Quality Assurance, Good Manufacturing Practices, and Biostatistics
VerifiedAdded on 2020/06/04
|41
|14882
|85
Homework Assignment
AI Summary
This assignment delves into the critical aspects of quality assurance and good manufacturing practices (GMP) within the pharmaceutical industry. It encompasses a wide range of topics, including antimicrobial preservative effectiveness, teratogenic activity testing, accuracy versus precision, and the different types of glass used in pharmaceutical manufacturing, along with their chemical resistance tests. The assignment also covers stability testing, ICH guidelines, acute toxicity testing, WHO guidelines for stability studies, and physical testing of ointments. Furthermore, it explores bioanalytical method validation, ICH guidelines for photostability studies, OECD guidelines for chronic toxicity, and quality tests for packaging materials. The second part focuses on biostatistics, including P-values, ANOVA, t-tests, hypothesis testing, correlation, and various statistical software applications. The third part addresses clinical trials, including efficacy guidelines, investigator responsibilities, GCP, IRB roles, WHO certificates, GCLP, CAPA, deviation handling, safety guidelines, clinical study reports, CTD, sponsor responsibilities, and documents required before clinical studies. The assignment concludes with sections on quality management systems, clinical trial protocols, quality assurance in clinical research, and risk management.

Quality Assurance & Good
Manufacturing Practices
Manufacturing Practices
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
INTRODUCTION...........................................................................................................................1
PART 1............................................................................................................................................1
Section A.....................................................................................................................................1
Section B.....................................................................................................................................1
1. Tests for the effectiveness of the antimicrobial preservatives................................................1
2. Different phases of teratogenic activity testing.......................................................................2
3. Difference between accuracy and precision............................................................................2
4. What are the different types of glass used in pharmaceutical manufacturing and discuss
their chemical resistance test.......................................................................................................3
5. Bracketing design for testing the new drug substance............................................................4
6.short note on following tests:...................................................................................................4
7. Describe the storage condition and testing frequency for long term and accelerated stability
study of the drug substance.........................................................................................................5
8. Why is the testing of Bacteriostasis and fungi stasis carried out before sterility testing.
Describe the inoculation method.................................................................................................6
9. Explain the ICH guideline for stability testing for new drugs................................................6
10. Describe the microbiological limit test for the Pseudomonas aeruginosa............................7
11. Explain quality control test for Metal containers, paper, paper boards and cardboards as
packaging material......................................................................................................................7
12. What is acute toxicity and what is the need of acute toxicity testing....................................8
13. Describe the pyrogen test for quality control testing of parenteral products........................9
14. Explain WHO guidelines for ongoing stability study for active pharmaceutical ingredients.
What is significant change..........................................................................................................9
15. Describe the physical test for the quality control testing of ointments...............................10
Section C...................................................................................................................................10
1. Describe the Bioanalytical method validation and the parameters involved........................10
2. Detail Note on ICH guidelines for photo stability study for new drugs and substance
product. .....................................................................................................................................11
3. OECD guidelines for chronic toxicity...................................................................................11
INTRODUCTION...........................................................................................................................1
PART 1............................................................................................................................................1
Section A.....................................................................................................................................1
Section B.....................................................................................................................................1
1. Tests for the effectiveness of the antimicrobial preservatives................................................1
2. Different phases of teratogenic activity testing.......................................................................2
3. Difference between accuracy and precision............................................................................2
4. What are the different types of glass used in pharmaceutical manufacturing and discuss
their chemical resistance test.......................................................................................................3
5. Bracketing design for testing the new drug substance............................................................4
6.short note on following tests:...................................................................................................4
7. Describe the storage condition and testing frequency for long term and accelerated stability
study of the drug substance.........................................................................................................5
8. Why is the testing of Bacteriostasis and fungi stasis carried out before sterility testing.
Describe the inoculation method.................................................................................................6
9. Explain the ICH guideline for stability testing for new drugs................................................6
10. Describe the microbiological limit test for the Pseudomonas aeruginosa............................7
11. Explain quality control test for Metal containers, paper, paper boards and cardboards as
packaging material......................................................................................................................7
