Purification Unit
62 Pages8801 Words40 Views
Added on 2023-03-17
About This Document
This section covers the purification unit of the plant, providing details of different phases, technology chosen, purpose, theory, and process description.
Purification Unit
Added on 2023-03-17
ShareRelated Documents
Purification Unit
This part will cover the purification unit of the plant. This segment will provide details
of different phases of purification unit and technology chosen. This section will also explain the
purpose, theory and detailed process description including design details.
The primary objective of purification station is to purify raw juice required for evaporation and
fermentation units. Raw sugar beets juice contains 2.5% by mass containments that contains of
various minerals, acids, colloids and nitrogenous compounds that can impact the operation of
downstream units including evaporators and fermenters. The objectives of this unit are
underneath.1
Elimination of every insoluble substance.
Elimination of specific soluble matter, for example, non-sugars.
Creation of thermostable nature juice with that of very low hardness.
Theory of Purification.
The product of the unit is thin juice and Calcium Carbonate is the by-product.
To precipitate and destabilize the non-sugars Milk of lime (MOL) is mixed with juice.
Carbonation gas, which is CO2 and air mixture is fed in 2 steps, which absorbs impurities and
colors. Lime is precipitated as calcium carbonate, CaCO3 a byproduct. It absorbs the color bodies
and impurities from the liquid by inclusion within the carbonate crystals2. The clarifier separates
the precipitated calcium carbonate. The overflow thin juice is carbonated in the second
carbonator reactor, and filtered before sending to evaporation unit of the refinery.
1 Beet Sugar Handbook Mosen Asadi page 214
Condensed Phase Thermochemistry Data. Water 24/02/2015] http://webbook.nist.gov/cgi/cbook.cgi?
ID=C7732185&Mask=2#Thermo-Condensed.
2 Beet Sugar Handbook Mosen Asadi page 234
This part will cover the purification unit of the plant. This segment will provide details
of different phases of purification unit and technology chosen. This section will also explain the
purpose, theory and detailed process description including design details.
The primary objective of purification station is to purify raw juice required for evaporation and
fermentation units. Raw sugar beets juice contains 2.5% by mass containments that contains of
various minerals, acids, colloids and nitrogenous compounds that can impact the operation of
downstream units including evaporators and fermenters. The objectives of this unit are
underneath.1
Elimination of every insoluble substance.
Elimination of specific soluble matter, for example, non-sugars.
Creation of thermostable nature juice with that of very low hardness.
Theory of Purification.
The product of the unit is thin juice and Calcium Carbonate is the by-product.
To precipitate and destabilize the non-sugars Milk of lime (MOL) is mixed with juice.
Carbonation gas, which is CO2 and air mixture is fed in 2 steps, which absorbs impurities and
colors. Lime is precipitated as calcium carbonate, CaCO3 a byproduct. It absorbs the color bodies
and impurities from the liquid by inclusion within the carbonate crystals2. The clarifier separates
the precipitated calcium carbonate. The overflow thin juice is carbonated in the second
carbonator reactor, and filtered before sending to evaporation unit of the refinery.
1 Beet Sugar Handbook Mosen Asadi page 214
Condensed Phase Thermochemistry Data. Water 24/02/2015] http://webbook.nist.gov/cgi/cbook.cgi?
ID=C7732185&Mask=2#Thermo-Condensed.
2 Beet Sugar Handbook Mosen Asadi page 234

Following reaction takes place.
Ca (OH )2 +CO2 yields
→
CaCO3 ↓+ H2 O
Thin Juice has following characteristics.3
Enhanced Purity
Less color
Reduced hardness
5ree of insoluble solids
Suitable settling and filterability
Improved thermal stability
Method of Purification
Two methods are widely used in industry Classical Purification and Defeco Purification.
Defeco purification includes consolidating more than two of the liming and carbonation cycle to
minimize required vessels, nevertheless this process requires a significant amount recycle (up to
700 times of incoming juice)4 of suspended solids, which causes additional capital costs.
Appendix 4.1 consists of detailed study of both methods and comparison sheet. Based on study
in appendix, Classical purification method is selected for this project.
