Protein Cystatin C: Structure, Function, Dysfunction and CAA Disease
VerifiedAdded on 2020/03/23
|10
|2156
|97
Report
AI Summary
This report delves into the multifaceted nature of Cystatin C, a protein with critical roles in human biology. It begins by outlining the normal function of Cystatin C as a proteinase inhibitor, particularly its regulation of cysteine proteases and its use as a biomarker for renal and cardiovascular health. The report then examines the protein's primary, secondary, tertiary, and quaternary structures, including the canonical cystatin fold and its specialized structural features. A significant portion of the report is dedicated to the analysis of protein dysfunction, specifically focusing on Cerebral Amyloid Angiopathy (CAA), an autosomal dominant disease caused by mutations in the cystatin C gene. The molecular basis of CAA is explored, detailing gene mutations, protein misfolding, and the formation of amyloid fibrils. The report also discusses the symptoms, physiological basis, and current and potential future treatments for CAA, including the use of anti-amyloid therapies like gantenerumab. Finally, the report provides a detailed list of references.

Running head: PROTEIN-CYSTATIN C
Protein-Cystatin C
Name of the Student
Name of the University
Author Note
Protein-Cystatin C
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PROTEIN-CYSTATIN C
Table of Contents
Normal Function of Protein Cystatin C and its Role in Human Biology...................................2
Primary Structure of Protein Cystatin C................................................................................2
Secondary Tertiary and Quaternary Structure........................................................................3
Analysis of Protein Dysfunction............................................................................................4
Details of protein family........................................................................................................4
Cerebral Amyloid Angiopathy (CAA).......................................................................................5
Molecular Basis of the Disease..............................................................................................5
Gene mutation........................................................................................................................5
Protein Dysfunction...............................................................................................................5
Disease Symptoms and Characteristics..................................................................................5
Physiological Basis of the Disease.........................................................................................6
Current and Future Potential of Treatment................................................................................6
References..................................................................................................................................8
Table of Contents
Normal Function of Protein Cystatin C and its Role in Human Biology...................................2
Primary Structure of Protein Cystatin C................................................................................2
Secondary Tertiary and Quaternary Structure........................................................................3
Analysis of Protein Dysfunction............................................................................................4
Details of protein family........................................................................................................4
Cerebral Amyloid Angiopathy (CAA).......................................................................................5
Molecular Basis of the Disease..............................................................................................5
Gene mutation........................................................................................................................5
Protein Dysfunction...............................................................................................................5
Disease Symptoms and Characteristics..................................................................................5
Physiological Basis of the Disease.........................................................................................6
Current and Future Potential of Treatment................................................................................6
References..................................................................................................................................8

PROTEIN-CYSTATIN C
Normal Function of Protein Cystatin C and its Role in Human Biology
The protein cystatin C is proteinase inhibitor. This ubiquitous protein is found in the
body fluids of all the mammals. The primary function of Protein Cystatin C is to regulate the
activity of cysteine via inhibiting its vital functions. Cysteine plays an important role in
regulating the intracellular catabolism of proteins and peptides. Protein Cystatin C shows
active inhibitory effect on several cysteine protease namely cathepsins B, H, K, L and S. This
ubiquitous protein undergoes proteolytic degradation under the influence of cathepsin D and
elastase. Cathepsins are lysosomal proteases and are popularly known as housekeeping genes
and they differ in their primary structural symmetry, substrate binding specificities and
biochemical characteristics (1).
Cystatin C is a biomarker used for the detection of normal renal function and
cardiovascular disease (1).
Primary Structure of Protein Cystatin C
Cystatin C belongs to the cystatin superfamily. It interacts reversibly with target
peptidases and such interactions is seemingly independent and depends only on the affinity
contributions from a wedge-shaped binding region that is constructed with the help of the two
loop-forming inhibitor segments and a corresponding binding region corresponding to the N-
terminal segment of that particular inhibitor (2).
