistry symposium.
Kundu, D. and et.al., 2015. The emerging chemistry of sodium ion batteries for electrochemical
energy storage. Angewandte Chemie International Edition. 54(11). pp.3431-3448.
Sula, A. and et.al., 2017. The complete structure of an activated open sodium channel. Nature
communications. 8. p.14205.
Online
Doe, 2018. Colorful Sodium Shell Diagram. [Online]. Available through:<
http://sleeperfurniture.co/colorful-sodium-shell-diagram.html>.
Lithium atomic Structure Diagram Fresh 2 6 Molecular and Ionic Pounds, 2013. [Online].
Available through:< http://myforgottencoast.com/lithium-atomic-structure-diagram/lithium-
atomic-structure-diagram-fresh-2-6-molecular-and-ionic-pounds-chemistry/>.
6
Books and Journals
Sridharan, R., 2017. Chemistry of sodium coolant for fast reactor applications. In Proceedings of
the thirteenth DAE-BRNS nuclear and radiochemistry symposium.
Kundu, D. and et.al., 2015. The emerging chemistry of sodium ion batteries for electrochemical
energy storage. Angewandte Chemie International Edition. 54(11). pp.3431-3448.
Sula, A. and et.al., 2017. The complete structure of an activated open sodium channel. Nature
communications. 8. p.14205.
Online
Doe, 2018. Colorful Sodium Shell Diagram. [Online]. Available through:<
http://sleeperfurniture.co/colorful-sodium-shell-diagram.html>.
Lithium atomic Structure Diagram Fresh 2 6 Molecular and Ionic Pounds, 2013. [Online].
Available through:< http://myforgottencoast.com/lithium-atomic-structure-diagram/lithium-
atomic-structure-diagram-fresh-2-6-molecular-and-ionic-pounds-chemistry/>.
6
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





