KB7005 Advanced Internal Combustion Engines Lab Assignment: Emissions
VerifiedAdded on 2023/04/21
|10
|1080
|358
Practical Assignment
AI Summary
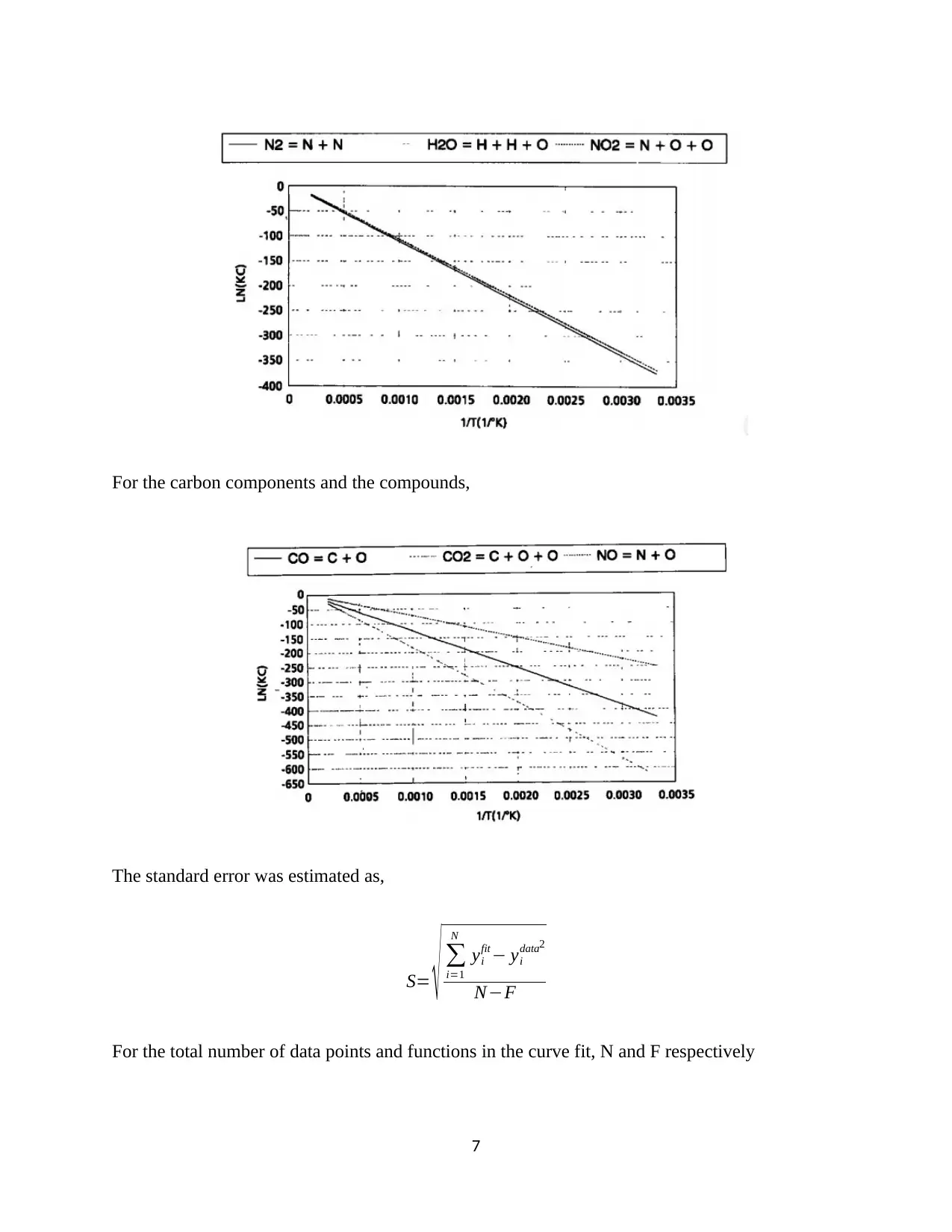
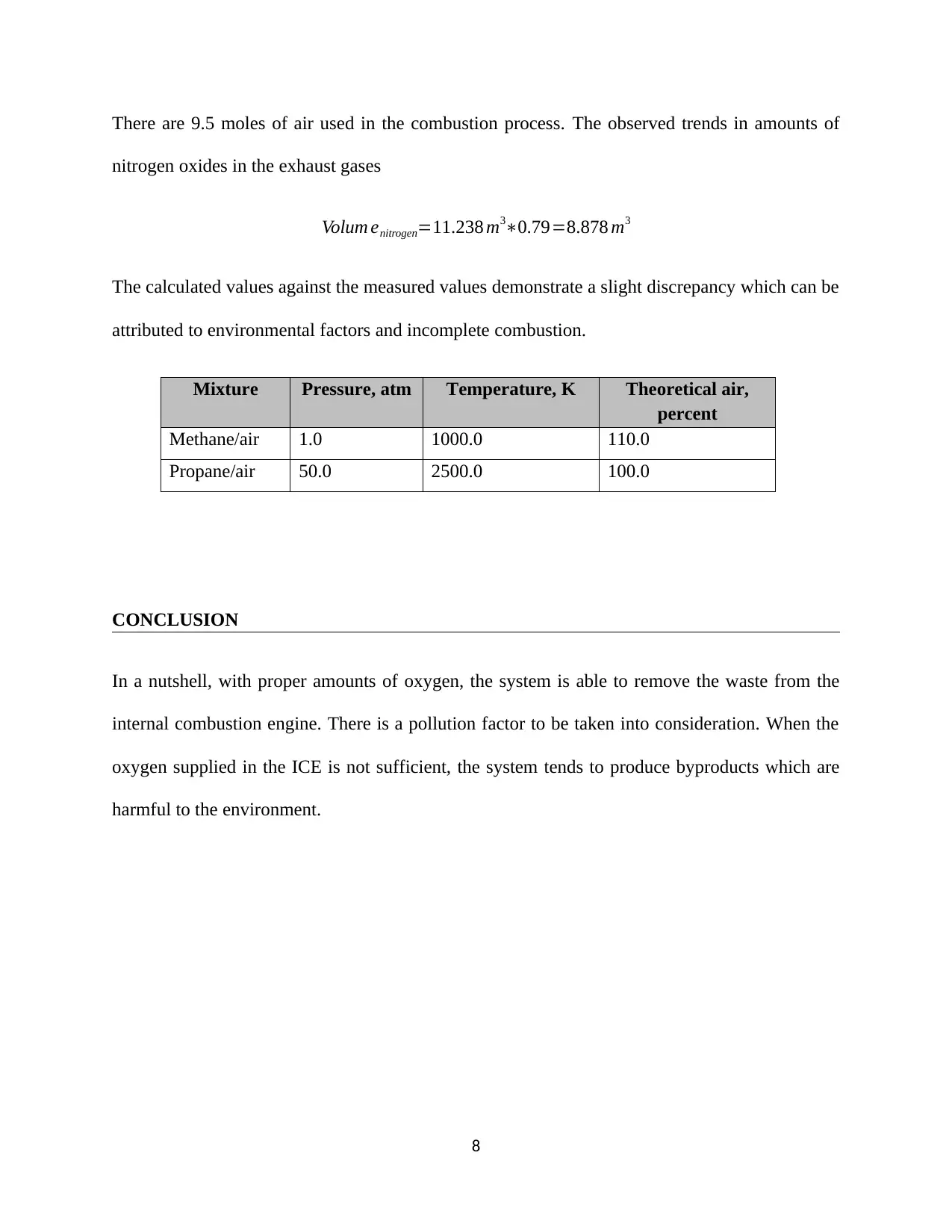
This assignment focuses on the experimental analysis of internal combustion (IC) engine performance and pollutant emissions. The experiment utilizes a Ricardo Hydra single-cylinder engine and the Horiba Mexa FT-one emission measurement system. The procedure includes preparing the exhaust gas sampling system, measuring intake conditions, and operating the engine in motoring mode. Data collected includes crank angle vs. in-cylinder pressure, and exhaust gas composition under various operating conditions. The assignment requires the calculation of the polytropic index, derivation of the relationship between oxygen content and equivalence ratio, estimation of combustion duration, and determination of burnt gas composition and thermo-equilibrium. The report also includes discussion of the experimental results, data analysis, and conclusions regarding the engine's performance and pollutant formation, with a focus on factors like oxygen supply and its impact on environmental byproducts. The experiment is designed to support the understanding of spark ignition engines and pollutant formation during combustion.