Classical Purification
In this process Milk of Lime (MOL), as aqueous Calcium dihydroxide Ca (OH )2 is
added. The lime addition helps to precipitate, neutralize and coagulate. This objective is achieved
in Preliming and Mainlining. In carbonation reactors carbon dioxide gas takes place in the form
3 Beet Sugar Handbook Mosen Asadi page 215
4 Beet Sugar Handbook Mosen Asadi page 241
Ca (OH )2 +CO2 yields
→
CaCO3 ↓+ H2 O
Thin Juice has following characteristics.3
Enhanced Purity
Less color
Reduced hardness
5ree of insoluble solids
Suitable settling and filterability
Improved thermal stability
Method of Purification
Two methods are widely used in industry Classical Purification and Defeco Purification.
Defeco purification includes consolidating more than two of the liming and carbonation cycle to
minimize required vessels, nevertheless this process requires a significant amount recycle (up to
700 times of incoming juice)4 of suspended solids, which causes additional capital costs.
Appendix 4.1 consists of detailed study of both methods and comparison sheet. Based on study
in appendix, Classical purification method is selected for this project.
Classical Purification
In this process Milk of Lime (MOL), as aqueous Calcium dihydroxide Ca (OH )2 is
added. The lime addition helps to precipitate, neutralize and coagulate. This objective is achieved
in Preliming and Mainlining. In carbonation reactors carbon dioxide gas takes place in the form
3 Beet Sugar Handbook Mosen Asadi page 215
4 Beet Sugar Handbook Mosen Asadi page 241

of bubble, this bubbles get through the limed mixtures in order to remove any high Ca (OH )2 by
the drizzle of calcium carbonate. The formation of calcium-carbonate results as a adsorbent for
various specific impurities to bind. Clarifier removes precipitates physically and overflow juice
is carbonated further before sending to downstream sections.
Classical Purification steps consist of:
Prelimer
Mainlimer
First Carbonation
Clarifier
2d carbonation
Ultrafiltration
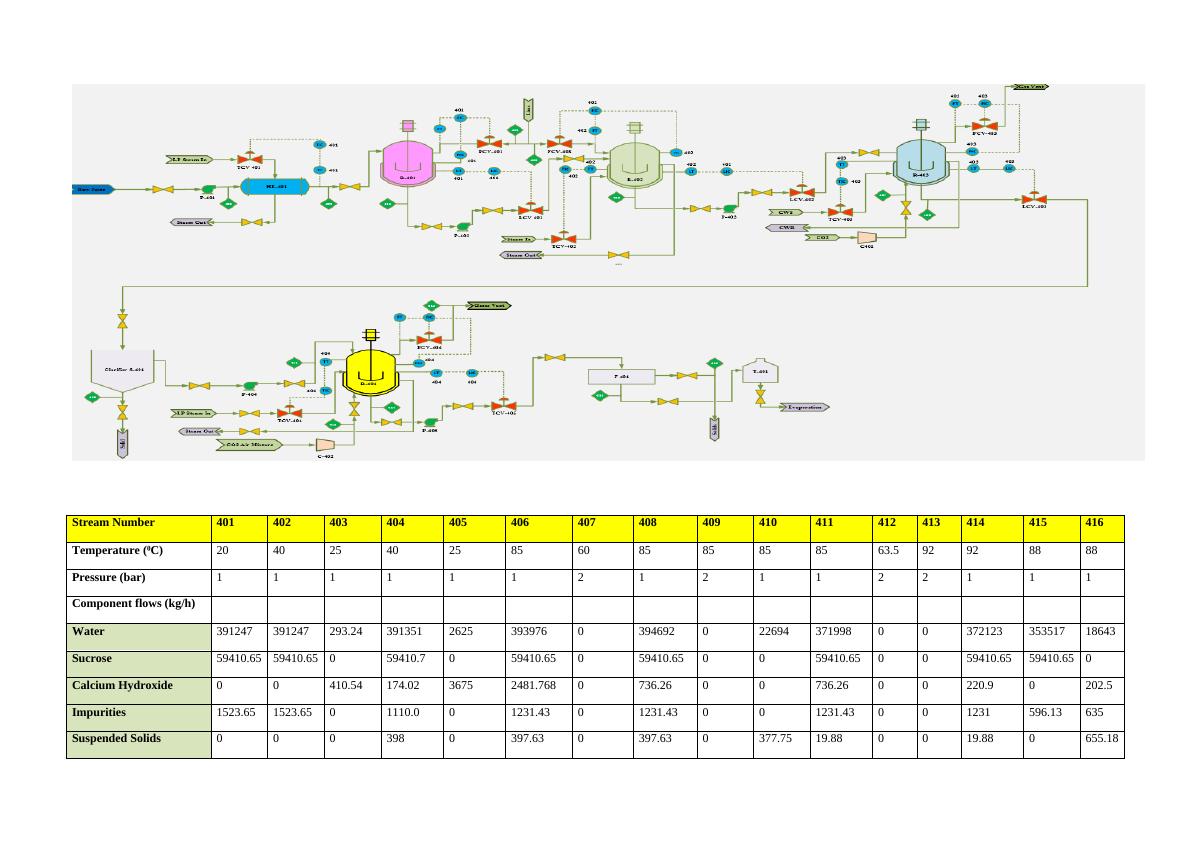
Overall Purification section will be able to produce 59411 Kg/Hr purified thin juice to
downstream evaporation Section.
