Organic Chemistry Synthesis Problems
VerifiedAdded on 2019/09/16
|4
|520
|187
Homework Assignment
AI Summary
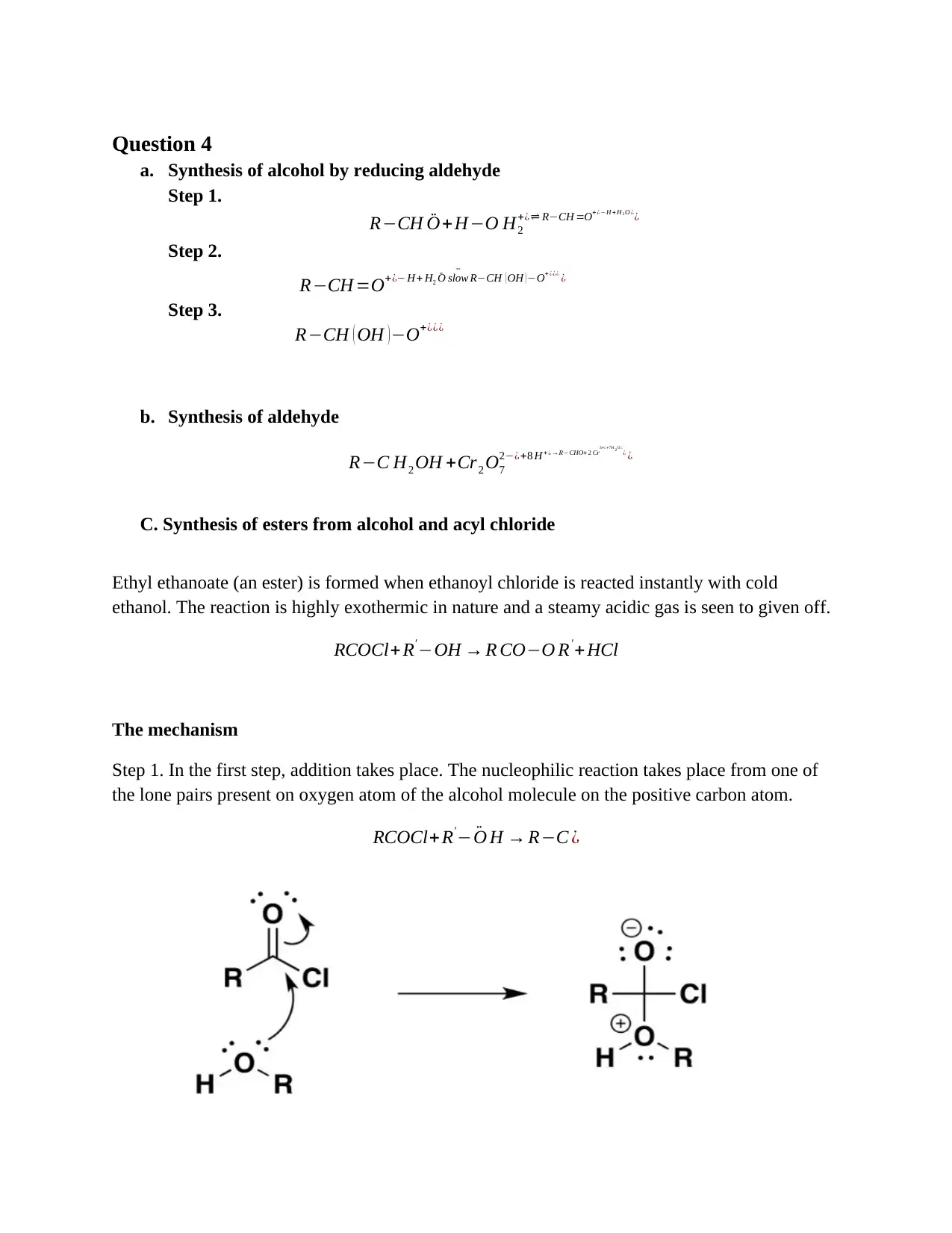
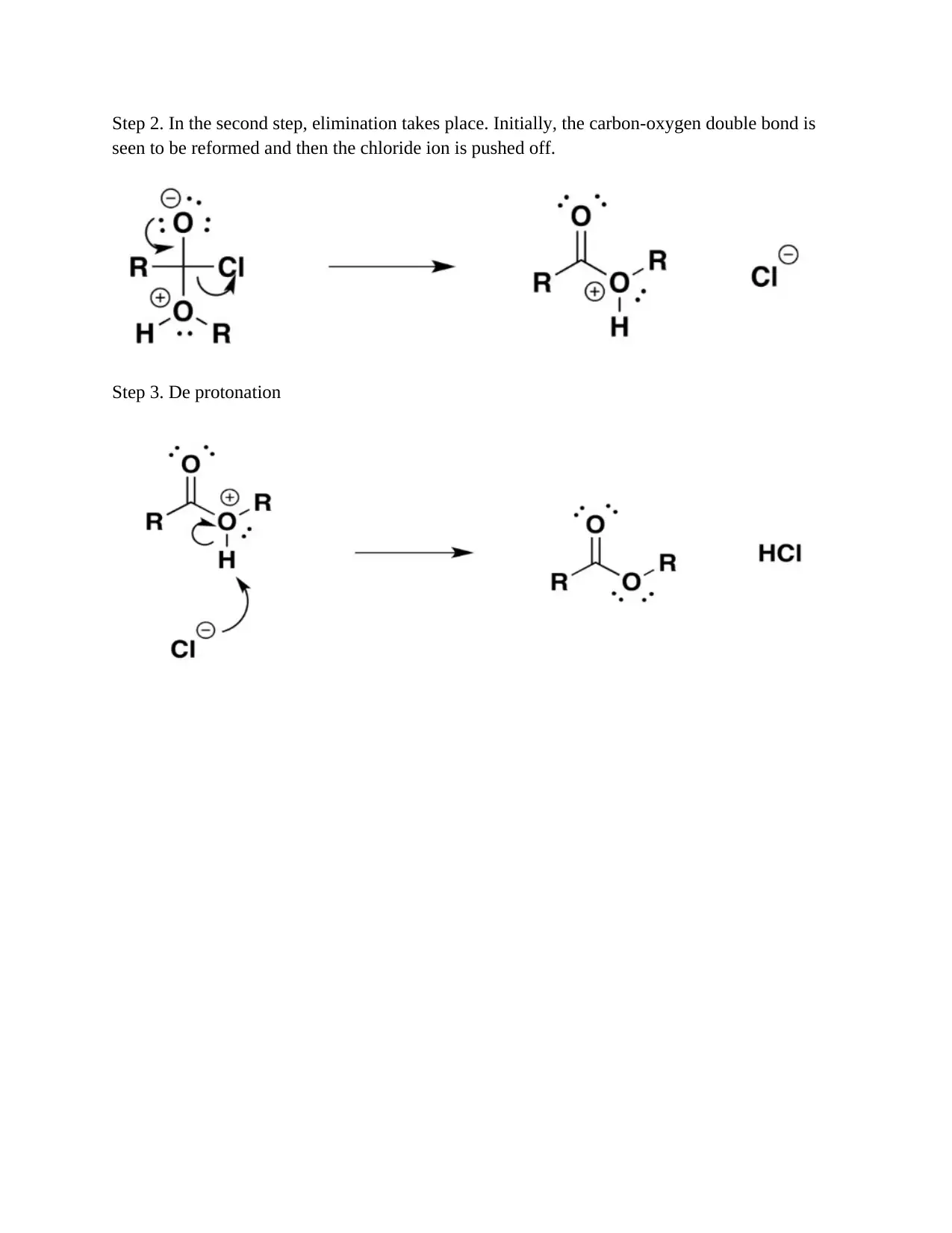
This chemistry assignment presents solved problems focusing on organic synthesis. It covers the synthesis of carboxylic acids from primary alcohols using acidified potassium dichromate, the synthesis of haloalkanes from alkenes (specifically ethene to bromoethane), the synthesis of alcohols by reducing aldehydes, and the synthesis of esters from alcohols and acyl chlorides (ethyl ethanoate example). Each synthesis includes a step-by-step mechanism and explanation, detailing the reactions and the underlying chemical principles. The problems demonstrate electrophilic addition, oxidation, and reduction reactions, along with detailed reaction mechanisms.
1 out of 4






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)