1 out of 10


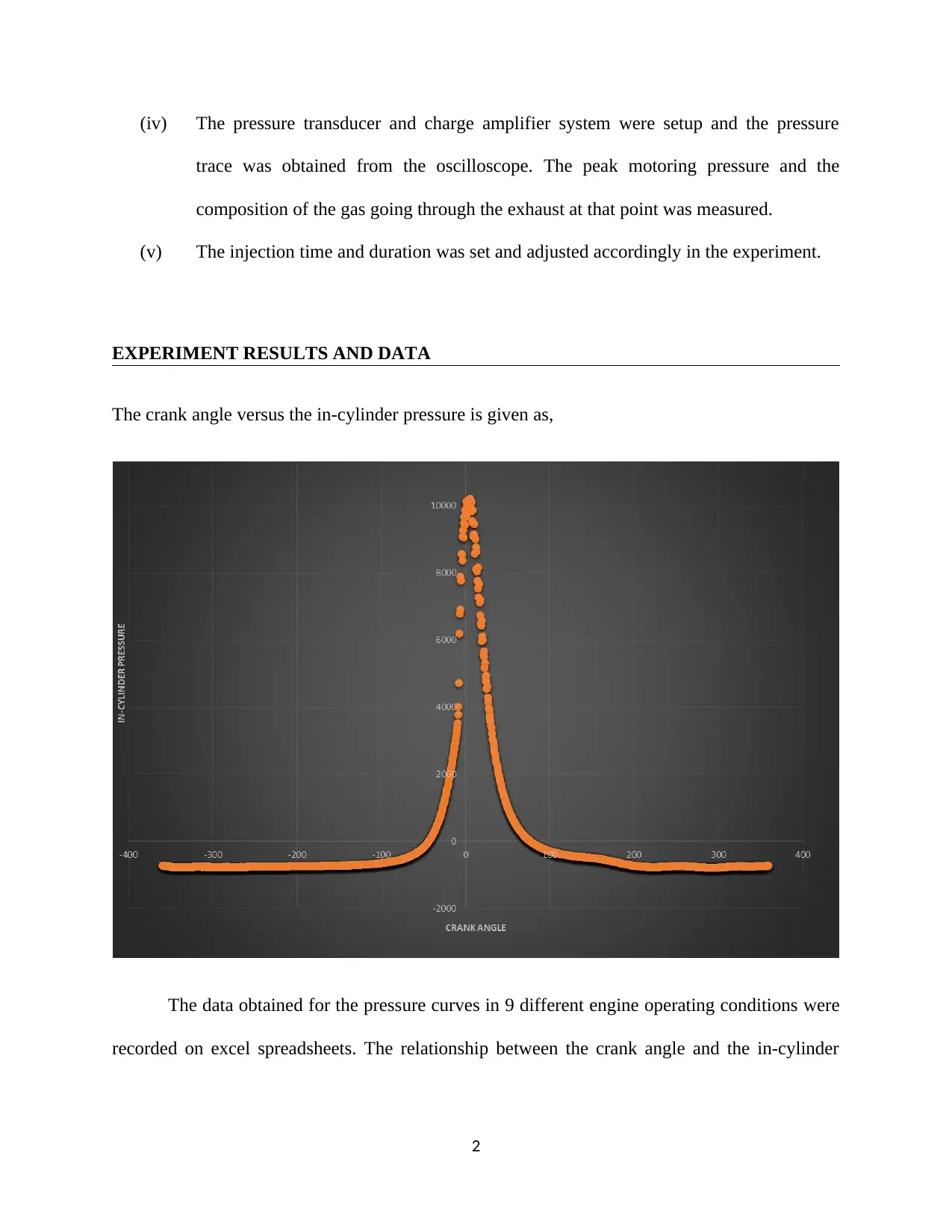