the drizzle of calcium carbonate. The formation of calcium-carbonate results as a adsorbent for
various specific impurities to bind. Clarifier removes precipitates physically and overflow juice
is carbonated further before sending to downstream sections.
Classical Purification steps consist of:
Prelimer
Mainlimer
First Carbonation
Clarifier
2d carbonation
Ultrafiltration
Overall Purification section will be able to produce 59411 Kg/Hr purified thin juice to
downstream evaporation Section.


Stream Number 401 402 403 404 405 406 407 408 409 410 411 412 413 414 415 416
Temperature ( C)⁰ 20 40 25 40 25 85 60 85 85 85 85 63.5 92 92 88 88
Pressure (bar) 1 1 1 1 1 1 2 1 2 1 1 2 2 1 1 1
Component flows (kg/h)
Water 391247 391247 293.24 391351 2625 393976 0 394692 0 22694 371998 0 0 372123 353517 18643
Sucrose 59410.65 59410.65 0 59410.7 0 59410.65 0 59410.65 0 0 59410.65 0 0 59410.65 59410.65 0
Calcium Hydroxide 0 0 410.54 174.02 3675 2481.768 0 736.26 0 0 736.26 0 0 220.9 0 202.5
Impurities 1523.65 1523.65 0 1110.0 0 1231.43 0 1231.43 0 0 1231.43 0 0 1231 596.13 635
Suspended Solids 0 0 0 398 0 397.63 0 397.63 0 377.75 19.88 0 0 19.88 0 655.18
Temperature ( C)⁰ 20 40 25 40 25 85 60 85 85 85 85 63.5 92 92 88 88
Pressure (bar) 1 1 1 1 1 1 2 1 2 1 1 2 2 1 1 1
Component flows (kg/h)
Water 391247 391247 293.24 391351 2625 393976 0 394692 0 22694 371998 0 0 372123 353517 18643
Sucrose 59410.65 59410.65 0 59410.7 0 59410.65 0 59410.65 0 0 59410.65 0 0 59410.65 59410.65 0
Calcium Hydroxide 0 0 410.54 174.02 3675 2481.768 0 736.26 0 0 736.26 0 0 220.9 0 202.5
Impurities 1523.65 1523.65 0 1110.0 0 1231.43 0 1231.43 0 0 1231.43 0 0 1231 596.13 635
Suspended Solids 0 0 0 398 0 397.63 0 397.63 0 377.75 19.88 0 0 19.88 0 655.18
CCC 0 0 0 0 0 9071 0 9071 0 9071 0 0 0 0 0 0
Carbon Dioxide 0 0 0 0 0 0 2618.62 872.78 0 0 524.3 220.9 0 0 0
Air 0 0 0 0 0 0 15541 15541 0 0 3093 3093 0 0 0
Calcium Carbonate 0 0 0 0 0 0 0 3968.64 0 3770.208 198.43 0 0 198.43 0 198.43
Total 452181.3 452181.3 703.78 452443.67 6300 466568.5 18159.62 469507.61 16413.78 35912.96 433594.65 3617.3 3313.9 433204.29 413523.8 20335
Purification Unit: Prepared and Reviewed : Inam Ullah
Khan
Carbon Dioxide 0 0 0 0 0 0 2618.62 872.78 0 0 524.3 220.9 0 0 0
Air 0 0 0 0 0 0 15541 15541 0 0 3093 3093 0 0 0
Calcium Carbonate 0 0 0 0 0 0 0 3968.64 0 3770.208 198.43 0 0 198.43 0 198.43
Total 452181.3 452181.3 703.78 452443.67 6300 466568.5 18159.62 469507.61 16413.78 35912.96 433594.65 3617.3 3313.9 433204.29 413523.8 20335
Purification Unit: Prepared and Reviewed : Inam Ullah
Khan
Process Description
1. Prelimer
This section will explain the details of design and vessel sizing, material and energy balance.