12. What is acute toxicity and what is the need of acute toxicity testing....................................8
13. Describe the pyrogen test for quality control testing of parenteral products........................9
14. Explain WHO guidelines for ongoing stability study for active pharmaceutical ingredients.
What is significant change..........................................................................................................9
15. Describe the physical test for the quality control testing of ointments...............................10
Section C...................................................................................................................................10
1. Describe the Bioanalytical method validation and the parameters involved........................10
2. Detail Note on ICH guidelines for photo stability study for new drugs and substance
product. .....................................................................................................................................11
3. OECD guidelines for chronic toxicity...................................................................................11

4.) List various packaging material of pharmaceutical industry and quality test for plastics.. .12
5. What is mutagenicity and its various tests............................................................................12
PART 2..........................................................................................................................................12
Quality Testing Tools and Techniques..........................................................................................12
Section A........................................................................................................................................12
1. P values> 0.05:......................................................................................................................12
2. ANOVA is an example of:....................................................................................................12
3. Test used in ANOVA............................................................................................................13
4. D) Non Bliend trial................................................................................................................13
5. A) RCT..................................................................................................................................13
Section B........................................................................................................................................13
1. Meaning of biostatistics and various related terms...............................................................13
2. Point estimation.....................................................................................................................13
3. Difference between statistical significance and P-values.....................................................14
4. T-test and differences between paired and unpaired test......................................................14
5. Hypothesis testing.................................................................................................................15
6. Differences between Pearson's Correlation Coefficient and Spearman's Correlation
Coefficient.................................................................................................................................15
7. Analysis of means and comparison of correlation with regression.......................................16
8. Explain:.................................................................................................................................16
9) What is meta analysis and how it is performed....................................................................17
10. SAS sorfware and its advantage over other........................................................................17
11. How to create scatter plot using minitab.............................................................................17
12. Enlist and explain various plots used in statistics...............................................................18
13. Difference between parametric and non para metric test and the type of data obtained from
it.................................................................................................................................................18
14. Enlist software's used for T test and T test in excel............................................................18
15. Difference between F test and Z test...................................................................................19
Section C...................................................................................................................................19
1. Different non parametric test used in statistics.....................................................................19
2. Explain RCT..........................................................................................................................19
5. What is mutagenicity and its various tests............................................................................12
PART 2..........................................................................................................................................12
Quality Testing Tools and Techniques..........................................................................................12
Section A........................................................................................................................................12
1. P values> 0.05:......................................................................................................................12
2. ANOVA is an example of:....................................................................................................12
3. Test used in ANOVA............................................................................................................13
4. D) Non Bliend trial................................................................................................................13
5. A) RCT..................................................................................................................................13
Section B........................................................................................................................................13
1. Meaning of biostatistics and various related terms...............................................................13
2. Point estimation.....................................................................................................................13
3. Difference between statistical significance and P-values.....................................................14
4. T-test and differences between paired and unpaired test......................................................14
5. Hypothesis testing.................................................................................................................15
6. Differences between Pearson's Correlation Coefficient and Spearman's Correlation
Coefficient.................................................................................................................................15
7. Analysis of means and comparison of correlation with regression.......................................16
8. Explain:.................................................................................................................................16
9) What is meta analysis and how it is performed....................................................................17
10. SAS sorfware and its advantage over other........................................................................17
11. How to create scatter plot using minitab.............................................................................17
12. Enlist and explain various plots used in statistics...............................................................18
13. Difference between parametric and non para metric test and the type of data obtained from
it.................................................................................................................................................18
14. Enlist software's used for T test and T test in excel............................................................18
15. Difference between F test and Z test...................................................................................19
Section C...................................................................................................................................19
1. Different non parametric test used in statistics.....................................................................19
2. Explain RCT..........................................................................................................................19
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3. Explain Graph pad prism software........................................................................................19
4. What is measurement of central tendencies and dispersion. Explain interval estimation.....20
5. Different types and advantage and disadvantage of Adaptive design and quasi experimental
design........................................................................................................................................20
PART 3..........................................................................................................................................21
Ques 1. Efficacy Guidelines no. E-2C (R2) and E 2A .............................................................21
Ques 2 Responsibilities of Investigator during clinical trials...................................................21
Ques 3. GCP..............................................................................................................................22
Ques 4 Roles and responsibilities of IRB.................................................................................22
Ques 5 Several certificates which are to be issued under WHO on quality of Pharamaceutical
