Reducing Heart Failure Readmissions: A Systematic Review of DMPs
VerifiedAdded on 2023/06/13
|13
|12524
|261
Literature Review
AI Summary
This literature review critically evaluates the efficacy of heart failure (HF) disease management programs (DMPs) in reducing hospital readmissions and mortality. It examines randomized controlled trials of HF DMPs, including home care, outpatient clinic interventions, structured telephone support, and telemonitoring. The review highlights the inconsistent results associated with different DMP approaches, noting that no specific type has consistently reduced HF hospitalizations. While home visits and outpatient clinic interventions may be effective, they face limitations in cost and accessibility. Telemanagement, particularly structured telephone support, shows promise in reducing readmissions, though not all-cause mortality. Non-invasive telemonitoring demonstrates significant reductions in all-cause mortality and HF hospitalizations, while invasive telemonitoring's efficacy remains inconclusive. The review suggests that the inconsistent outcomes of HF DMPs may stem from applying a uniform approach to diverse patient populations, emphasizing the need for personalized, flexible strategies tailored to individual patient needs. Desklib provides access to a wealth of similar documents and study resources for students.

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction
in any medium, provided the original work is properly cited
126
Review Cardiol Res. 2014;5(5):126-138
ressElmer
Reducing Heart Failure Hospital Readmissions: A
Systematic Review of Disease Management Programs
Janardhana Gorthia, Claire B. Huntera, Ayran N. Moossa, Venkata M. Allaa, Daniel E. Hillemana, b
Abstract
The recent enactment of the Patient Protection and Affordable Care
Act which established the federal Hospital Readmissions Reduction
Program (HRRP) has accelerated efforts to develop heart failure (HF)
disease management programs (DMPs) that reduce readmissions in
patients hospitalized for HF. This systematic review identified ran-
domized controlled trials of HF DMPs which included home care,
outpatient clinic interventions, structured telephone support, and
non-invasive and invasive telemonitoring. These different types of
DMPs have been associated with conflicting results. No specific type
of DMP has produced consistent benefit in reducing HF hospitaliza-
tions. Although probably effective at reducing readmissions, home
visits and outpatient clinic interventions have substantial limitations
including cost and accessibility. Telemanagement has the potential
to reach a large number of patients at a reasonable cost. Structured
telephone support follow-up has been shown to significantly reduce
HF readmissions, but does not significantly reduce all-cause mortality
or all-cause hospitalization. A meta-analysis of 11 non-invasive te-
lemonitoring studies demonstrated significant reductions in all-cause
mortality and HF hospitalizations. Invasive telemonitoring is a poten-
tially effective means of reducing HF hospitalizations, but only one
study using pulmonary artery pressure monitoring was able to dem-
onstrate a reduction in HF hospitalizations. Other studies using inva-
sive hemodynamic monitoring have failed to demonstrate changes in
rates of readmission or mortality. The efficacy of HF DMPs is associ-
ated with inconsistent results. Our review should not be interpreted to
indicate that HF DMPs are universally ineffective. Rather, our data
suggest that one approach applied to a broad spectrum of different pa-
tient types may produce an erratic impact on readmissions and clini-
cal outcomes. HF DMPs should include the flexibility to meet the
individualized needs of specific patients.
Keywords: Heart failure; Hospitalizations; Heart failure clinics; Tele-
Manuscript accepted for publication October 24, 2014
aThe Creighton University Cardiac Center, Creighton University School of
Medicine, Omaha, NE, USA
bCorresponding Author: Daniel E. Hilleman, Creighton University Cardiac
Center, 3006 Webster Street, Omaha, NE 68131, USA.
Email: hilleman@creighton.edu
doi: http://dx.doi.org/10.14740/cr362w
management; Telemonitoring
Introduction
The medical and financial burden of heart failure (HF) hos-
pitalizations has led to a substantive body of research charac-
terizing the timing and etiology of readmissions, identifying
methods that predict readmission, and evaluating strategies
that reduce readmissions. Findings from epidemiologic sur-
veys of HF patients indicate that 30% of readmissions occur
during the first 2 months after hospital discharge, 50% of re-
admissions occur within the last 2 months prior to death, and
the remaining 20% of readmissions occur between these time
periods [1, 2]. This pattern of readmissions has been referred
to as the “three-phase terrain” of HF readmissions [3].
The Patient Protection and Affordable Care Act estab-
lished the federal Hospital Readmissions Reduction Program
(HRRP) through which Medicare payments to hospitals that
have excess readmissions following an admission for HF, my-
ocardial infarction, or pneumonia would be reduced [4]. The
HRRP took effect on October 1, 2012 using claims data from
July 2008 through June 2011. The CMS defines a readmission
as any hospital admission that occurs within 30 days of a dis-
charge from the same or other hospital [5]. Excess readmis-
sions are calculated by comparing a hospital’s rate of readmis-
sion for an applicable condition against the national average
for similar hospitals. For fiscal year 2013, excessive readmis-
sions can result in a maximal loss of up to 1% of Medicare re-
imbursement for the coming year [6]. The HRRP is expanding
in 2015 to include readmissions for chronic obstructive pulmo-
nary disease, coronary artery bypass graft surgery, percutane-
ous coronary interventions, and other vascular interventions
with penalties increasing to a maximum payment withholding
of 3% [7].
The ability of HF disease management programs (DMPs)
to routinely reduce all-cause hospital readmissions at 30 days
has not been documented. Many HF DMPs have reported
morbidity and/or mortality outcomes or have used different
follow-up time points [3]. Many programs have not focused
on clinical outcomes or reductions in unplanned healthcare
contacts, but rather have evaluated the rate at which a DMP
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction
in any medium, provided the original work is properly cited
126
Review Cardiol Res. 2014;5(5):126-138
ressElmer
Reducing Heart Failure Hospital Readmissions: A
Systematic Review of Disease Management Programs
Janardhana Gorthia, Claire B. Huntera, Ayran N. Moossa, Venkata M. Allaa, Daniel E. Hillemana, b
Abstract
The recent enactment of the Patient Protection and Affordable Care
Act which established the federal Hospital Readmissions Reduction
Program (HRRP) has accelerated efforts to develop heart failure (HF)
disease management programs (DMPs) that reduce readmissions in
patients hospitalized for HF. This systematic review identified ran-
domized controlled trials of HF DMPs which included home care,
outpatient clinic interventions, structured telephone support, and
non-invasive and invasive telemonitoring. These different types of
DMPs have been associated with conflicting results. No specific type
of DMP has produced consistent benefit in reducing HF hospitaliza-
tions. Although probably effective at reducing readmissions, home
visits and outpatient clinic interventions have substantial limitations
including cost and accessibility. Telemanagement has the potential
to reach a large number of patients at a reasonable cost. Structured
telephone support follow-up has been shown to significantly reduce
HF readmissions, but does not significantly reduce all-cause mortality
or all-cause hospitalization. A meta-analysis of 11 non-invasive te-
lemonitoring studies demonstrated significant reductions in all-cause
mortality and HF hospitalizations. Invasive telemonitoring is a poten-
tially effective means of reducing HF hospitalizations, but only one
study using pulmonary artery pressure monitoring was able to dem-
onstrate a reduction in HF hospitalizations. Other studies using inva-
sive hemodynamic monitoring have failed to demonstrate changes in
rates of readmission or mortality. The efficacy of HF DMPs is associ-
ated with inconsistent results. Our review should not be interpreted to
indicate that HF DMPs are universally ineffective. Rather, our data
suggest that one approach applied to a broad spectrum of different pa-
tient types may produce an erratic impact on readmissions and clini-
cal outcomes. HF DMPs should include the flexibility to meet the
individualized needs of specific patients.
Keywords: Heart failure; Hospitalizations; Heart failure clinics; Tele-
Manuscript accepted for publication October 24, 2014
aThe Creighton University Cardiac Center, Creighton University School of
Medicine, Omaha, NE, USA
bCorresponding Author: Daniel E. Hilleman, Creighton University Cardiac
Center, 3006 Webster Street, Omaha, NE 68131, USA.
Email: hilleman@creighton.edu
doi: http://dx.doi.org/10.14740/cr362w
management; Telemonitoring
Introduction
The medical and financial burden of heart failure (HF) hos-
pitalizations has led to a substantive body of research charac-
terizing the timing and etiology of readmissions, identifying
methods that predict readmission, and evaluating strategies
that reduce readmissions. Findings from epidemiologic sur-
veys of HF patients indicate that 30% of readmissions occur
during the first 2 months after hospital discharge, 50% of re-
admissions occur within the last 2 months prior to death, and
the remaining 20% of readmissions occur between these time
periods [1, 2]. This pattern of readmissions has been referred
to as the “three-phase terrain” of HF readmissions [3].