Agitation design will along with process control strategy will be elaborated.
Preliming stage is foundation step for downstream stages of purification, due to complex and
challenging role of Preliming which contains number of chemical reactions, Prelimer is selected
for detailed design.
Prelimer main roles include:5
Neutralization of juice
Precipitation of nonsugars
Destabilization of the colloids
Theory
The addition of the lime causes precipitation and neutralization. In Preliming, very less quantity
of lime (about 0.2 to 0.7% on juice) is added. The Prelimer operating parameters include, pH 8.5,
temperature 40°C6. Residence time is about 10 to 15 minutes.
Six-second order reactions occur in the Prelimer. Three Precipitation reactions and three
neutralization reactions take place. The precipitation takes place among Ca (OH )2 , and oxalate
minerals of sodium, potassium and magnesium with each forming calcium oxalate precipitate
5 Beet Sugar Handbook Mosen Asadi page 228
6 Beet Sugar Handbook Mosen Asadi page 241
1. Prelimer
This section will explain the details of design and vessel sizing, material and energy balance.
Agitation design will along with process control strategy will be elaborated.
Preliming stage is foundation step for downstream stages of purification, due to complex and
challenging role of Preliming which contains number of chemical reactions, Prelimer is selected
for detailed design.
Prelimer main roles include:5
Neutralization of juice
Precipitation of nonsugars
Destabilization of the colloids
Theory
The addition of the lime causes precipitation and neutralization. In Preliming, very less quantity
of lime (about 0.2 to 0.7% on juice) is added. The Prelimer operating parameters include, pH 8.5,
temperature 40°C6. Residence time is about 10 to 15 minutes.
Six-second order reactions occur in the Prelimer. Three Precipitation reactions and three
neutralization reactions take place. The precipitation takes place among Ca (OH )2 , and oxalate
minerals of sodium, potassium and magnesium with each forming calcium oxalate precipitate
5 Beet Sugar Handbook Mosen Asadi page 228
6 Beet Sugar Handbook Mosen Asadi page 241

and the corresponding metal hydroxide. The lime causes the neutralization of oxalic, citric and
tartaric acid which each produce a calcium precipitate and water.
In Pre Liming, 4 processes are taking place and include precipitation, neutralization, coagulation
and decomposition. Ca (OH )2 Solution reacts with various impurities. Precipitation reduces
mineral content and neutralization reduces acidity in the juice. The mount of diffusive juice is
very complex. On the other hand, the process is made into simpler form to six major reactions
that occur in the Prelimer.7 Appendix 4.2 explains the details of reactions and their
stoichiometry.
Design
Horizontal and vertical both nature of prelimers are used within the industry. Vertical
type is selected for the process as vertical prelimers required low space and the process of mixing
is also very effective. Juice fed from the top and flows down due to gravity and milk of lime is
fed from the bottom. Lime is spurred towards the juice by influence of baffles. It comprises of
the seven compartments that includes a shaft mounted towards the stirring paddles within each of
the compartment for effective mixing8.
7 Beet Sugar Handbook Mosen Asadi page 231
8 Beet Sugar Handbook Mosen Asadi page 233
tartaric acid which each produce a calcium precipitate and water.
In Pre Liming, 4 processes are taking place and include precipitation, neutralization, coagulation
and decomposition. Ca (OH )2 Solution reacts with various impurities. Precipitation reduces
mineral content and neutralization reduces acidity in the juice. The mount of diffusive juice is
very complex. On the other hand, the process is made into simpler form to six major reactions
that occur in the Prelimer.7 Appendix 4.2 explains the details of reactions and their
stoichiometry.
Design
Horizontal and vertical both nature of prelimers are used within the industry. Vertical
type is selected for the process as vertical prelimers required low space and the process of mixing
is also very effective. Juice fed from the top and flows down due to gravity and milk of lime is
fed from the bottom. Lime is spurred towards the juice by influence of baffles. It comprises of
the seven compartments that includes a shaft mounted towards the stirring paddles within each of
the compartment for effective mixing8.
7 Beet Sugar Handbook Mosen Asadi page 231
8 Beet Sugar Handbook Mosen Asadi page 233

End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Purification Process in Deskliblg...
|19
|4769
|65