products.....................................................................................................................................23
Ques 6 GCLP............................................................................................................................23
Ques 7 CAPA............................................................................................................................23
Ques 8. Meaning of Following Term:.......................................................................................24
Ques 9 Deviation Handling under Quality management system..............................................24
Ques 10 Guidelines no. S1 A and S3 A for safety....................................................................25
Ques.11 Guideline E3 related to structure as well as content of clinical study report.............25
Ques 12. Meaning of CTD.......................................................................................................26
Ques 13 Roles as well as responsibilities of sponsors in clinical trials....................................26
Ques 14 Documents that are to be prepared before the execution of clinical study ................27
Ques 15. Guideline no. ICHQ 10..............................................................................................27
SECTION C...................................................................................................................................28
Ques 1 Quality management system.........................................................................................28
Ques. 2 Clinical Trial protocol .................................................................................................30
3. How the quality can be assured in clinical research.............................................................31
4. Seven steps of CAPA in pharmaceutical industry................................................................31
5. Explain ICH Tripartite Guideline ODF Quality risk management (Q9)...............................32
CONCLUSION..............................................................................................................................32
REFERENCES..............................................................................................................................33
4. What is measurement of central tendencies and dispersion. Explain interval estimation.....20
5. Different types and advantage and disadvantage of Adaptive design and quasi experimental
design........................................................................................................................................20
PART 3..........................................................................................................................................21
Ques 1. Efficacy Guidelines no. E-2C (R2) and E 2A .............................................................21
Ques 2 Responsibilities of Investigator during clinical trials...................................................21
Ques 3. GCP..............................................................................................................................22
Ques 4 Roles and responsibilities of IRB.................................................................................22
Ques 5 Several certificates which are to be issued under WHO on quality of Pharamaceutical
products.....................................................................................................................................23
Ques 6 GCLP............................................................................................................................23
Ques 7 CAPA............................................................................................................................23
Ques 8. Meaning of Following Term:.......................................................................................24
Ques 9 Deviation Handling under Quality management system..............................................24
Ques 10 Guidelines no. S1 A and S3 A for safety....................................................................25
Ques.11 Guideline E3 related to structure as well as content of clinical study report.............25
Ques 12. Meaning of CTD.......................................................................................................26
Ques 13 Roles as well as responsibilities of sponsors in clinical trials....................................26
Ques 14 Documents that are to be prepared before the execution of clinical study ................27
Ques 15. Guideline no. ICHQ 10..............................................................................................27
SECTION C...................................................................................................................................28
Ques 1 Quality management system.........................................................................................28
Ques. 2 Clinical Trial protocol .................................................................................................30
3. How the quality can be assured in clinical research.............................................................31
4. Seven steps of CAPA in pharmaceutical industry................................................................31
5. Explain ICH Tripartite Guideline ODF Quality risk management (Q9)...............................32
CONCLUSION..............................................................................................................................32
REFERENCES..............................................................................................................................33
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

INTRODUCTION
The better quality management and manufacturing practices is very necessary that helps
in the better productivity and profitability. The better quality will help a business organisation in
gaining an effective customer satisfaction level. This report will cover the better microbial part
and its effectiveness, the difference between precision and accuracy and different pharmaceutical
packaging material is been taken into consideration (Newton And et.al., 2015. ). Various stability
testing are been taken into consideration and ICH guidelines are been taken in effective
consideration. Apart from this, the acute toxicity and its various tests are been discussed. The
WHO guidelines are been discussed. Other than this the various bio analytical measures are been
taken into effective consideration with OECD guidelines are been discussed.
PART 1
Section A
2. C) Pseudomonas aeruginosa
3. D) Photo stability testing of the new drugs or substance.
4. D) 14 Days
5. C) Weight Variation test.
6. C) 8.5 ml
Section B
1. Tests for the effectiveness of the antimicrobial preservatives.
The antimicrobial preservatives are very necessary and vital in the pharmaceutical
industry as it helps in the safeguarding the life saving drugs from getting contaminated. This will
help in producing and manufacturing the high quality drugs and medicines to customers. The
antimicrobial preservatives will protect the medicines and various types of drugs from the getting
affected by the microorganisms and keep the medicine sterile and viable in various
environmental condition (Haleem And et.al., 2015). The major function of the antimicrobial
preservative is to prevent the growth of the bacteria and other contaminations in the vital drugs
and medicines. This will help the customers to get the safe and high quality drugs and
medications in a very effective way.
The better quality management and manufacturing practices is very necessary that helps
in the better productivity and profitability. The better quality will help a business organisation in
gaining an effective customer satisfaction level. This report will cover the better microbial part
and its effectiveness, the difference between precision and accuracy and different pharmaceutical
packaging material is been taken into consideration (Newton And et.al., 2015. ). Various stability
testing are been taken into consideration and ICH guidelines are been taken in effective
consideration. Apart from this, the acute toxicity and its various tests are been discussed. The
WHO guidelines are been discussed. Other than this the various bio analytical measures are been
taken into effective consideration with OECD guidelines are been discussed.
PART 1
Section A
2. C) Pseudomonas aeruginosa
3. D) Photo stability testing of the new drugs or substance.
4. D) 14 Days
5. C) Weight Variation test.
6. C) 8.5 ml
Section B
1. Tests for the effectiveness of the antimicrobial preservatives.
The antimicrobial preservatives are very necessary and vital in the pharmaceutical
industry as it helps in the safeguarding the life saving drugs from getting contaminated. This will
help in producing and manufacturing the high quality drugs and medicines to customers. The
antimicrobial preservatives will protect the medicines and various types of drugs from the getting
affected by the microorganisms and keep the medicine sterile and viable in various
environmental condition (Haleem And et.al., 2015). The major function of the antimicrobial
preservative is to prevent the growth of the bacteria and other contaminations in the vital drugs
and medicines. This will help the customers to get the safe and high quality drugs and
medications in a very effective way.

Such preservatives are added in almost every sort of the drugs and medicines including
the parenteral otic, nasal, ophthalmologic, oral and topical sort of drugs in solid and aqueous
bases. It helps in gaining the better handling of the different operations which helps in the
effective handling of the various operations. It helps in increasing the shelf life of the drugs and
medicines and reduces the constraint of the temperature specific storing of the medications at a
clinic or pharmaceutical shop. This it helps in maintain the quality of drugs for prolonged period
(Alli, 2016).
