Detailed Report on Pyruvate Kinase: Structure, Function, Regulation
VerifiedAdded on 2023/06/15
|5
|1058
|187
Report
AI Summary
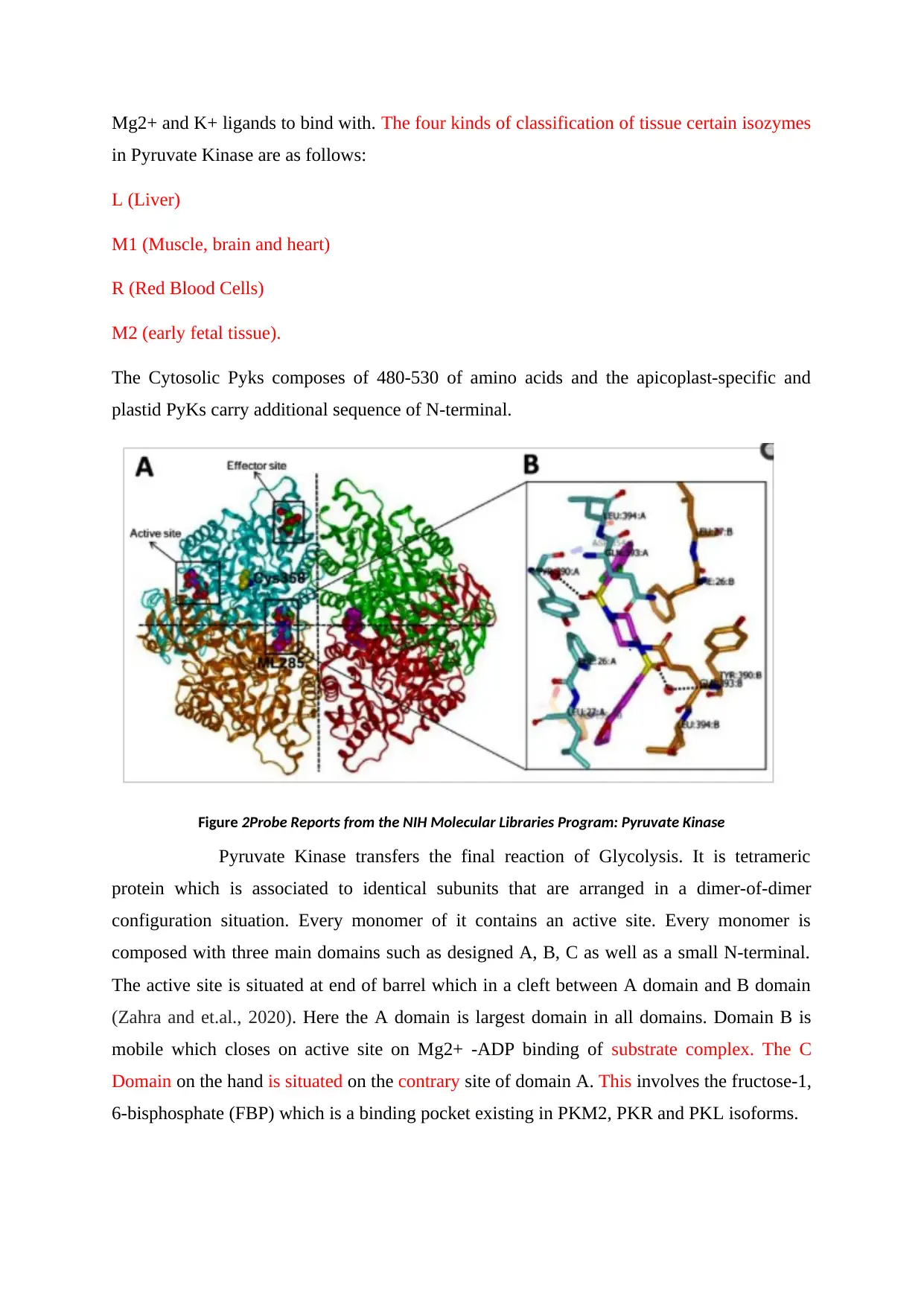
This report provides a detailed analysis of Pyruvate Kinase (protein code 3EOW) from Leishmania Mexicana (LmPYK), an enzyme crucial in the final stage of glycolysis. The report elucidates the enzyme's function in catalyzing the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding ATP and pyruvate. It discusses the protein's various binding sites, regulation via fructose 2,6-bisphosphate, and its existence in four tissue-specific isozymes with distinct kinetic properties. The analysis covers the protein's structure, including its all-beta protein class, PK beta-barrel domain, and tetrameric quaternary structure with metal-binding sites for Mg2+ and K+ ligands. A comparison with human hemoglobin highlights both similarities and differences in subunit structure and ligand binding. The report further discusses the temperature-dependent conformational states and immunological cross-reactivity with heat shock proteins, offering a comprehensive overview of Pyruvate Kinase's structure, function, and regulation.
1 out of 5





![[object Object]](/_next/static/media/star-bottom.7253800d.svg)