Protein Cystatin C is present as a single copy in the human genome with a size of 5 kb
(approx.) It is a non-glycosylated polypeptide chain of 120-residue. cystatin family exhibits
low molecular weight and shares homology in their amino-acid sequences. In spite of their
sequential homology, there exists a difference in the post translational modification. The
nature of the post-translational modification of cystatin gene (alpha, beta and gamma) is
Normal Function of Protein Cystatin C and its Role in Human Biology
The protein cystatin C is proteinase inhibitor. This ubiquitous protein is found in the
body fluids of all the mammals. The primary function of Protein Cystatin C is to regulate the
activity of cysteine via inhibiting its vital functions. Cysteine plays an important role in
regulating the intracellular catabolism of proteins and peptides. Protein Cystatin C shows
active inhibitory effect on several cysteine protease namely cathepsins B, H, K, L and S. This
ubiquitous protein undergoes proteolytic degradation under the influence of cathepsin D and
elastase. Cathepsins are lysosomal proteases and are popularly known as housekeeping genes
and they differ in their primary structural symmetry, substrate binding specificities and
biochemical characteristics (1).
Cystatin C is a biomarker used for the detection of normal renal function and
cardiovascular disease (1).
Primary Structure of Protein Cystatin C
Cystatin C belongs to the cystatin superfamily. It interacts reversibly with target
peptidases and such interactions is seemingly independent and depends only on the affinity
contributions from a wedge-shaped binding region that is constructed with the help of the two
loop-forming inhibitor segments and a corresponding binding region corresponding to the N-
terminal segment of that particular inhibitor (2).
Protein Cystatin C is present as a single copy in the human genome with a size of 5 kb
(approx.) It is a non-glycosylated polypeptide chain of 120-residue. cystatin family exhibits
low molecular weight and shares homology in their amino-acid sequences. In spite of their
sequential homology, there exists a difference in the post translational modification. The
nature of the post-translational modification of cystatin gene (alpha, beta and gamma) is
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
PROTEIN-CYSTATIN C
different from each other. The modification is dependent on their localizations. This
difference in post translational modification helps in the generation of diverse biological
functions (2).
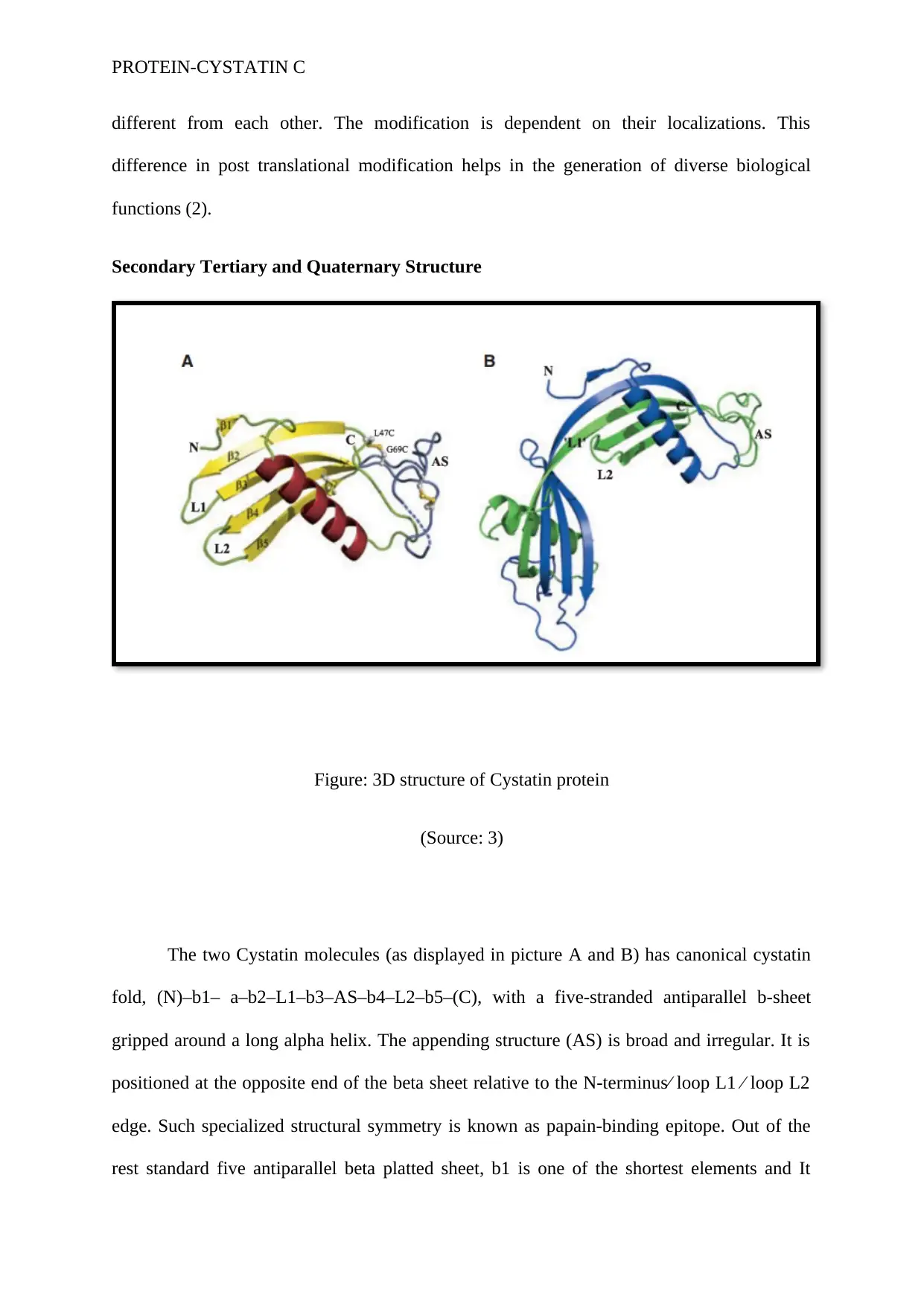
Secondary Tertiary and Quaternary Structure