The Patient Protection and Affordable Care Act estab-
lished the federal Hospital Readmissions Reduction Program
(HRRP) through which Medicare payments to hospitals that
have excess readmissions following an admission for HF, my-
ocardial infarction, or pneumonia would be reduced [4]. The
HRRP took effect on October 1, 2012 using claims data from
July 2008 through June 2011. The CMS defines a readmission
as any hospital admission that occurs within 30 days of a dis-
charge from the same or other hospital [5]. Excess readmis-
sions are calculated by comparing a hospital’s rate of readmis-
sion for an applicable condition against the national average
for similar hospitals. For fiscal year 2013, excessive readmis-
sions can result in a maximal loss of up to 1% of Medicare re-
imbursement for the coming year [6]. The HRRP is expanding
in 2015 to include readmissions for chronic obstructive pulmo-
nary disease, coronary artery bypass graft surgery, percutane-
ous coronary interventions, and other vascular interventions
with penalties increasing to a maximum payment withholding
of 3% [7].
The ability of HF disease management programs (DMPs)
to routinely reduce all-cause hospital readmissions at 30 days
has not been documented. Many HF DMPs have reported
morbidity and/or mortality outcomes or have used different
follow-up time points [3]. Many programs have not focused
on clinical outcomes or reductions in unplanned healthcare
contacts, but rather have evaluated the rate at which a DMP
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org 127
Gorthi et al Cardiol Res. 2014;5(5):126-138
has been successful in changing the process of care in HF pa-
tients [8]. Since the inception of the HRRP, greater emphasis
has been place on HF DMPs [9].
The purpose of the present systematic review was to criti-
cally evaluate all available studies meeting minimal inclusion
criteria to define the efficacy of DMPs in reducing hospitali-
zations and/or mortality in patients with chronic HF. The re-
cent HRRP initiative has provided substantial motivation to
minimize hospital readmissions in patients discharged with a
diagnosis of HF.
Methods
Studies were identified using the guidelines defined by the
Cochrane Handbook or Systemic Reviews and the Meta-anal-
ysis of Observational Studies in Epidemiology (MOOSE) [10,
11]. The on-line databases of PubMed (Medline), EBSCOHost,
and the Cochrane Library were searched from January 1975
through August 2014 for studies reporting the outcomes of HF
DMPs. The medical subject heading terms used in the search
included HF DMPs, HF, hemodynamics, structured telephone
support, telemonitoring, telemanagement, and implantable
hemodynamic devices. A manual search of the bibliographies
of the identified reports and reviews was also performed.
Only studies published in English were included in the
analysis. Studies published only as abstracts were excluded.
Only prospective, randomized studies including a minimum
of 50 patients were included. Eligible studies had to report
either hospitalizations (all-cause or heart failure specific) or
mortality (all-cause or cardiovascular). Efficacy was based on
study reported outcomes concerning hospital readmissions or
mortality comparing the intervention and control or usual care
treatment arms. Studies using pre- and post-disease manage-
ment intervention analyses were excluded. Studies that were
published as preliminary reports that were subsequently re-
ported in a later publication with a larger sample size were
not included in this analysis. In addition, studies reporting on
patients with disease states other than HF which did not report
outcomes for HF patients separately from other patient types
were also excluded.
Results
In-home care interventions
A total of eight randomized controlled studies meeting eligi-
bility criteria evaluating the efficacy of in-home visits as part
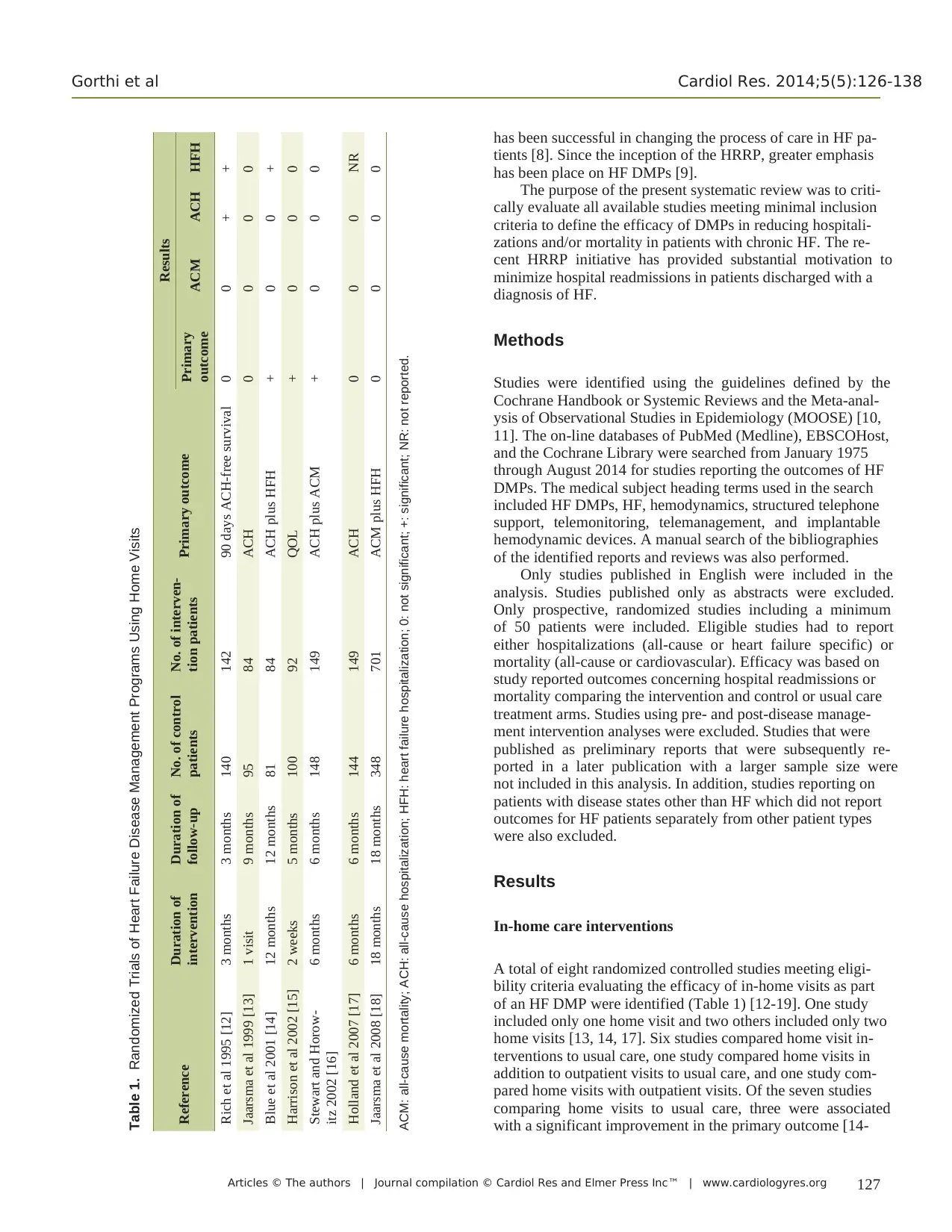
of an HF DMP were identified (Table 1) [12-19]. One study
included only one home visit and two others included only two
home visits [13, 14, 17]. Six studies compared home visit in-
terventions to usual care, one study compared home visits in
addition to outpatient visits to usual care, and one study com-
pared home visits with outpatient visits. Of the seven studies
comparing home visits to usual care, three were associated
with a significant improvement in the primary outcome [14-
Table 1. Randomized Trials of Heart Failure Disease Management Programs Using Home Visits
Reference Duration of
intervention
Duration of
follow-up
No. of control
patients
No. of interven-
tion patients Primary outcome
Results
Primary
outcome ACM ACH HFH
Rich et al 1995 [12] 3 months 3 months 140 142 90 days ACH-free survival 0 0 + +
Jaarsma et al 1999 [13] 1 visit 9 months 95 84 ACH 0 0 0 0
Blue et al 2001 [14] 12 months 12 months 81 84 ACH plus HFH + 0 0 +
Harrison et al 2002 [15] 2 weeks 5 months 100 92 QOL + 0 0 0
Stewart and Horow-
itz 2002 [16]
6 months 6 months 148 149 ACH plus ACM + 0 0 0
Holland et al 2007 [17] 6 months 6 months 144 149 ACH 0 0 0 NR
Jaarsma et al 2008 [18] 18 months 18 months 348 701 ACM plus HFH 0 0 0 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported.
Gorthi et al Cardiol Res. 2014;5(5):126-138
has been successful in changing the process of care in HF pa-
tients [8]. Since the inception of the HRRP, greater emphasis
has been place on HF DMPs [9].
The purpose of the present systematic review was to criti-
cally evaluate all available studies meeting minimal inclusion
criteria to define the efficacy of DMPs in reducing hospitali-
zations and/or mortality in patients with chronic HF. The re-
cent HRRP initiative has provided substantial motivation to
minimize hospital readmissions in patients discharged with a
diagnosis of HF.