2. Different phases of teratogenic activity testing
The teratogens are the effective chemical or drugs that lead to the severe toxicity and
inhibition in the body. The effective testing of the toxic material is very necessary for the
safeguarding the life of an individual. The presence or the consumption of any sort of teratogenic
component like alcohol or other chemical may lead to the rise in the toxicity of the individual,
which may lead to the severe consequences. The testing involves the various phases such as:
Animal test system: This involves the testing of the teratogen drugs on the animals to
understand its consequences on the human body.
Whole Embryo culture test: It involves the process of culturing or growing of embryo
of an animal like frog or mouse to carry out the teratogenic compounds and its
implication on user.
3. Difference between accuracy and precision
The accuracy and provision plays a very vital and crucial role in the maintaining and
developing of the proper product or service. In the pharmaceutical field, the accuracy and
precision plays a very crucial and vital role in development of the high quality product and
services (Nally, 2016.). The major difference between accuracy and precision is as follows:
Accuracy Precision
Accuracy indicates the difference between the
measurements set and the actual value
achieved.
Precision is the variation that is visible when
the value is measured from the same device,
again and again.
It indicates the closeness of the value achieved It indicates the measure that how closely the
the parenteral otic, nasal, ophthalmologic, oral and topical sort of drugs in solid and aqueous
bases. It helps in gaining the better handling of the different operations which helps in the
effective handling of the various operations. It helps in increasing the shelf life of the drugs and
medicines and reduces the constraint of the temperature specific storing of the medications at a
clinic or pharmaceutical shop. This it helps in maintain the quality of drugs for prolonged period
(Alli, 2016).
2. Different phases of teratogenic activity testing
The teratogens are the effective chemical or drugs that lead to the severe toxicity and
inhibition in the body. The effective testing of the toxic material is very necessary for the
safeguarding the life of an individual. The presence or the consumption of any sort of teratogenic
component like alcohol or other chemical may lead to the rise in the toxicity of the individual,
which may lead to the severe consequences. The testing involves the various phases such as:
Animal test system: This involves the testing of the teratogen drugs on the animals to
understand its consequences on the human body.
Whole Embryo culture test: It involves the process of culturing or growing of embryo
of an animal like frog or mouse to carry out the teratogenic compounds and its
implication on user.
3. Difference between accuracy and precision
The accuracy and provision plays a very vital and crucial role in the maintaining and
developing of the proper product or service. In the pharmaceutical field, the accuracy and
precision plays a very crucial and vital role in development of the high quality product and
services (Nally, 2016.). The major difference between accuracy and precision is as follows:
Accuracy Precision
Accuracy indicates the difference between the
measurements set and the actual value
achieved.
Precision is the variation that is visible when
the value is measured from the same device,
again and again.
It indicates the closeness of the value achieved It indicates the measure that how closely the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
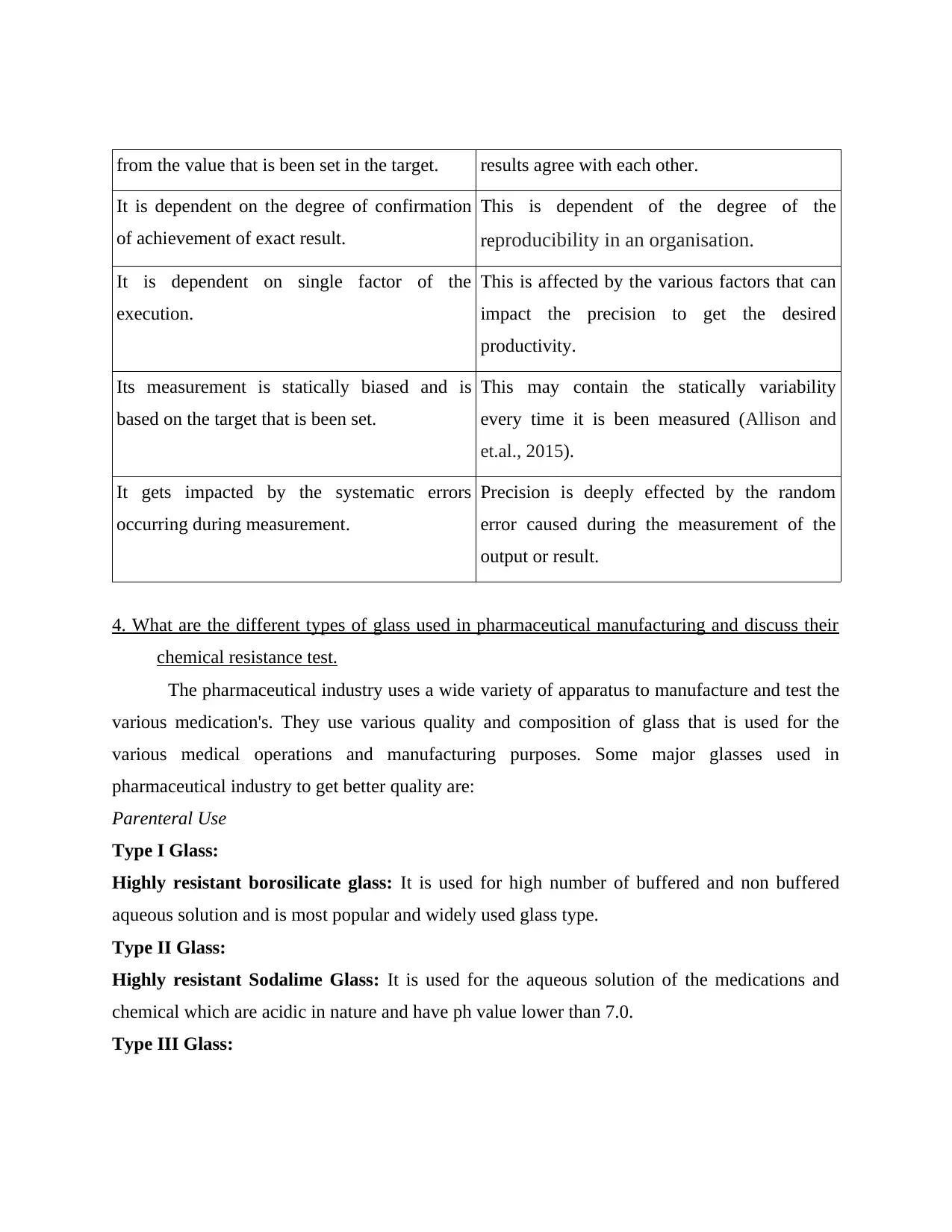
from the value that is been set in the target. results agree with each other.