Figure: 3D structure of Cystatin protein
(Source: 3)
The two Cystatin molecules (as displayed in picture A and B) has canonical cystatin
fold, (N)–b1– a–b2–L1–b3–AS–b4–L2–b5–(C), with a five-stranded antiparallel b-sheet
gripped around a long alpha helix. The appending structure (AS) is broad and irregular. It is
positioned at the opposite end of the beta sheet relative to the N-terminus⁄ loop L1 ⁄ loop L2
edge. Such specialized structural symmetry is known as papain-binding epitope. Out of the
rest standard five antiparallel beta platted sheet, b1 is one of the shortest elements and It
different from each other. The modification is dependent on their localizations. This
difference in post translational modification helps in the generation of diverse biological
functions (2).
Secondary Tertiary and Quaternary Structure
Figure: 3D structure of Cystatin protein
(Source: 3)
The two Cystatin molecules (as displayed in picture A and B) has canonical cystatin
fold, (N)–b1– a–b2–L1–b3–AS–b4–L2–b5–(C), with a five-stranded antiparallel b-sheet
gripped around a long alpha helix. The appending structure (AS) is broad and irregular. It is
positioned at the opposite end of the beta sheet relative to the N-terminus⁄ loop L1 ⁄ loop L2
edge. Such specialized structural symmetry is known as papain-binding epitope. Out of the
rest standard five antiparallel beta platted sheet, b1 is one of the shortest elements and It
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PROTEIN-CYSTATIN C
comprises of two residues. In both the molecules displayed in the above picture, the first 11
residues are completely disordered. The following two AS residues, which differ in each
molecule are found to be Proline78-Leucine79 in A and Leucine80-Aspartate81 in B. Some
of the disulfide bridges exists in a partially broken form in the tertiary structure (3).
Analysis of Protein Dysfunction
Cerebral Amyloid Angiopathy (CAA) occurs due to the mutation in one of the three
exons of cystatin protein. The luciene residue at 68th position of the gene is the principal
target for gene mutation. Generally, heterogeneous point mutation occurs at this particular
site which results in the formation of truncated protein from the amino terminal domain. The
post translational pathway of protein folding (elastase or a serine protease) fails to recognise
this truncated protein, leading to protein misfolding. Moreover, this luciene variant at 68th
position (Leu68Gln) of cystatin C gene is the major building block of amyloid fibrils. The
misfolded protein gets deposited over the cerebral/spinal arteries as amyloid fibrils leading to
disease manifestation (4).