Methods
Studies were identified using the guidelines defined by the
Cochrane Handbook or Systemic Reviews and the Meta-anal-
ysis of Observational Studies in Epidemiology (MOOSE) [10,
11]. The on-line databases of PubMed (Medline), EBSCOHost,
and the Cochrane Library were searched from January 1975
through August 2014 for studies reporting the outcomes of HF
DMPs. The medical subject heading terms used in the search
included HF DMPs, HF, hemodynamics, structured telephone
support, telemonitoring, telemanagement, and implantable
hemodynamic devices. A manual search of the bibliographies
of the identified reports and reviews was also performed.
Only studies published in English were included in the
analysis. Studies published only as abstracts were excluded.
Only prospective, randomized studies including a minimum
of 50 patients were included. Eligible studies had to report
either hospitalizations (all-cause or heart failure specific) or
mortality (all-cause or cardiovascular). Efficacy was based on
study reported outcomes concerning hospital readmissions or
mortality comparing the intervention and control or usual care
treatment arms. Studies using pre- and post-disease manage-
ment intervention analyses were excluded. Studies that were
published as preliminary reports that were subsequently re-
ported in a later publication with a larger sample size were
not included in this analysis. In addition, studies reporting on
patients with disease states other than HF which did not report
outcomes for HF patients separately from other patient types
were also excluded.
Results
In-home care interventions
A total of eight randomized controlled studies meeting eligi-
bility criteria evaluating the efficacy of in-home visits as part
of an HF DMP were identified (Table 1) [12-19]. One study
included only one home visit and two others included only two
home visits [13, 14, 17]. Six studies compared home visit in-
terventions to usual care, one study compared home visits in
addition to outpatient visits to usual care, and one study com-
pared home visits with outpatient visits. Of the seven studies
comparing home visits to usual care, three were associated
with a significant improvement in the primary outcome [14-
Table 1. Randomized Trials of Heart Failure Disease Management Programs Using Home Visits
Reference Duration of
intervention
Duration of
follow-up
No. of control
patients
No. of interven-
tion patients Primary outcome
Results
Primary
outcome ACM ACH HFH
Rich et al 1995 [12] 3 months 3 months 140 142 90 days ACH-free survival 0 0 + +
Jaarsma et al 1999 [13] 1 visit 9 months 95 84 ACH 0 0 0 0
Blue et al 2001 [14] 12 months 12 months 81 84 ACH plus HFH + 0 0 +
Harrison et al 2002 [15] 2 weeks 5 months 100 92 QOL + 0 0 0
Stewart and Horow-
itz 2002 [16]
6 months 6 months 148 149 ACH plus ACM + 0 0 0
Holland et al 2007 [17] 6 months 6 months 144 149 ACH 0 0 0 NR
Jaarsma et al 2008 [18] 18 months 18 months 348 701 ACM plus HFH 0 0 0 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported.

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org128
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
16]. None of the studies were able to demonstrate a significant
reduction in all-cause mortality. One study was able to dem-
onstrate a reduction in all-cause hospitalization which was
driven by a reduction in HF hospitalizations [12]. Two studies
significantly reduced HF hospitalizations [12, 14]. Three stud-
ies evaluating home visits failed to demonstrate a significant
improvement in hospitalization or mortality [13, 17, 18].
In the largest published study to incorporate home visits
into the disease management intervention, home visits had no
favorable impact on outcomes [18]. The Coordinating Study
Evaluating Outcomes of Advising and Counseling in Heart
Failure (COACH) randomized 1,023 patients with NYHA
class II/III HF to one of three interventions including a control
group (n = 339), a basic support group (n = 340), and an inten-
sive support group (n = 344). All three interventions included
four visits to a cardiologist over an 18-month follow-up period
after an HF hospital discharge. The basic support intervention
included nine additional visits to an HF specialist nurse at an
outpatient clinic. The intensive support intervention included
18 additional visits to an HF specialist nurse at an outpatient
clinic, two home visits by the nurse specialist with one occur-
ring in the first month after discharge, and two multidiscipli-
nary advice sessions. The usual care group included only the
four outpatient visits to a cardiologist. The primary endpoint
of the composite of HF readmission or all-cause mortality oc-
curred in 141 (42%) control patients, 138 (38%) patients in the
basic support group, and 132 (38%) patients in the intensive
support group. Analysis of the time to the first event deter-
mined hazard ratios of 0.96 (95% CI 0.76 - 1.21; P = 0.73)
and 0.93 (95% CI 0.73 - 1.17; P = 0.53) for the composite
outcome comparing basic and intensive support against the
control group. All-cause mortality and hospitalizations were
not different among the patients randomized to the three inter-
ventions. The frequency of healthcare contacts initiated by the
patient was greater than prescribed in the protocol in all three
interventions. This was the greatest in the basic support group
where the increase in healthcare contacts was 40% while the
increase in the control group was 33%. The increase was only
10% greater than prescribed in the intensive support group.
The most recently published trial including home visits
was a randomized comparison against patients who were seen
in a walk-in specialty HF clinic. The WHICH (Which Heart
Failure Intervention Is Most Cost-Effective & Consumer
Friendly in Reducing Hospital Care) study randomized 143 pa-
tients to a home-based intervention (HBI) and 137 patients to
a specialized HF clinic-based intervention (CBI) with a 12- to
18-month follow-up [19]. The primary outcome was the com-
posite of all-cause unplanned hospitalizations or death. Since
there was no control group in this study, conclusions about the
relative effectiveness of the either DMP cannot be reached.
There was no significant difference in the primary composite
outcome between the HBI (71%) and the CBI (76%) (adjusted
hazard ratio 0.97; 95% CI 0.73 - 1.30; P = 0.86). There were
also no significant differences in unplanned hospitalizations
between the HBI (67%) and the CBI (69%) (P = 0.88) or in
all-cause mortality between the HBI (22%) and the CBI (28%)
(P = 0.25). Patients in the HBI group did have a significantly
shorter median duration of days during hospitalizations. The
Table 2. Randomized Trials of Heart Failure Disease Management Programs Using Outpatient Visits
Reference Duration of
intervention
Duration of
follow-up
No. of control
patients
No. of interven-
tion patients
Primary
outcome
Results
Primary
outcome ACM ACH HFH
Cline et al 1998 [20] 12 months 12 months 110 80 Time to
readmission
+ 0 0 NR
Ekman et al 1998 [21] 6 months 6 months 79 79 ACH plus ACM 0 0 0 0
Kasper et al 2002 [22] 6 months 6 months 98 102 ACM plus HFH 0 0 0 0
Doughty et al 2002 [23] 12 months 12 months 97 100 ACH plus ACM 0 0 + 0
Ledwidge et al 2002 [24] 3 months 3 months 47 51 Cost benefit + 0 + +
Capomolla et al 2002 [25] 12 months 12 months 122 112 Cost utility + + + NR
Stromberg et al 2003 [26] 12 months 12 months 54 52 ACM plus ACH + + + 0
de la Porte et al 2007 [27] 12 months 12 months 122 118 ACM plus HFH + 0 + +
Powell et al 2010 [28] 12 months 31 months 451 451 ACM plus HFH 0 0 0 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported.
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
16]. None of the studies were able to demonstrate a significant
reduction in all-cause mortality. One study was able to dem-
onstrate a reduction in all-cause hospitalization which was
driven by a reduction in HF hospitalizations [12]. Two studies
significantly reduced HF hospitalizations [12, 14]. Three stud-
ies evaluating home visits failed to demonstrate a significant
improvement in hospitalization or mortality [13, 17, 18].
In the largest published study to incorporate home visits
into the disease management intervention, home visits had no
favorable impact on outcomes [18]. The Coordinating Study
Evaluating Outcomes of Advising and Counseling in Heart
Failure (COACH) randomized 1,023 patients with NYHA
class II/III HF to one of three interventions including a control
group (n = 339), a basic support group (n = 340), and an inten-
sive support group (n = 344). All three interventions included
four visits to a cardiologist over an 18-month follow-up period
after an HF hospital discharge. The basic support intervention
included nine additional visits to an HF specialist nurse at an
outpatient clinic. The intensive support intervention included
18 additional visits to an HF specialist nurse at an outpatient
clinic, two home visits by the nurse specialist with one occur-
ring in the first month after discharge, and two multidiscipli-
nary advice sessions. The usual care group included only the
four outpatient visits to a cardiologist. The primary endpoint
of the composite of HF readmission or all-cause mortality oc-
curred in 141 (42%) control patients, 138 (38%) patients in the
basic support group, and 132 (38%) patients in the intensive
support group. Analysis of the time to the first event deter-
mined hazard ratios of 0.96 (95% CI 0.76 - 1.21; P = 0.73)
and 0.93 (95% CI 0.73 - 1.17; P = 0.53) for the composite
outcome comparing basic and intensive support against the
control group. All-cause mortality and hospitalizations were
not different among the patients randomized to the three inter-
ventions. The frequency of healthcare contacts initiated by the
patient was greater than prescribed in the protocol in all three
interventions. This was the greatest in the basic support group
where the increase in healthcare contacts was 40% while the
increase in the control group was 33%. The increase was only
10% greater than prescribed in the intensive support group.