It is dependent on the degree of confirmation
of achievement of exact result.
This is dependent of the degree of the
reproducibility in an organisation.
It is dependent on single factor of the
execution.
This is affected by the various factors that can
impact the precision to get the desired
productivity.
Its measurement is statically biased and is
based on the target that is been set.
This may contain the statically variability
every time it is been measured (Allison and
et.al., 2015).
It gets impacted by the systematic errors
occurring during measurement.
Precision is deeply effected by the random
error caused during the measurement of the
output or result.
4. What are the different types of glass used in pharmaceutical manufacturing and discuss their
chemical resistance test.
The pharmaceutical industry uses a wide variety of apparatus to manufacture and test the
various medication's. They use various quality and composition of glass that is used for the
various medical operations and manufacturing purposes. Some major glasses used in
pharmaceutical industry to get better quality are:
Parenteral Use
Type I Glass:
Highly resistant borosilicate glass: It is used for high number of buffered and non buffered
aqueous solution and is most popular and widely used glass type.
Type II Glass:
Highly resistant Sodalime Glass: It is used for the aqueous solution of the medications and
chemical which are acidic in nature and have ph value lower than 7.0.
Type III Glass:
It is dependent on the degree of confirmation
of achievement of exact result.
This is dependent of the degree of the
reproducibility in an organisation.
It is dependent on single factor of the
execution.
This is affected by the various factors that can
impact the precision to get the desired
productivity.
Its measurement is statically biased and is
based on the target that is been set.
This may contain the statically variability
every time it is been measured (Allison and
et.al., 2015).
It gets impacted by the systematic errors
occurring during measurement.
Precision is deeply effected by the random
error caused during the measurement of the
output or result.
4. What are the different types of glass used in pharmaceutical manufacturing and discuss their
chemical resistance test.
The pharmaceutical industry uses a wide variety of apparatus to manufacture and test the
various medication's. They use various quality and composition of glass that is used for the
various medical operations and manufacturing purposes. Some major glasses used in
pharmaceutical industry to get better quality are:
Parenteral Use
Type I Glass:
Highly resistant borosilicate glass: It is used for high number of buffered and non buffered
aqueous solution and is most popular and widely used glass type.
Type II Glass:
Highly resistant Sodalime Glass: It is used for the aqueous solution of the medications and
chemical which are acidic in nature and have ph value lower than 7.0.
Type III Glass:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Moderately resistant Sodalime Glass: It is mostly used for the less reactive medications having
the tangibility of dry powder form or as oily solution (Parikh, 2016).
Non Parenteral Use
Type IV Glass:
General purpose Sodalime Glass: It is used for the non parenteral purposes and utilised for the
tablets, oral medications and other external drugs.
Various evaluation parameters are been taken into the effective consideration for the
better testing of the Glass components that are been used in the pharmaceutical industry. Some
major tests that are been followed are:
Crushed glass test: The container is well crushed and the sieved to get the uniform
particles. A definite quantity of mixture is taken and is treated with the various solutions
to test is reactivity with the various solutions. This helps to determine that the glass is
treated or not.
Whole container test: In this, the complete apparatus is been tested for its tensile
strngth, reactivity and smoothness in order to use it effectively for the various
pharmaceutical functions in manufacturing of high quality medications (Lee, and et.al.,
2015).
5. Bracketing design for testing the new drug substance.
The bracketing and matrix design function is used to reduce the sample size of the drugs
or the medication that is been used by the pharmaceutical organisation for the testing the
reactiveness and stability of the drugs in various chemical composition. It helps in getting the
appropriate output and reducing the wastage of the medication that is been produced and
manufactured. It reduces the number of sample size required to get the appropriate output. It
helps in getting the effective data about the estimated shelf life of a drug. It helps in the better
testing of the batches of the smaller quantity of different drugs and medicines. The stability
owning samples are been effectively used to have a better productivity and revenue generation
(Govindaraghavan and Sucher, 2015).