Details of protein family
Human Cystatin C Gene or CST3 produce Cystatin Protein upon translation. The
same gene is also responsible for the generation of cystatin protein found in mammals. It is
located in chromosome number 20 in humans. Protein Cystatin C falls under the group of
proteinase inhibitors and manipulates the mode of action of cysteine proteinases via
inhibiting their activity (2). Three cystatin loci namely CST1 (cystatin SN), CST2 (cystatin
SA) and CSTP1 (a cystatin pseudo gene) together constitute CST3. CST3 gene is made up of
two introns (non-coding region) and three exons (coding region). The introns in the gene
cover a base air of 2252 and 1254 bp respectively. Introns here are located in between the
nucleotide triplets encoding the amino acid residues 55-56 and 93-94 of the mature protein
respectively. The respective positions of the introns are identical with cystatin SN and
comprises of two residues. In both the molecules displayed in the above picture, the first 11
residues are completely disordered. The following two AS residues, which differ in each
molecule are found to be Proline78-Leucine79 in A and Leucine80-Aspartate81 in B. Some
of the disulfide bridges exists in a partially broken form in the tertiary structure (3).
Analysis of Protein Dysfunction
Cerebral Amyloid Angiopathy (CAA) occurs due to the mutation in one of the three
exons of cystatin protein. The luciene residue at 68th position of the gene is the principal
target for gene mutation. Generally, heterogeneous point mutation occurs at this particular
site which results in the formation of truncated protein from the amino terminal domain. The
post translational pathway of protein folding (elastase or a serine protease) fails to recognise
this truncated protein, leading to protein misfolding. Moreover, this luciene variant at 68th
position (Leu68Gln) of cystatin C gene is the major building block of amyloid fibrils. The
misfolded protein gets deposited over the cerebral/spinal arteries as amyloid fibrils leading to
disease manifestation (4).
Details of protein family
Human Cystatin C Gene or CST3 produce Cystatin Protein upon translation. The
same gene is also responsible for the generation of cystatin protein found in mammals. It is
located in chromosome number 20 in humans. Protein Cystatin C falls under the group of
proteinase inhibitors and manipulates the mode of action of cysteine proteinases via
inhibiting their activity (2). Three cystatin loci namely CST1 (cystatin SN), CST2 (cystatin
SA) and CSTP1 (a cystatin pseudo gene) together constitute CST3. CST3 gene is made up of
two introns (non-coding region) and three exons (coding region). The introns in the gene
cover a base air of 2252 and 1254 bp respectively. Introns here are located in between the
nucleotide triplets encoding the amino acid residues 55-56 and 93-94 of the mature protein
respectively. The respective positions of the introns are identical with cystatin SN and

PROTEIN-CYSTATIN C
cystatin SA genes. However, the first intron of CST3 gene is comparatively bigger than the
first introns present in the cystatin SN and cystatin SA genes and thus creating a structural
gene difference. The approximate size of the gene though remains similar amounting to about
is 4.3 kb (2).
Cerebral Amyloid Angiopathy (CAA)
Cerebral Amyloid Angiopathy (CAA), an autosomal dominant disease and is
commonly known as hereditary cystatin C amyloid angiopathy (HCCAA). The disease is a
combination of amyloidosis with hereditary cerebral haemorrhage. The disease causes
amyloid deposition in the spinal or cerebral arteries along with arterioles. The deposited
amyloid fibrils block the proper neuronal function, leading to recurrent haemorrhagic strokes.
Such shocks cause severe brain damage, and even lead to fatal stroke (5).
Molecular Basis of the Disease
This dominant disease results from a point mutation. The truncated version of the
cystatin c gene is a result of heterogeneous point mutation. Elastase or a serine protease fails
to recognise the truncated protein, leading to protein misfolding. The misfolded or unfolded
protein gets deposited over the cerebral/spinal arteries and is then known as amyloid fibrils
(2).