The most recently published trial including home visits
was a randomized comparison against patients who were seen
in a walk-in specialty HF clinic. The WHICH (Which Heart
Failure Intervention Is Most Cost-Effective & Consumer
Friendly in Reducing Hospital Care) study randomized 143 pa-
tients to a home-based intervention (HBI) and 137 patients to
a specialized HF clinic-based intervention (CBI) with a 12- to
18-month follow-up [19]. The primary outcome was the com-
posite of all-cause unplanned hospitalizations or death. Since
there was no control group in this study, conclusions about the
relative effectiveness of the either DMP cannot be reached.
There was no significant difference in the primary composite
outcome between the HBI (71%) and the CBI (76%) (adjusted
hazard ratio 0.97; 95% CI 0.73 - 1.30; P = 0.86). There were
also no significant differences in unplanned hospitalizations
between the HBI (67%) and the CBI (69%) (P = 0.88) or in
all-cause mortality between the HBI (22%) and the CBI (28%)
(P = 0.25). Patients in the HBI group did have a significantly
shorter median duration of days during hospitalizations. The
Table 2. Randomized Trials of Heart Failure Disease Management Programs Using Outpatient Visits
Reference Duration of
intervention
Duration of
follow-up
No. of control
patients
No. of interven-
tion patients
Primary
outcome
Results
Primary
outcome ACM ACH HFH
Cline et al 1998 [20] 12 months 12 months 110 80 Time to
readmission
+ 0 0 NR
Ekman et al 1998 [21] 6 months 6 months 79 79 ACH plus ACM 0 0 0 0
Kasper et al 2002 [22] 6 months 6 months 98 102 ACM plus HFH 0 0 0 0
Doughty et al 2002 [23] 12 months 12 months 97 100 ACH plus ACM 0 0 + 0
Ledwidge et al 2002 [24] 3 months 3 months 47 51 Cost benefit + 0 + +
Capomolla et al 2002 [25] 12 months 12 months 122 112 Cost utility + + + NR
Stromberg et al 2003 [26] 12 months 12 months 54 52 ACM plus ACH + + + 0
de la Porte et al 2007 [27] 12 months 12 months 122 118 ACM plus HFH + 0 + +
Powell et al 2010 [28] 12 months 31 months 451 451 ACM plus HFH 0 0 0 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org 129
Gorthi et al Cardiol Res. 2014;5(5):126-138
median duration of hospital length of stay with HBI was 4.0
days (interquartile range of 2.0 - 7.0 days) compared to 6.0
days (interquartile range 3.5 - 13 days) with CBI (P = 0.004).
Although the HBI was not associated with a significant im-
provement in the primary outcome compared to the CBI, the
shorter hospital stay with HBI was associated with a lower
overall healthcare cost (P = 0.03). The costs of providing the
patient interventions were not significantly different between
HBI ($1,813 per patient) and CBI ($1,829 per patient).
Outpatient visit interventions
A total of 11 randomized controlled studies meeting eligibil-
ity criteria evaluating the efficacy of outpatient clinic visits
as part of an HF DMP were identified (Table 2) [18-28]. Two
of these trials were previously discussed: the COACH study
which found no benefit of frequent visits to a nurse specialist in
an outpatient setting compared to usual care and the WHICH
study comparing HBI and CBI [18, 19]. Of the remaining
nine studies, the primary outcome was significantly improved
in five studies [20, 24-27]. However, only two of these stud-
ies used hospitalizations or mortality in the primary outcome
[26, 27]. Three other studies achieved a statistically significant
improvement in their primary outcome [20, 24, 25]. The pri-
mary outcomes in these studies were time to readmission, cost-
benefit, and cost-utility. All-cause mortality was significantly
reduced in two studies, but one of these studies only enrolled
a total of 106 patients [25, 26]. Of the seven studies reporting
HF-related readmissions, only two significantly reduced those
events. The most consistent effect found in the studies utilizing
outpatient clinic visits was a significant reduction in all-cause
hospitalization which was achieved in five of the nine studies.
In the largest study using outpatient clinic visits, the Heart
Failure Adherence and Retention Trial (HART), 902 patients
with NYHA class II/III HF were randomized to one of two
interventions [28]. The self-management plus education inter-
vention included 18 two-hour group meetings offered over the
first year after randomization. The HF education alone group
received 18 “Heart Failure Tip Sheets” mailed on the same
schedule as the group meetings. Telephone calls were made
within 2 - 3 days after each mailing to ensure receipt and com-
prehension. Patients were followed for a minimum of 2 years
(1 year of treatment and 1 year of post-treatment follow-up).
The rate of the primary composite outcome of HF hospitaliza-
tion plus all-cause mortality was not different in the self-man-
agement plus education group (163 events, 40%) compared
to the education alone group (171 events, 41%) after a mean
follow-up of 2.56 years (odd ratio 0.95; 95% CI 0.72 - 1.26).
There were also no significant differences in the secondary
endpoints of death, HF hospitalization, all-cause hospitaliza-
tion, or quality of life.
Structured telephone support interventions
Disease management interventions relying on outpatient or
home visits are resource intensive, costly, and are limited in
the numbers of patients that can be impacted. This is especially
true for patients in geographically remote areas or those with
transportation limitations. Telemanagement using phone calls
or the more complex transmission of patient-related clinical
data (telemonitoring) over telephone or internet connections
have the potential to reach unlimited numbers of HF patients.
A total of 13 randomized controlled studies meeting eli-
gibility criteria evaluating the efficacy of structured telephone
support as part of an HF DMP were identified (Table 3) [29-
41]. All but two studies used hospitalization or mortality in the
primary efficacy outcome [33, 34]. In these two studies, time
to hospitalization for HF and medication adherence were the
primary outcomes, and neither achieved their primary efficacy
endpoint. In the 11 studies using hospitalization, mortality, or
both as the primary efficacy endpoint, four studies achieved
their primary efficacy endpoint [29, 30, 35, 37]. Two studies
were associated with a significant reduction in all-cause mor-
tality, one study was associated with a significant reduction in
all-cause hospitalization, and four studies were associated with
a significant reduction in HF hospitalizations [29-31, 35-37,
39]. A 2007 meta-analysis which pooled the results of 10 stud-
ies of structured telephone support concluded that telephone
follow-up significantly reduced HF readmissions, but did not
significantly reduce all-cause mortality or all-cause hospitali-
zation [42]. Two of the structured telephone support studies
were randomized comparisons against non-invasive telemoni-
toring DMPs [36, 40]. These studies are discussed further un-
der the non-invasive telemonitoring intervention section.
Non-invasive telemonitoring interventions
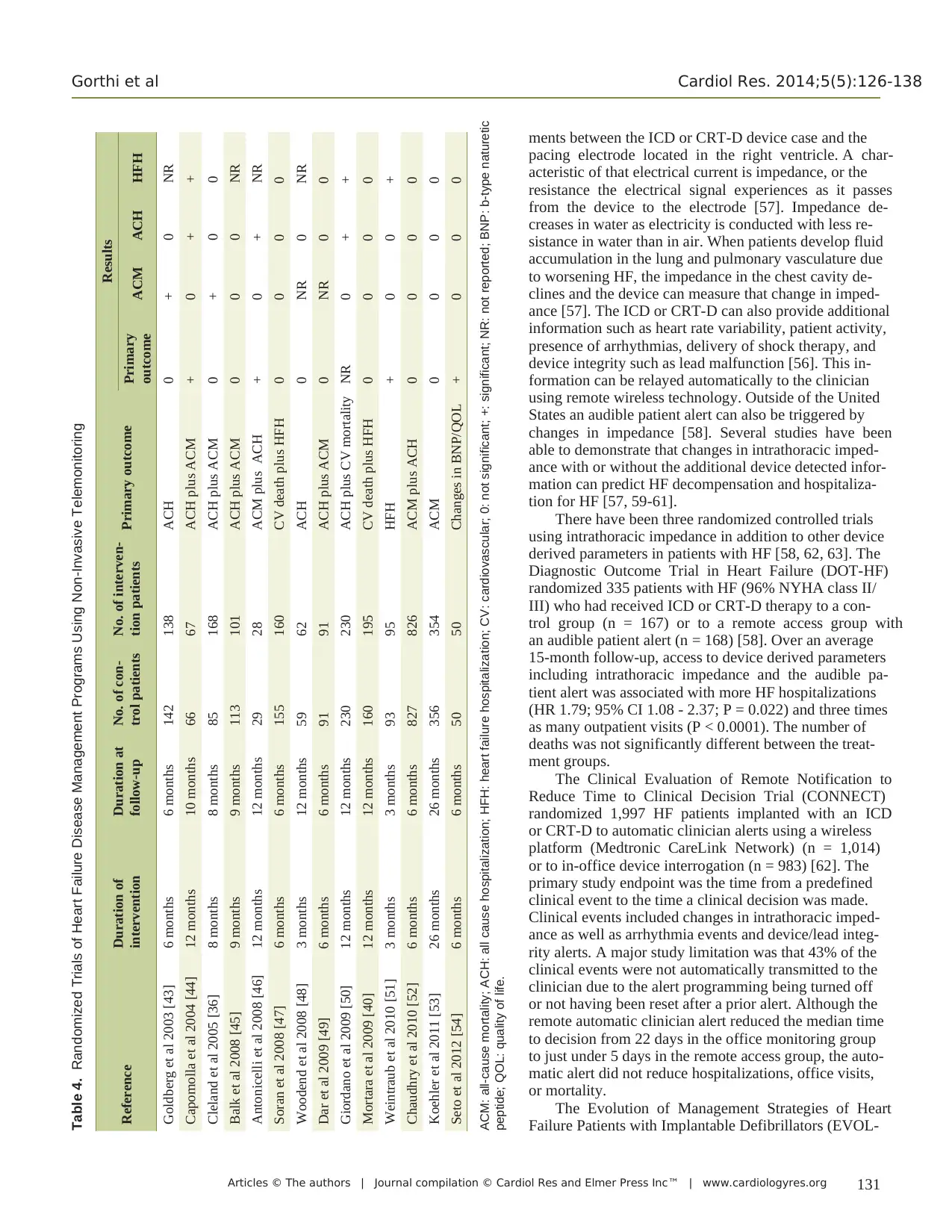
A total of 14 randomized controlled studies meeting eligibility
criteria evaluating the efficacy of non-invasive telemonitoring
support as part of an HF DMP were identified (Table 4) [36,
40, 43-54]. Thirteen of the 14 studies used a primary efficacy
endpoint that included hospitalizations, mortality, or both. The
lone study that did not include hospitalizations or mortality in
the primary outcome used changes in b-type naturetic peptide
levels and quality of life [54]. This study did demonstrate a
significant improvement in both of the primary endpoints us-
ing a mobile-phone-based telemonitoring system.