The samples are effectively manufactured, labelled ad stored, reducing the number of the
sample required for testing and thus saves the production and management cost as the production
the tangibility of dry powder form or as oily solution (Parikh, 2016).
Non Parenteral Use
Type IV Glass:
General purpose Sodalime Glass: It is used for the non parenteral purposes and utilised for the
tablets, oral medications and other external drugs.
Various evaluation parameters are been taken into the effective consideration for the
better testing of the Glass components that are been used in the pharmaceutical industry. Some
major tests that are been followed are:
Crushed glass test: The container is well crushed and the sieved to get the uniform
particles. A definite quantity of mixture is taken and is treated with the various solutions
to test is reactivity with the various solutions. This helps to determine that the glass is
treated or not.
Whole container test: In this, the complete apparatus is been tested for its tensile
strngth, reactivity and smoothness in order to use it effectively for the various
pharmaceutical functions in manufacturing of high quality medications (Lee, and et.al.,
2015).
5. Bracketing design for testing the new drug substance.
The bracketing and matrix design function is used to reduce the sample size of the drugs
or the medication that is been used by the pharmaceutical organisation for the testing the
reactiveness and stability of the drugs in various chemical composition. It helps in getting the
appropriate output and reducing the wastage of the medication that is been produced and
manufactured. It reduces the number of sample size required to get the appropriate output. It
helps in getting the effective data about the estimated shelf life of a drug. It helps in the better
testing of the batches of the smaller quantity of different drugs and medicines. The stability
owning samples are been effectively used to have a better productivity and revenue generation
(Govindaraghavan and Sucher, 2015).
The samples are effectively manufactured, labelled ad stored, reducing the number of the
sample required for testing and thus saves the production and management cost as the production

and storage cost of the various drugs and medicines are quite high and expensive. The bracketing
will help in the better handling of the production design, setting up of goals and improve the
product quality. It involves the following of the various factors such as strength and package
size. And get the better stability of the various drugs on the intermediate level (Vives, Oliver-
Vila and Pla, 2015).
6.short note on following tests:
1) Self Sealability test for closures: It is the effective test that is been used to test the
various drugs, medications and apparatus that are been produced by the pharmaceutical
institution. It helps in the better testing of the various closures which are intended to be
used with the water close the vials with the prepared closures. For each closure test, a
new hypodermic needle of external diameter at 0.8 mm is used. It will pierce the closure
for about 10 times, each time at a different site. Then this vial is immersed in 0.1% w/v
solution of methylene Blue and the external pressure is reduced by 27KPa for some time
(Allison and et.al., 2017). Then the atmospheric pressures is restored and the vials are
been left into the solution for some time. After this, the externals of the vials are been
rinsed. The vial will not have any traces of the coloured solution.
2) Disintegration test for tablets: It is been conducted to test the disintegration of a tablet
or a capsule, when placed in a liquid medium under the experimental condition. The
purpose of this disintegration process is not to imply the complete dissolution f the
constituent part of the tablet or even its active constituent particles. It will not be used to
have the proper or complete solution of the various components of the medicine. It may
involve the soft mass of the constituent particle, with no palpable firm core. This will
help in the better rise in the productivity and profitability of the company and have the
better understanding of the various impact of the medication (Felix, C.W., 2018).
7. Describe the storage condition and testing frequency for long term and accelerated stability
study of the drug substance.
The effective storage and testing frequency is needed to be taken into effective
consideration for the better handling of the various operations at a pharmaceutical company.
These are as follows:
will help in the better handling of the production design, setting up of goals and improve the
product quality. It involves the following of the various factors such as strength and package
size. And get the better stability of the various drugs on the intermediate level (Vives, Oliver-
Vila and Pla, 2015).
6.short note on following tests:
1) Self Sealability test for closures: It is the effective test that is been used to test the
various drugs, medications and apparatus that are been produced by the pharmaceutical
institution. It helps in the better testing of the various closures which are intended to be
used with the water close the vials with the prepared closures. For each closure test, a
new hypodermic needle of external diameter at 0.8 mm is used. It will pierce the closure
for about 10 times, each time at a different site. Then this vial is immersed in 0.1% w/v
solution of methylene Blue and the external pressure is reduced by 27KPa for some time
(Allison and et.al., 2017). Then the atmospheric pressures is restored and the vials are
been left into the solution for some time. After this, the externals of the vials are been
rinsed. The vial will not have any traces of the coloured solution.
2) Disintegration test for tablets: It is been conducted to test the disintegration of a tablet
or a capsule, when placed in a liquid medium under the experimental condition. The
purpose of this disintegration process is not to imply the complete dissolution f the
constituent part of the tablet or even its active constituent particles. It will not be used to
have the proper or complete solution of the various components of the medicine. It may
involve the soft mass of the constituent particle, with no palpable firm core. This will
help in the better rise in the productivity and profitability of the company and have the
better understanding of the various impact of the medication (Felix, C.W., 2018).