Gene mutation
Cystatin C, a Leu68Gln variant is the principal component of the amyloid fibrils.
CAA, an autosomal dominant disorder, results out of heterozygous point mutation, in the
cystatin C gene (7).
cystatin SA genes. However, the first intron of CST3 gene is comparatively bigger than the
first introns present in the cystatin SN and cystatin SA genes and thus creating a structural
gene difference. The approximate size of the gene though remains similar amounting to about
is 4.3 kb (2).
Cerebral Amyloid Angiopathy (CAA)
Cerebral Amyloid Angiopathy (CAA), an autosomal dominant disease and is
commonly known as hereditary cystatin C amyloid angiopathy (HCCAA). The disease is a
combination of amyloidosis with hereditary cerebral haemorrhage. The disease causes
amyloid deposition in the spinal or cerebral arteries along with arterioles. The deposited
amyloid fibrils block the proper neuronal function, leading to recurrent haemorrhagic strokes.
Such shocks cause severe brain damage, and even lead to fatal stroke (5).
Molecular Basis of the Disease
This dominant disease results from a point mutation. The truncated version of the
cystatin c gene is a result of heterogeneous point mutation. Elastase or a serine protease fails
to recognise the truncated protein, leading to protein misfolding. The misfolded or unfolded
protein gets deposited over the cerebral/spinal arteries and is then known as amyloid fibrils
(2).
Gene mutation
Cystatin C, a Leu68Gln variant is the principal component of the amyloid fibrils.
CAA, an autosomal dominant disorder, results out of heterozygous point mutation, in the
cystatin C gene (7).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PROTEIN-CYSTATIN C
Protein Dysfunction
CST3 is commonly known as human cystatin C gene. It is located in chromosome 20
and its mutation is responsible for hereditary cystatin C amyloid angiopathy (6). The
misfolded protein fails to functions properly and leading to the generation of complications.
Disease Symptoms and Characteristics
In CAA, blood leaks out from several vessels of the brain. This leakage of the blood
outside the blood vessels leads to the formation of blood clot and the brain cells or neurons
suddenly stops working. Due to the malfunction of the neurons, the primary symptoms,
which are expressed in case of CAA, are weakness or paralysis of the limbs. These symptoms
are gradually followed by difficulty in speaking, loss of sensation, loss of body balance. In
extreme cases, patients pass into coma. Sudden leakage of the blood vessels through the
sensitive tissues results in the generation of unbearable headache. In rare cases there occurs
seizures, short spells and other temporary neurologic symptoms like tingling of face and
weakness in limbs (8).
Patients with CAA have focal neurological deficit with large parenchymal
hematomas. Symptoms like intracranial pressure are common along with alterations in
consciousness. CAA also causes mass lesion and intimal vessel thickening leading to
transient neurological symptoms like leukoencephalopathy and seizures. Such symptoms may
last for a minutes or an hour (9).
Physiological Basis of the Disease
CAA is defined as a condition of vasculopathy. It causes deposition of amyloid fibrils
inside the arterioles and the arteries of the cerebral cortex. Staining of the affected brain
tissue with hematoxylin and eosin revealed hyaline thickening in the blood vessels of the
brain along with luminal narrowing. CAA is also detected in progressive dementia after a
Protein Dysfunction
CST3 is commonly known as human cystatin C gene. It is located in chromosome 20
and its mutation is responsible for hereditary cystatin C amyloid angiopathy (6). The
misfolded protein fails to functions properly and leading to the generation of complications.
Disease Symptoms and Characteristics
In CAA, blood leaks out from several vessels of the brain. This leakage of the blood
outside the blood vessels leads to the formation of blood clot and the brain cells or neurons
suddenly stops working. Due to the malfunction of the neurons, the primary symptoms,
which are expressed in case of CAA, are weakness or paralysis of the limbs. These symptoms
are gradually followed by difficulty in speaking, loss of sensation, loss of body balance. In
extreme cases, patients pass into coma. Sudden leakage of the blood vessels through the
sensitive tissues results in the generation of unbearable headache. In rare cases there occurs
seizures, short spells and other temporary neurologic symptoms like tingling of face and
weakness in limbs (8).