Of the remaining 13 studies, three achieved their primary
efficacy endpoint [44, 46, 51]. Twelve of the 13 studies report-
ed the effect of the DMP on cardiac or all-cause mortality with
only two studies demonstrating a significantly positive effect
on this outcome [36, 43]. Three studies significantly reduced
all-cause hospitalizations [44, 46, 50]. Ten of the 14 studies re-
ported rates of HF hospitalizations with three of the 10 studies
demonstrating significant reductions in these hospitalizations
[44, 50, 51].
A Cochrane database review conducted a meta-analysis
published in 2010 which included a total of 27 controlled
studies including 11 studies using non-invasive telemonitor-
ing (2,710 patients) and 16 studies using structured telephone
support (5,613 patients) [55]. All-cause mortality was signifi-
cantly reduced by non-invasive telemonitoring (RR 0.66; 95%
CI 0.54 - 0.81; P < 0.001). While structured telephone support
Gorthi et al Cardiol Res. 2014;5(5):126-138
median duration of hospital length of stay with HBI was 4.0
days (interquartile range of 2.0 - 7.0 days) compared to 6.0
days (interquartile range 3.5 - 13 days) with CBI (P = 0.004).
Although the HBI was not associated with a significant im-
provement in the primary outcome compared to the CBI, the
shorter hospital stay with HBI was associated with a lower
overall healthcare cost (P = 0.03). The costs of providing the
patient interventions were not significantly different between
HBI ($1,813 per patient) and CBI ($1,829 per patient).
Outpatient visit interventions
A total of 11 randomized controlled studies meeting eligibil-
ity criteria evaluating the efficacy of outpatient clinic visits
as part of an HF DMP were identified (Table 2) [18-28]. Two
of these trials were previously discussed: the COACH study
which found no benefit of frequent visits to a nurse specialist in
an outpatient setting compared to usual care and the WHICH
study comparing HBI and CBI [18, 19]. Of the remaining
nine studies, the primary outcome was significantly improved
in five studies [20, 24-27]. However, only two of these stud-
ies used hospitalizations or mortality in the primary outcome
[26, 27]. Three other studies achieved a statistically significant
improvement in their primary outcome [20, 24, 25]. The pri-
mary outcomes in these studies were time to readmission, cost-
benefit, and cost-utility. All-cause mortality was significantly
reduced in two studies, but one of these studies only enrolled
a total of 106 patients [25, 26]. Of the seven studies reporting
HF-related readmissions, only two significantly reduced those
events. The most consistent effect found in the studies utilizing
outpatient clinic visits was a significant reduction in all-cause
hospitalization which was achieved in five of the nine studies.
In the largest study using outpatient clinic visits, the Heart
Failure Adherence and Retention Trial (HART), 902 patients
with NYHA class II/III HF were randomized to one of two
interventions [28]. The self-management plus education inter-
vention included 18 two-hour group meetings offered over the
first year after randomization. The HF education alone group
received 18 “Heart Failure Tip Sheets” mailed on the same
schedule as the group meetings. Telephone calls were made
within 2 - 3 days after each mailing to ensure receipt and com-
prehension. Patients were followed for a minimum of 2 years
(1 year of treatment and 1 year of post-treatment follow-up).
The rate of the primary composite outcome of HF hospitaliza-
tion plus all-cause mortality was not different in the self-man-
agement plus education group (163 events, 40%) compared
to the education alone group (171 events, 41%) after a mean
follow-up of 2.56 years (odd ratio 0.95; 95% CI 0.72 - 1.26).
There were also no significant differences in the secondary
endpoints of death, HF hospitalization, all-cause hospitaliza-
tion, or quality of life.
Structured telephone support interventions
Disease management interventions relying on outpatient or
home visits are resource intensive, costly, and are limited in
the numbers of patients that can be impacted. This is especially
true for patients in geographically remote areas or those with
transportation limitations. Telemanagement using phone calls
or the more complex transmission of patient-related clinical
data (telemonitoring) over telephone or internet connections
have the potential to reach unlimited numbers of HF patients.
A total of 13 randomized controlled studies meeting eli-
gibility criteria evaluating the efficacy of structured telephone
support as part of an HF DMP were identified (Table 3) [29-
41]. All but two studies used hospitalization or mortality in the
primary efficacy outcome [33, 34]. In these two studies, time
to hospitalization for HF and medication adherence were the
primary outcomes, and neither achieved their primary efficacy
endpoint. In the 11 studies using hospitalization, mortality, or
both as the primary efficacy endpoint, four studies achieved
their primary efficacy endpoint [29, 30, 35, 37]. Two studies
were associated with a significant reduction in all-cause mor-
tality, one study was associated with a significant reduction in
all-cause hospitalization, and four studies were associated with
a significant reduction in HF hospitalizations [29-31, 35-37,
39]. A 2007 meta-analysis which pooled the results of 10 stud-
ies of structured telephone support concluded that telephone
follow-up significantly reduced HF readmissions, but did not
significantly reduce all-cause mortality or all-cause hospitali-
zation [42]. Two of the structured telephone support studies
were randomized comparisons against non-invasive telemoni-
toring DMPs [36, 40]. These studies are discussed further un-
der the non-invasive telemonitoring intervention section.
Non-invasive telemonitoring interventions
A total of 14 randomized controlled studies meeting eligibility
criteria evaluating the efficacy of non-invasive telemonitoring
support as part of an HF DMP were identified (Table 4) [36,
40, 43-54]. Thirteen of the 14 studies used a primary efficacy
endpoint that included hospitalizations, mortality, or both. The
lone study that did not include hospitalizations or mortality in
the primary outcome used changes in b-type naturetic peptide
levels and quality of life [54]. This study did demonstrate a
significant improvement in both of the primary endpoints us-
ing a mobile-phone-based telemonitoring system.
Of the remaining 13 studies, three achieved their primary
efficacy endpoint [44, 46, 51]. Twelve of the 13 studies report-
ed the effect of the DMP on cardiac or all-cause mortality with
only two studies demonstrating a significantly positive effect
on this outcome [36, 43]. Three studies significantly reduced
all-cause hospitalizations [44, 46, 50]. Ten of the 14 studies re-
ported rates of HF hospitalizations with three of the 10 studies
demonstrating significant reductions in these hospitalizations
[44, 50, 51].
A Cochrane database review conducted a meta-analysis
published in 2010 which included a total of 27 controlled
studies including 11 studies using non-invasive telemonitor-
ing (2,710 patients) and 16 studies using structured telephone
support (5,613 patients) [55]. All-cause mortality was signifi-
cantly reduced by non-invasive telemonitoring (RR 0.66; 95%
CI 0.54 - 0.81; P < 0.001). While structured telephone support
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org130
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
reduced all-cause mortality, the effect was not statistically
significant (RR 0.88; 95% CI 0.76 - 1.01; P = 0.08). HF
hospitalizations were significantly reduced by both te-
lemonitoring (RR 0.79; 95% CI 0.67 - 0.94; P = 0.008)
and structured telephone support (RR 0.77; 95% CI 0.68
- 0.87; P < 0.0001).