7. Describe the storage condition and testing frequency for long term and accelerated stability
study of the drug substance.
The effective storage and testing frequency is needed to be taken into effective
consideration for the better handling of the various operations at a pharmaceutical company.
These are as follows:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Testing frequency: To facilitate the effective long term understanding of the operations
to gain a better productivity by having a better knowledge of the stability profiler of
active pharmaceutical ingredient (API). The average shelf life of an API is 12 months
that will help in the effective long term storage condition (Cusato and et.al., 2014). The
components can be retested and can be expanded to the shelf life of the various
operations in a better way. This includes the initial and final time points and requires a 6
month study for the better understanding of the different operations. It helps in getting the
effective change criteria. The change in the sample can be made either by the adding
them in the final point and have a better study design on a regular interval. This process
may involve the minimum of 4 time points including the initial and final time points for
example on 0,6,9 and 12 months regularly in a one year process (Doe, 2017).
Storage conditions: An API is usually tested in the evaluated storage condition of an
organisation with appropriate tolerable conditions. It involves the testing of the thermal
stability and the sensitivity towards moisture. The length of the testing must be sufficient
to cover the shipment and storage of the medication in a very effective way. The storage
condition must be able to compensate the temperature variation and environmental
changes to increase the stability of the API. It must also involve the test period which is
used for the assessment of the medication manufactured by the pharmaceutical firm to
maintain its quality (Fries, 2016).
8. Why is the testing of Bacteriostasis and fungi stasis carried out before sterility testing.
Describe the inoculation method.
The bacteriostasis and fungi stasis is been effectively carried out before the sterility
testing as it will help in the better detection of the presence of any bacteria, fungus or other
microorganisms in the API or the medication. This will help in the better identification and
taking of the different measures to meet the sterility standard of the medication. The sterile
distribution of the medication is very necessary as it helps in the better management of the
different operations which will help in the better productivity. Besides this, the presence of the
bacteria or fungus in the medication is the indication of presence of contamination which can
have harmful consequences and deteriorate the quality of product (Luthringer And et.al., 2015).
to gain a better productivity by having a better knowledge of the stability profiler of
active pharmaceutical ingredient (API). The average shelf life of an API is 12 months
that will help in the effective long term storage condition (Cusato and et.al., 2014). The
components can be retested and can be expanded to the shelf life of the various
operations in a better way. This includes the initial and final time points and requires a 6
month study for the better understanding of the different operations. It helps in getting the
effective change criteria. The change in the sample can be made either by the adding
them in the final point and have a better study design on a regular interval. This process
may involve the minimum of 4 time points including the initial and final time points for
example on 0,6,9 and 12 months regularly in a one year process (Doe, 2017).
Storage conditions: An API is usually tested in the evaluated storage condition of an
organisation with appropriate tolerable conditions. It involves the testing of the thermal
stability and the sensitivity towards moisture. The length of the testing must be sufficient
to cover the shipment and storage of the medication in a very effective way. The storage
condition must be able to compensate the temperature variation and environmental
changes to increase the stability of the API. It must also involve the test period which is
used for the assessment of the medication manufactured by the pharmaceutical firm to
maintain its quality (Fries, 2016).
8. Why is the testing of Bacteriostasis and fungi stasis carried out before sterility testing.
Describe the inoculation method.
The bacteriostasis and fungi stasis is been effectively carried out before the sterility
testing as it will help in the better detection of the presence of any bacteria, fungus or other
microorganisms in the API or the medication. This will help in the better identification and
taking of the different measures to meet the sterility standard of the medication. The sterile
distribution of the medication is very necessary as it helps in the better management of the
different operations which will help in the better productivity. Besides this, the presence of the
bacteria or fungus in the medication is the indication of presence of contamination which can
have harmful consequences and deteriorate the quality of product (Luthringer And et.al., 2015).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

The inoculation process involves the proper induction of the artificial immunity against a
disease or a microorganism, to prevent any sort of infection to the user. It is been induced in the
persons body in the form of vaccination that will help in building a better resistance against an
infection or disease. The introduction of the microorganism in body will lead the immunity
system of body to produce antibodies against them, improving the immunity. Also, it will help in
rise of the production of the sterile medications or vaccinations of better quality by a
pharmaceutical institution (Aung and Chang, 2014).
9. Explain the ICH guideline for stability testing for new drugs
The International Conference of Harmonisation (ICH) has developed the effective
guidelines and measures that helps in the better handling of the different operations and measures
that will help in the better handling of the different operations of various medications and drugs
(Clegg and Perry, 2017).
It involves the various measures that will help in the better handling of the different
operations at the pharmaceutical institution such as:
The proper storing temperature for the drugs and medications is below 30° C.
The sample taken from the batch of the medication must be sterile and free from any sort
of contamination, in order to retain the quality.
The exposed or contaminated drugs must be disposed off immediately in order to prevent
any sort of severe consequences.