Patients with CAA have focal neurological deficit with large parenchymal
hematomas. Symptoms like intracranial pressure are common along with alterations in
consciousness. CAA also causes mass lesion and intimal vessel thickening leading to
transient neurological symptoms like leukoencephalopathy and seizures. Such symptoms may
last for a minutes or an hour (9).
Physiological Basis of the Disease
CAA is defined as a condition of vasculopathy. It causes deposition of amyloid fibrils
inside the arterioles and the arteries of the cerebral cortex. Staining of the affected brain
tissue with hematoxylin and eosin revealed hyaline thickening in the blood vessels of the
brain along with luminal narrowing. CAA is also detected in progressive dementia after a
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

PROTEIN-CYSTATIN C
course of few weeks or months. Dementia which occurs in association with CAA is
characterized by severe deposition of vascular amyloid fibrils along with cortical
haemorrhages. This haemorrhages causes destruction of the white matter. In case of
Alzheimer disease, the amyloid fibrils are deposited in the parenchyma leading to neuritic
dystrophy loss of synaptic activity (9).
Current and Future Potential of Treatment
CAA is largely untreated because in the majority of the cases, it remains unidentified.
In order to treat haemorrhage associated with CAA, the therapy is limited to withdrawal of
antiplatelet agents and anticoagulant agents. Here anticoagulants like warfarin are used.
However, prolong used of warfarin is not safe and such treatments may give rise of ischemic
heart attack (10).
Clearance of the amyloid plaques occurs via antiamyloid therapy. Current research is
trying to employ gantenerumab as the principal drug for the removal of amyloid plaques.
Gantenerumab is a fully human anti-A antibody derived from human IgG1. This potent
antibody has high affinity towards fibrilar amyloid beta fibrils and binds specifically to
amyloid plaques and thereby promoting their clearance (11).
course of few weeks or months. Dementia which occurs in association with CAA is
characterized by severe deposition of vascular amyloid fibrils along with cortical
haemorrhages. This haemorrhages causes destruction of the white matter. In case of
Alzheimer disease, the amyloid fibrils are deposited in the parenchyma leading to neuritic
dystrophy loss of synaptic activity (9).
Current and Future Potential of Treatment
CAA is largely untreated because in the majority of the cases, it remains unidentified.
In order to treat haemorrhage associated with CAA, the therapy is limited to withdrawal of
antiplatelet agents and anticoagulant agents. Here anticoagulants like warfarin are used.
However, prolong used of warfarin is not safe and such treatments may give rise of ischemic
heart attack (10).
Clearance of the amyloid plaques occurs via antiamyloid therapy. Current research is
trying to employ gantenerumab as the principal drug for the removal of amyloid plaques.
Gantenerumab is a fully human anti-A antibody derived from human IgG1. This potent
antibody has high affinity towards fibrilar amyloid beta fibrils and binds specifically to
amyloid plaques and thereby promoting their clearance (11).

PROTEIN-CYSTATIN C
References
1. Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. cerebral amyloid
angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination
failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy.
Neuropathology and applied neurobiology. 2013 Oct 1;39(6):593-611.
2. Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins:
from structure, function and regulation to new frontiers. Biochimica et Biophysica
Acta (BBA)-Proteins and Proteomics. 2012 Jan 31;1824(1):68-88.
3. Kolodziejczyk R, Michalska K, Hernandez‐Santoyo A, Wahlbom M, Grubb A,
Jaskolski M. Crystal structure of human cystatin C stabilized against amyloid
formation. The FEBS journal. 2010 Apr 1;277(7):1726-37.
4. Rajagopalan P, Refsum H, Hua X, Toga AW, Jack CR, Weiner MW, Thompson PM,
Alzheimer's Disease Neuroimaging Initiative. Mapping creatinine-and cystatin C-
related white matter brain deficits in the elderly. Neurobiology of aging. 2013 Apr
30;34(4):1221-30.