There were two randomized controlled studies com-
paring structured telephone support against non-invasive
telemonitoring. The Trans-European Network-Home
Care Management System (TEN-HMS) study rand-
omized 426 patients to usual care (n = 85), structured tel-
ephone support (n = 173), or to non-invasive telemonitor-
ing (n = 168) [36]. Telemonitoring included twice daily
transmission of weight, blood pressure, heart rate, and
cardiac rhythm. The primary endpoints of all-cause mor-
tality plus all-cause hospitalization as well as all-cause
and HF hospitalizations were not different between either
of the intervention groups compared to usual care. The
differences in these endpoints were also not significant
between telephone support and telemonitoring. However,
both intervention groups were associated with significant
reductions in all-cause mortality compared to usual care.
The second randomized trial comparing structured
telephone support and telemonitoring randomized 160
patients to usual care and 301 patients to one of three in-
tervention groups [40]. Strategy 1 employed structured
telephone support alone (n = 104), strategy 2 employed
structured telephone support plus weekly transmission
of vital signs including changes in weight, blood pres-
sure and symptoms (n = 96), and strategy 3 employed the
same intervention used in strategy 2 plus a monthly 24-h
cardiorespiratory recording (n = 101). The cardiorespi-
ratory recording included 24-h continuous electrocardio-
graphic monitoring and physical activity. All-cause hos-
pitalization, HF hospitalization, and mortality were not
significantly reduced in the more intensive strategy 2 and
3 intervention groups compared to strategy 1 patients.
Invasive telemonitoring interventions
Four different types of invasive hemodynamic monitor-
ing interventions have been evaluated in patients with HF
[56]. These include intrathoracic impedance monitoring,
pulmonary artery pressure monitoring, right ventricular
pressure monitoring, and left atrial pressure monitoring.
There are relatively few randomized, controlled trials us-
ing invasive hemodynamic monitoring for the prevention
of hospital readmission in patients with HF (Table 5).
The largest numbers of studies published to date
evaluated intrathoracic impedance monitoring with or
without the addition of other physiologic variables. Many
patients with severe HF have indications for implantable
cardioverter defibrillator (ICD) or cardiac resynchroniza-
tion therapy with defibrillator (CRT-D) therapy [56]. In-
trathoracic impedance monitoring is calculated using an
algorithm (OptiVol, Medtronic, Minneapolis, MN, USA)
that performs a series of electrical impedance measure-
Table 3. Randomized Trials of Heart Failure Disease Management Programs Using Structured Telephone Support
Reference Duration of
intervention
Duration of
follow-up
No. of con-
trol patients
No. of interven-
tion patients
Primary
outcome
Results
Primary outcome ACM ACH HFH
Gattis et al 1999 [29] 6 months 6 months 91 90 ACM plus HFH + 0 NR +
Riegel et al 2002 [30] 6 months 6 months 228 130 HFH + 0 0 +
Krumholz et al 2002 [31] 12 months 12 months 44 44 ACH plus ACM 0 0 0 +
Laramee et al 2003 [32] 3 months 3 months 146 141 ACH 0 0 0 0
Tsuyuki et al 2004 [33] 6 months 6 months 136 140 Medication
adherence
0 0 0 0
DeBusk et al 2004 [34] 12 months 12 months 234 228 Time to HFH 0 0 0 0
Galbreath et al 2004 [35] 18 months 18 months 359 710 ACM + + 0 0
Cleland et al 2005 [36] 8 months 8 months 85 173 ACM plus ACH 0 + 0 0
GESICA Investiga-
tors 2005 [37]
16 months 16 months 758 760 ACM plus HFH + 0 0 +
Riegel et al 2006 [38] 6 months 6 months 65 69 ACH 0 0 0 0
Sisk et al 2006 [39] 12 months 12 months 203 203 ACM plus ACH 0 0 + 0
Mortara et al 2009 [40] 12 months 12 months 160 106 Cardiac death
plus HFH
0 0 0 0
DeWalt et al 2012 [41] 12 months 12 months 302 303 ACH plus ACM 0 NR NR 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported.
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
reduced all-cause mortality, the effect was not statistically
significant (RR 0.88; 95% CI 0.76 - 1.01; P = 0.08). HF
hospitalizations were significantly reduced by both te-
lemonitoring (RR 0.79; 95% CI 0.67 - 0.94; P = 0.008)
and structured telephone support (RR 0.77; 95% CI 0.68
- 0.87; P < 0.0001).
There were two randomized controlled studies com-
paring structured telephone support against non-invasive
telemonitoring. The Trans-European Network-Home
Care Management System (TEN-HMS) study rand-
omized 426 patients to usual care (n = 85), structured tel-
ephone support (n = 173), or to non-invasive telemonitor-
ing (n = 168) [36]. Telemonitoring included twice daily
transmission of weight, blood pressure, heart rate, and
cardiac rhythm. The primary endpoints of all-cause mor-
tality plus all-cause hospitalization as well as all-cause
and HF hospitalizations were not different between either
of the intervention groups compared to usual care. The
differences in these endpoints were also not significant
between telephone support and telemonitoring. However,
both intervention groups were associated with significant
reductions in all-cause mortality compared to usual care.
The second randomized trial comparing structured
telephone support and telemonitoring randomized 160
patients to usual care and 301 patients to one of three in-
tervention groups [40]. Strategy 1 employed structured
telephone support alone (n = 104), strategy 2 employed
structured telephone support plus weekly transmission
of vital signs including changes in weight, blood pres-
sure and symptoms (n = 96), and strategy 3 employed the
same intervention used in strategy 2 plus a monthly 24-h
cardiorespiratory recording (n = 101). The cardiorespi-
ratory recording included 24-h continuous electrocardio-
graphic monitoring and physical activity. All-cause hos-
pitalization, HF hospitalization, and mortality were not
significantly reduced in the more intensive strategy 2 and
3 intervention groups compared to strategy 1 patients.
Invasive telemonitoring interventions
Four different types of invasive hemodynamic monitor-
ing interventions have been evaluated in patients with HF
[56]. These include intrathoracic impedance monitoring,
pulmonary artery pressure monitoring, right ventricular
pressure monitoring, and left atrial pressure monitoring.
There are relatively few randomized, controlled trials us-
ing invasive hemodynamic monitoring for the prevention
of hospital readmission in patients with HF (Table 5).
The largest numbers of studies published to date
evaluated intrathoracic impedance monitoring with or
without the addition of other physiologic variables. Many
patients with severe HF have indications for implantable
cardioverter defibrillator (ICD) or cardiac resynchroniza-
tion therapy with defibrillator (CRT-D) therapy [56]. In-
trathoracic impedance monitoring is calculated using an
algorithm (OptiVol, Medtronic, Minneapolis, MN, USA)
that performs a series of electrical impedance measure-
Table 3. Randomized Trials of Heart Failure Disease Management Programs Using Structured Telephone Support
Reference Duration of
intervention
Duration of
follow-up
No. of con-
trol patients
No. of interven-
tion patients
Primary
outcome
Results
Primary outcome ACM ACH HFH
Gattis et al 1999 [29] 6 months 6 months 91 90 ACM plus HFH + 0 NR +
Riegel et al 2002 [30] 6 months 6 months 228 130 HFH + 0 0 +
Krumholz et al 2002 [31] 12 months 12 months 44 44 ACH plus ACM 0 0 0 +
Laramee et al 2003 [32] 3 months 3 months 146 141 ACH 0 0 0 0
Tsuyuki et al 2004 [33] 6 months 6 months 136 140 Medication
adherence
0 0 0 0
DeBusk et al 2004 [34] 12 months 12 months 234 228 Time to HFH 0 0 0 0
Galbreath et al 2004 [35] 18 months 18 months 359 710 ACM + + 0 0
Cleland et al 2005 [36] 8 months 8 months 85 173 ACM plus ACH 0 + 0 0
GESICA Investiga-
tors 2005 [37]
16 months 16 months 758 760 ACM plus HFH + 0 0 +
Riegel et al 2006 [38] 6 months 6 months 65 69 ACH 0 0 0 0
Sisk et al 2006 [39] 12 months 12 months 203 203 ACM plus ACH 0 0 + 0
Mortara et al 2009 [40] 12 months 12 months 160 106 Cardiac death
plus HFH
0 0 0 0
DeWalt et al 2012 [41] 12 months 12 months 302 303 ACH plus ACM 0 NR NR 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported.
Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org 131
Gorthi et al Cardiol Res. 2014;5(5):126-138
ments between the ICD or CRT-D device case and the
pacing electrode located in the right ventricle. A char-
acteristic of that electrical current is impedance, or the
resistance the electrical signal experiences as it passes
from the device to the electrode [57]. Impedance de-
creases in water as electricity is conducted with less re-
sistance in water than in air. When patients develop fluid
accumulation in the lung and pulmonary vasculature due
to worsening HF, the impedance in the chest cavity de-
clines and the device can measure that change in imped-
ance [57]. The ICD or CRT-D can also provide additional
information such as heart rate variability, patient activity,
presence of arrhythmias, delivery of shock therapy, and
device integrity such as lead malfunction [56]. This in-
formation can be relayed automatically to the clinician
using remote wireless technology. Outside of the United
States an audible patient alert can also be triggered by
changes in impedance [58]. Several studies have been
able to demonstrate that changes in intrathoracic imped-
ance with or without the additional device detected infor-
mation can predict HF decompensation and hospitaliza-
tion for HF [57, 59-61].