No alterations or other illegal methods should be used to gain a better productivity and
profitability in a better way (Webster, Castellano and Onuma, 2017).
The packaging must be effectively done in order to avoid any sort of wastage or
contamination.
The manufacturing and expiry dates must be inscribed on the drug or medication and
must be disposed off in a better way to prevent any misuse of drugs.
The drug must be properly tested and effective instructions must be provided with it
regarding its effective usage or consumption of the drug or the medicine.
disease or a microorganism, to prevent any sort of infection to the user. It is been induced in the
persons body in the form of vaccination that will help in building a better resistance against an
infection or disease. The introduction of the microorganism in body will lead the immunity
system of body to produce antibodies against them, improving the immunity. Also, it will help in
rise of the production of the sterile medications or vaccinations of better quality by a
pharmaceutical institution (Aung and Chang, 2014).
9. Explain the ICH guideline for stability testing for new drugs
The International Conference of Harmonisation (ICH) has developed the effective
guidelines and measures that helps in the better handling of the different operations and measures
that will help in the better handling of the different operations of various medications and drugs
(Clegg and Perry, 2017).
It involves the various measures that will help in the better handling of the different
operations at the pharmaceutical institution such as:
The proper storing temperature for the drugs and medications is below 30° C.
The sample taken from the batch of the medication must be sterile and free from any sort
of contamination, in order to retain the quality.
The exposed or contaminated drugs must be disposed off immediately in order to prevent
any sort of severe consequences.
No alterations or other illegal methods should be used to gain a better productivity and
profitability in a better way (Webster, Castellano and Onuma, 2017).
The packaging must be effectively done in order to avoid any sort of wastage or
contamination.
The manufacturing and expiry dates must be inscribed on the drug or medication and
must be disposed off in a better way to prevent any misuse of drugs.
The drug must be properly tested and effective instructions must be provided with it
regarding its effective usage or consumption of the drug or the medicine.

10. Describe the microbiological limit test for the Pseudomonas aeruginosa
This test is been conducted to gain a better productivity and identify the presence of a
microbial organism that can cause Pseudomonas aeruginosa. The specimen of the pharmaceutical
item is been effectively collected for the limit testing. Then the specimen is well incubated to see
the extent or limit of microbial growth in the sample. The sample is thermostatically kept of a
room temperature ideal for the microbial growth i.e. 24° to 35° C for about 24 to 48 hours. This
will show case effectively the various operational quality measures and help the pharmaceutical
firm to identify whether the sample is meeting the quality standards or not (Cole And et.al.,
2017).
11. Explain quality control test for Metal containers, paper, paper boards and cardboards as
packaging material
Various tests are been run over the different components or items, that can be used as the
packaging material for the drugs and medications. This will involve the variety of tests such as:
Spectrophotometry: It involves the better process where the intensity of the light is been
taken into effective consideration depending of the amount absorbed by the solution or
chemical drug. It is based on the principle that compounds absorbs the light of certain
wavelength.
Chromatographic Methods: the chromatography method is a measure to test separation
of the compounds. The material used for the packaging of the drugs and medications
must be safe to use and prevent chromatographic segregation of a solution in a drug or
medication (Altenstetter, 2017).
Thermal analysis techniques: it is the test to identify the impact of the temperature on
an item that is used as the packaging material for the drugs or medication. The element
must be abler to withstand the change in the temperature or other environmental factors
in a better way.
Leak detection: the material used for the storing and packaging of drugs is required to be
leak proof to avoid the spoiling and wastage of drug or medication.
This test is been conducted to gain a better productivity and identify the presence of a
microbial organism that can cause Pseudomonas aeruginosa. The specimen of the pharmaceutical
item is been effectively collected for the limit testing. Then the specimen is well incubated to see
the extent or limit of microbial growth in the sample. The sample is thermostatically kept of a
room temperature ideal for the microbial growth i.e. 24° to 35° C for about 24 to 48 hours. This
will show case effectively the various operational quality measures and help the pharmaceutical
firm to identify whether the sample is meeting the quality standards or not (Cole And et.al.,
2017).
11. Explain quality control test for Metal containers, paper, paper boards and cardboards as
packaging material
Various tests are been run over the different components or items, that can be used as the
packaging material for the drugs and medications. This will involve the variety of tests such as:
Spectrophotometry: It involves the better process where the intensity of the light is been
taken into effective consideration depending of the amount absorbed by the solution or
chemical drug. It is based on the principle that compounds absorbs the light of certain
wavelength.
Chromatographic Methods: the chromatography method is a measure to test separation
of the compounds. The material used for the packaging of the drugs and medications
must be safe to use and prevent chromatographic segregation of a solution in a drug or
medication (Altenstetter, 2017).
Thermal analysis techniques: it is the test to identify the impact of the temperature on
an item that is used as the packaging material for the drugs or medication. The element
must be abler to withstand the change in the temperature or other environmental factors
in a better way.
Leak detection: the material used for the storing and packaging of drugs is required to be
leak proof to avoid the spoiling and wastage of drug or medication.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 41
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