5. Yamada M, Naiki H. Cerebral amyloid angiopathy. Progress in molecular biology and
translational science. 2012;107:41-78.
6. Lamontagne M, Timens W, Hao K, Bossé Y, Laviolette M, Steiling K, Campbell JD,
Couture C, Conti M, Sherwood K, Hogg JC. Genetic regulation of gene expression in
the lung identifies CST3 and CD22 as potential causal genes for airflow obstruction.
Thorax. 2014 Sep 2:thoraxjnl-2014.
7. Magister Š, Kos J. Cystatins in immune system. Journal of Cancer. 2013;4(1):45.
8. Cerebral Amyloid Angiopathy [FAQ] [Internet]. Angiopathy.org. 2017 [cited 26
September 2017]. Available from: http://www.angiopathy.org/faq.html#symptoms
References
1. Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. cerebral amyloid
angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination
failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy.
Neuropathology and applied neurobiology. 2013 Oct 1;39(6):593-611.
2. Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins:
from structure, function and regulation to new frontiers. Biochimica et Biophysica
Acta (BBA)-Proteins and Proteomics. 2012 Jan 31;1824(1):68-88.
3. Kolodziejczyk R, Michalska K, Hernandez‐Santoyo A, Wahlbom M, Grubb A,
Jaskolski M. Crystal structure of human cystatin C stabilized against amyloid
formation. The FEBS journal. 2010 Apr 1;277(7):1726-37.
4. Rajagopalan P, Refsum H, Hua X, Toga AW, Jack CR, Weiner MW, Thompson PM,
Alzheimer's Disease Neuroimaging Initiative. Mapping creatinine-and cystatin C-
related white matter brain deficits in the elderly. Neurobiology of aging. 2013 Apr
30;34(4):1221-30.
5. Yamada M, Naiki H. Cerebral amyloid angiopathy. Progress in molecular biology and
translational science. 2012;107:41-78.
6. Lamontagne M, Timens W, Hao K, Bossé Y, Laviolette M, Steiling K, Campbell JD,
Couture C, Conti M, Sherwood K, Hogg JC. Genetic regulation of gene expression in
the lung identifies CST3 and CD22 as potential causal genes for airflow obstruction.
Thorax. 2014 Sep 2:thoraxjnl-2014.
7. Magister Š, Kos J. Cystatins in immune system. Journal of Cancer. 2013;4(1):45.
8. Cerebral Amyloid Angiopathy [FAQ] [Internet]. Angiopathy.org. 2017 [cited 26
September 2017]. Available from: http://www.angiopathy.org/faq.html#symptoms
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

PROTEIN-CYSTATIN C
9. Mehndiratta P, Manjila S, Ostergard T, Eisele S, Cohen ML, Sila C, Selman WR.
Cerebral amyloid angiopathy–associated intracerebral hemorrhage: pathology and
management. Neurosurgical focus. 2012 Apr;32(4):E7
10. Greenberg SM. Cerebral amyloid angiopathy Prospects for clinical diagnosis and
treatment. Neurology. 1998 Sep 1;51(3):690-4..
11. Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk
WE, Ashford E, Yoo K, Xu ZX, Loetscher H. Mechanism of amyloid removal in
patients with Alzheimer disease treated with gantenerumab. Archives of neurology.
2012 Feb 13;69(2):198-207.
9. Mehndiratta P, Manjila S, Ostergard T, Eisele S, Cohen ML, Sila C, Selman WR.
Cerebral amyloid angiopathy–associated intracerebral hemorrhage: pathology and
management. Neurosurgical focus. 2012 Apr;32(4):E7
10. Greenberg SM. Cerebral amyloid angiopathy Prospects for clinical diagnosis and
treatment. Neurology. 1998 Sep 1;51(3):690-4..
11. Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk
WE, Ashford E, Yoo K, Xu ZX, Loetscher H. Mechanism of amyloid removal in
patients with Alzheimer disease treated with gantenerumab. Archives of neurology.
2012 Feb 13;69(2):198-207.
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