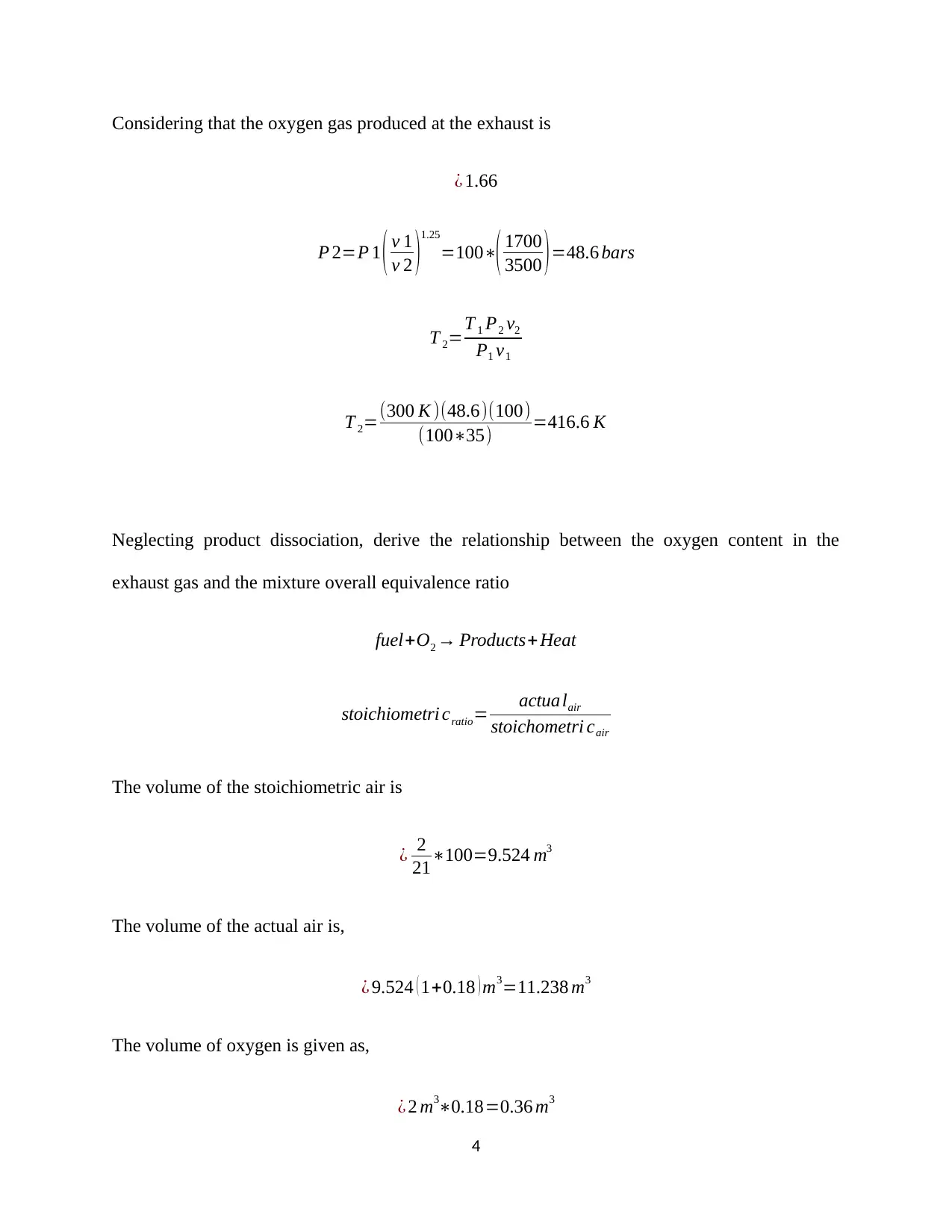
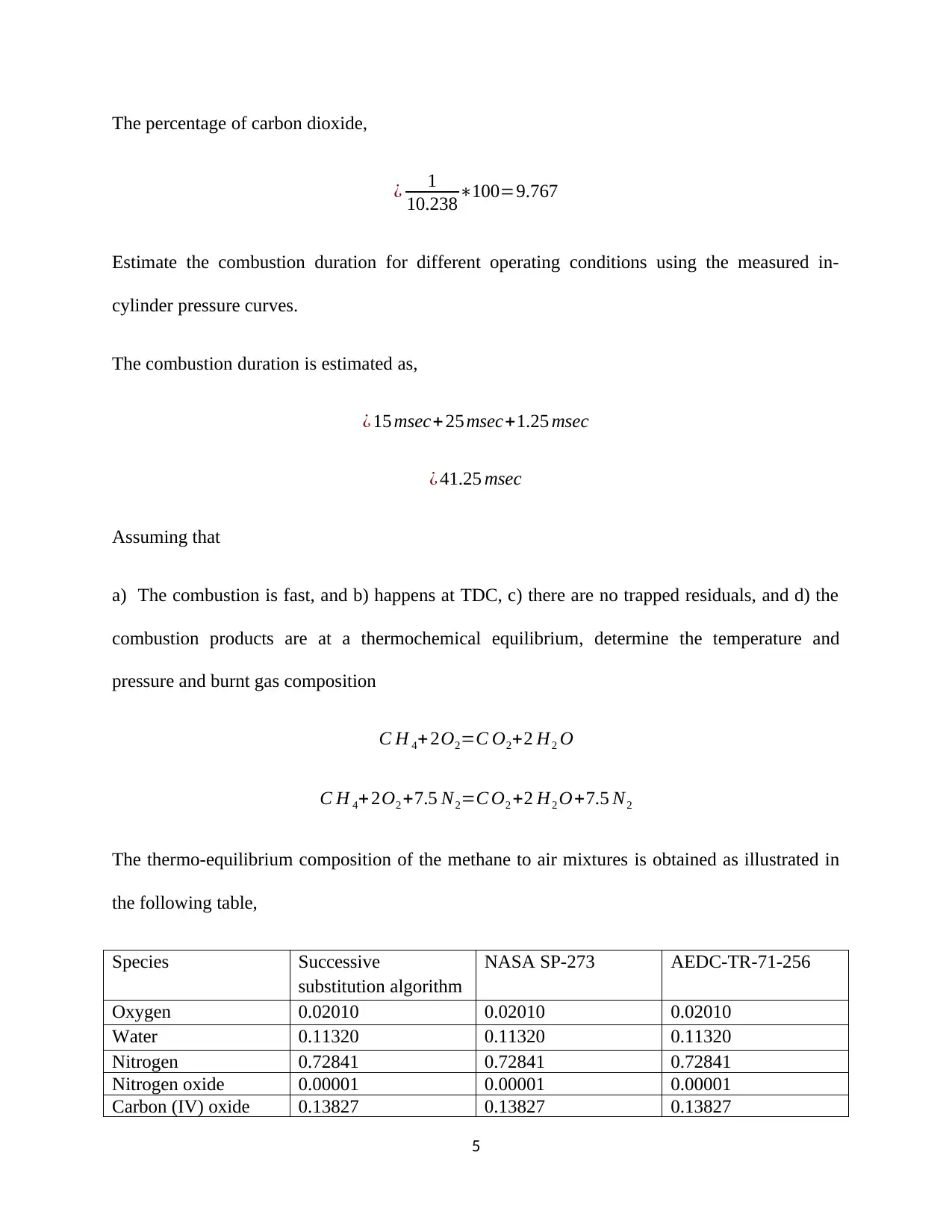