There have been three randomized controlled trials
using intrathoracic impedance in addition to other device
derived parameters in patients with HF [58, 62, 63]. The
Diagnostic Outcome Trial in Heart Failure (DOT-HF)
randomized 335 patients with HF (96% NYHA class II/
III) who had received ICD or CRT-D therapy to a con-
trol group (n = 167) or to a remote access group with
an audible patient alert (n = 168) [58]. Over an average
15-month follow-up, access to device derived parameters
including intrathoracic impedance and the audible pa-
tient alert was associated with more HF hospitalizations
(HR 1.79; 95% CI 1.08 - 2.37; P = 0.022) and three times
as many outpatient visits (P < 0.0001). The number of
deaths was not significantly different between the treat-
ment groups.
The Clinical Evaluation of Remote Notification to
Reduce Time to Clinical Decision Trial (CONNECT)
randomized 1,997 HF patients implanted with an ICD
or CRT-D to automatic clinician alerts using a wireless
platform (Medtronic CareLink Network) (n = 1,014)
or to in-office device interrogation (n = 983) [62]. The
primary study endpoint was the time from a predefined
clinical event to the time a clinical decision was made.
Clinical events included changes in intrathoracic imped-
ance as well as arrhythmia events and device/lead integ-
rity alerts. A major study limitation was that 43% of the
clinical events were not automatically transmitted to the
clinician due to the alert programming being turned off
or not having been reset after a prior alert. Although the
remote automatic clinician alert reduced the median time
to decision from 22 days in the office monitoring group
to just under 5 days in the remote access group, the auto-
matic alert did not reduce hospitalizations, office visits,
or mortality.
The Evolution of Management Strategies of Heart
Failure Patients with Implantable Defibrillators (EVOL-
Table 4. Randomized Trials of Heart Failure Disease Management Programs Using Non-Invasive Telemonitoring
Reference Duration of
intervention
Duration at
follow-up
No. of con-
trol patients
No. of interven-
tion patients Primary outcome
Results
Primary
outcome ACM ACH HFH
Goldberg et al 2003 [43] 6 months 6 months 142 138 ACH 0 + 0 NR
Capomolla et al 2004 [44] 12 months 10 months 66 67 ACH plus ACM + 0 + +
Cleland et al 2005 [36] 8 months 8 months 85 168 ACH plus ACM 0 + 0 0
Balk et al 2008 [45] 9 months 9 months 113 101 ACH plus ACM 0 0 0 NR
Antonicelli et al 2008 [46] 12 months 12 months 29 28 ACM plus ACH + 0 + NR
Soran et al 2008 [47] 6 months 6 months 155 160 CV death plus HFH 0 0 0 0
Woodend et al 2008 [48] 3 months 12 months 59 62 ACH 0 NR 0 NR
Dar et al 2009 [49] 6 months 6 months 91 91 ACH plus ACM 0 NR 0 0
Giordano et al 2009 [50] 12 months 12 months 230 230 ACH plus CV mortality NR 0 + +
Mortara et al 2009 [40] 12 months 12 months 160 195 CV death plus HFH 0 0 0 0
Weintraub et al 2010 [51] 3 months 3 months 93 95 HFH + 0 0 +
Chaudhry et al 2010 [52] 6 months 6 months 827 826 ACM plus ACH 0 0 0 0
Koehler et al 2011 [53] 26 months 26 months 356 354 ACM 0 0 0 0
Seto et al 2012 [54] 6 months 6 months 50 50 Changes in BNP/QOL + 0 0 0
ACM: all-cause mortality; ACH: all cause hospitalization; HFH: heart failure hospitalization; CV: cardiovascular; 0: not significant; +: significant; NR: not reported; BNP: b-type naturetic
peptide; QOL: quality of life.
Gorthi et al Cardiol Res. 2014;5(5):126-138
ments between the ICD or CRT-D device case and the
pacing electrode located in the right ventricle. A char-
acteristic of that electrical current is impedance, or the
resistance the electrical signal experiences as it passes
from the device to the electrode [57]. Impedance de-
creases in water as electricity is conducted with less re-
sistance in water than in air. When patients develop fluid
accumulation in the lung and pulmonary vasculature due
to worsening HF, the impedance in the chest cavity de-
clines and the device can measure that change in imped-
ance [57]. The ICD or CRT-D can also provide additional
information such as heart rate variability, patient activity,
presence of arrhythmias, delivery of shock therapy, and
device integrity such as lead malfunction [56]. This in-
formation can be relayed automatically to the clinician
using remote wireless technology. Outside of the United
States an audible patient alert can also be triggered by
changes in impedance [58]. Several studies have been
able to demonstrate that changes in intrathoracic imped-
ance with or without the additional device detected infor-
mation can predict HF decompensation and hospitaliza-
tion for HF [57, 59-61].
There have been three randomized controlled trials
using intrathoracic impedance in addition to other device
derived parameters in patients with HF [58, 62, 63]. The
Diagnostic Outcome Trial in Heart Failure (DOT-HF)
randomized 335 patients with HF (96% NYHA class II/
III) who had received ICD or CRT-D therapy to a con-
trol group (n = 167) or to a remote access group with
an audible patient alert (n = 168) [58]. Over an average
15-month follow-up, access to device derived parameters
including intrathoracic impedance and the audible pa-
tient alert was associated with more HF hospitalizations
(HR 1.79; 95% CI 1.08 - 2.37; P = 0.022) and three times
as many outpatient visits (P < 0.0001). The number of
deaths was not significantly different between the treat-
ment groups.
The Clinical Evaluation of Remote Notification to
Reduce Time to Clinical Decision Trial (CONNECT)
randomized 1,997 HF patients implanted with an ICD
or CRT-D to automatic clinician alerts using a wireless
platform (Medtronic CareLink Network) (n = 1,014)
or to in-office device interrogation (n = 983) [62]. The
primary study endpoint was the time from a predefined
clinical event to the time a clinical decision was made.
Clinical events included changes in intrathoracic imped-
ance as well as arrhythmia events and device/lead integ-
rity alerts. A major study limitation was that 43% of the
clinical events were not automatically transmitted to the
clinician due to the alert programming being turned off
or not having been reset after a prior alert. Although the
remote automatic clinician alert reduced the median time
to decision from 22 days in the office monitoring group
to just under 5 days in the remote access group, the auto-
matic alert did not reduce hospitalizations, office visits,
or mortality.
The Evolution of Management Strategies of Heart
Failure Patients with Implantable Defibrillators (EVOL-
Table 4. Randomized Trials of Heart Failure Disease Management Programs Using Non-Invasive Telemonitoring
Reference Duration of
intervention
Duration at
follow-up
No. of con-
trol patients
No. of interven-
tion patients Primary outcome
Results
Primary
outcome ACM ACH HFH
Goldberg et al 2003 [43] 6 months 6 months 142 138 ACH 0 + 0 NR
Capomolla et al 2004 [44] 12 months 10 months 66 67 ACH plus ACM + 0 + +
Cleland et al 2005 [36] 8 months 8 months 85 168 ACH plus ACM 0 + 0 0
Balk et al 2008 [45] 9 months 9 months 113 101 ACH plus ACM 0 0 0 NR
Antonicelli et al 2008 [46] 12 months 12 months 29 28 ACM plus ACH + 0 + NR
Soran et al 2008 [47] 6 months 6 months 155 160 CV death plus HFH 0 0 0 0
Woodend et al 2008 [48] 3 months 12 months 59 62 ACH 0 NR 0 NR
Dar et al 2009 [49] 6 months 6 months 91 91 ACH plus ACM 0 NR 0 0
Giordano et al 2009 [50] 12 months 12 months 230 230 ACH plus CV mortality NR 0 + +
Mortara et al 2009 [40] 12 months 12 months 160 195 CV death plus HFH 0 0 0 0
Weintraub et al 2010 [51] 3 months 3 months 93 95 HFH + 0 0 +
Chaudhry et al 2010 [52] 6 months 6 months 827 826 ACM plus ACH 0 0 0 0
Koehler et al 2011 [53] 26 months 26 months 356 354 ACM 0 0 0 0
Seto et al 2012 [54] 6 months 6 months 50 50 Changes in BNP/QOL + 0 0 0
ACM: all-cause mortality; ACH: all cause hospitalization; HFH: heart failure hospitalization; CV: cardiovascular; 0: not significant; +: significant; NR: not reported; BNP: b-type naturetic
peptide; QOL: quality of life.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org132
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
VO) study randomized 200 patients with HF and an ICD/
CRT-D to remote monitoring using the Medtronic CareLink
wireless feature with intrathoracic impedance and other device
alerts (n = 98) or to office follow-up (n = 101) [63]. In the
office follow-up treatment group, remote automatic clinician
alerts were programmed off, but audible patient alerts were
turned on. In the remote monitoring group, the audible alert
was programmed off. The primary study endpoint was the rate
of emergency department or urgent in-office visits for HF, ar-
rhythmias, or ICD alerts. At the end of 16 months, 75 events
occurred in the remote group compared to 117 in the in-office
group (RR 0.65; 95% CI 0.49 - 0.88; P = 0.005). This signifi-
cant difference resulted from a reduction in visits for HF (48
vs. 92 visits). Visits for arrhythmias and ICD alerts were not
different between the two groups. There were also no signifi-
cant differences in all-cause or HF hospitalizations. The time
to clinical decision in this study was approximately 1.5 days
in the remote access group and 25 days in the in-office group.