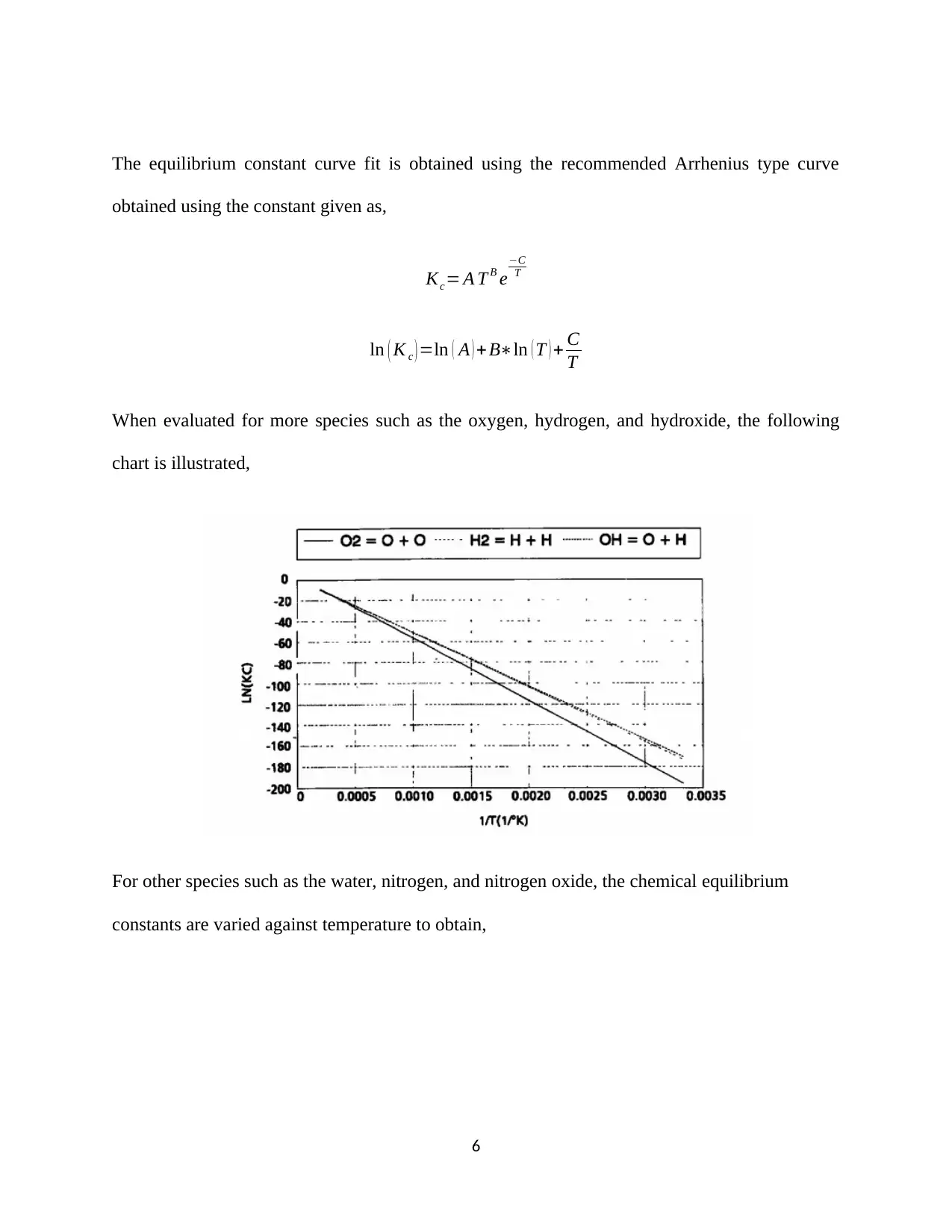




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)