There has been one randomized trial evaluating pulmo-
nary artery pressure monitoring using a wireless, passive,
radiofrequency sensor implanted into a distal branch of the
descending pulmonary artery [64]. The CardioMEMS Heart
Sensor Allows Monitoring of Pressure to Improve Outcomes
in NYHA class III Heart Failure Patients (CHAMPION) study
randomized 550 patients with the wireless pressure monitor to
a treatment group in which clinicians were given access to the
pressure results (n = 270) or to a control group in which clini-
cians did not receive pressure results (n = 280). The primary
study endpoint was HF hospitalizations at 6 months. The rate
of HF hospitalizations was significantly reduced in the treat-
ment group at 6 months and at the end of the entire follow-up
period (15 months). At 6 months, there were 84 HF hospitali-
zations in the treatment group and 120 in the control group
(28% RRR; P = 0.0002). At 15 months, there was a 37% reduc-
tion in HF hospitalizations in the treatment group compared to
the control group (P < 0.0001). All-cause hospitalization and
mortality were not reported.
Despite the favorable outcome of the CHAMPION study,
a Food and Drug Administration (FDA) Advisory Panel ini-
tially recommended against approval of the CardioMEMS HF
device in 2011 [65]. There was concern that a wider dispar-
ity in the distribution of HF hospitalizations occurred with a
substantial proportion of patients not being hospitalized. The
Advisory Panel raised concerns that the observed variance was
larger than the observed means. Another major concern was
that the treatment group received excessive treatment support
from investigators who had frequent communications with
physicians caring for patients in the treatment group but not
in the control group. Following a second FDA Advisory Panel
meeting in 2013 during which a post-marketing efficacy and
safety evaluation program was recommended, the FDA ap-
proved the CardioMEMS HF System - P100045TM in May
2014. This system was approved for implant in NYHA class
III HF patients who have been hospitalized for HF in the previ-
ous year. The device has coverage for inpatient reimbursement
through the Center for Medicare and Medicaid Services.
There were two randomized, controlled trials evaluating
the benefit of right ventricular pressure monitoring in patients
Table 5. Randomized Trials of Heart Failure Disease Management Programs Using Invasive Hemodynamic Monitoring
Reference Type of hemody-
namic monitoring
Duration of
intervention
Duration of
follow-up
No. of control
patients
No. of interven-
tion patients
Primary
outcome
Results
Primary
outcome
ACM ACH HFH
Van Veldhuisen
et al 2011 [58]
Intrathoracic
impedance
14.5 months 14.5 months 167 168 ACM plus HFH 0 0 0 0
Crossley et al
2011 [62]
Intrathoracic
Impedance
15 months 15 months 983 1014 Time to clini-
cal decision
+ 0 0 0
Landolina et
al 2012 [63]
Intrathoracic
impedance
16 months 16 months 101 99 ED and ur-
gent OPV
+ NR 0 0
Abraham et al
2011 [64]
Pulmonary ar-
tery pressures
15 months 15 months 270 280 HFH + 0 0 +
Bourge et al
2008 [66]
Right ventricu-
lar pressures
6 months 6 months 140 134 HF-related
urgent events
0 NR NR 0
Adamson et al
2011 [67]
Right ventricu-
lar pressures
12 months 12 months 198 202 HF-related
urgent events
0 NR 0 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported; ED: emergency department; OPV: outpa-
tient visits.
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
VO) study randomized 200 patients with HF and an ICD/
CRT-D to remote monitoring using the Medtronic CareLink
wireless feature with intrathoracic impedance and other device
alerts (n = 98) or to office follow-up (n = 101) [63]. In the
office follow-up treatment group, remote automatic clinician
alerts were programmed off, but audible patient alerts were
turned on. In the remote monitoring group, the audible alert
was programmed off. The primary study endpoint was the rate
of emergency department or urgent in-office visits for HF, ar-
rhythmias, or ICD alerts. At the end of 16 months, 75 events
occurred in the remote group compared to 117 in the in-office
group (RR 0.65; 95% CI 0.49 - 0.88; P = 0.005). This signifi-
cant difference resulted from a reduction in visits for HF (48
vs. 92 visits). Visits for arrhythmias and ICD alerts were not
different between the two groups. There were also no signifi-
cant differences in all-cause or HF hospitalizations. The time
to clinical decision in this study was approximately 1.5 days
in the remote access group and 25 days in the in-office group.
There has been one randomized trial evaluating pulmo-
nary artery pressure monitoring using a wireless, passive,
radiofrequency sensor implanted into a distal branch of the
descending pulmonary artery [64]. The CardioMEMS Heart
Sensor Allows Monitoring of Pressure to Improve Outcomes
in NYHA class III Heart Failure Patients (CHAMPION) study
randomized 550 patients with the wireless pressure monitor to
a treatment group in which clinicians were given access to the
pressure results (n = 270) or to a control group in which clini-
cians did not receive pressure results (n = 280). The primary
study endpoint was HF hospitalizations at 6 months. The rate
of HF hospitalizations was significantly reduced in the treat-
ment group at 6 months and at the end of the entire follow-up
period (15 months). At 6 months, there were 84 HF hospitali-
zations in the treatment group and 120 in the control group
(28% RRR; P = 0.0002). At 15 months, there was a 37% reduc-
tion in HF hospitalizations in the treatment group compared to
the control group (P < 0.0001). All-cause hospitalization and
mortality were not reported.
Despite the favorable outcome of the CHAMPION study,
a Food and Drug Administration (FDA) Advisory Panel ini-
tially recommended against approval of the CardioMEMS HF
device in 2011 [65]. There was concern that a wider dispar-
ity in the distribution of HF hospitalizations occurred with a
substantial proportion of patients not being hospitalized. The
Advisory Panel raised concerns that the observed variance was
larger than the observed means. Another major concern was
that the treatment group received excessive treatment support
from investigators who had frequent communications with
physicians caring for patients in the treatment group but not
in the control group. Following a second FDA Advisory Panel
meeting in 2013 during which a post-marketing efficacy and
safety evaluation program was recommended, the FDA ap-
proved the CardioMEMS HF System - P100045TM in May
2014. This system was approved for implant in NYHA class
III HF patients who have been hospitalized for HF in the previ-
ous year. The device has coverage for inpatient reimbursement
through the Center for Medicare and Medicaid Services.
There were two randomized, controlled trials evaluating
the benefit of right ventricular pressure monitoring in patients
Table 5. Randomized Trials of Heart Failure Disease Management Programs Using Invasive Hemodynamic Monitoring
Reference Type of hemody-
namic monitoring
Duration of
intervention
Duration of
follow-up
No. of control
patients
No. of interven-
tion patients
Primary
outcome
Results
Primary
outcome
ACM ACH HFH
Van Veldhuisen
et al 2011 [58]
Intrathoracic
impedance
14.5 months 14.5 months 167 168 ACM plus HFH 0 0 0 0
Crossley et al
2011 [62]
Intrathoracic
Impedance
15 months 15 months 983 1014 Time to clini-
cal decision
+ 0 0 0
Landolina et
al 2012 [63]
Intrathoracic
impedance
16 months 16 months 101 99 ED and ur-
gent OPV
+ NR 0 0
Abraham et al
2011 [64]
Pulmonary ar-
tery pressures
15 months 15 months 270 280 HFH + 0 0 +
Bourge et al
2008 [66]
Right ventricu-
lar pressures
6 months 6 months 140 134 HF-related
urgent events
0 NR NR 0
Adamson et al
2011 [67]
Right ventricu-
lar pressures
12 months 12 months 198 202 HF-related
urgent events
0 NR 0 0
ACM: all-cause mortality; ACH: all-cause hospitalization; HFH: heart failure hospitalization; 0: not significant; +: significant; NR: not reported; ED: emergency department; OPV: outpa-
tient visits.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org 133
Gorthi et al Cardiol Res. 2014;5(5):126-138
with HF [66, 67]. The Chronicle Offers Management to Pa-
tients with Advanced Signs and Symptoms of Heart Failure
(COMPASS-HF) [66]. This study randomized 274 NYHA
class III/IV HF patients who had an implantable continuous
hemodynamic monitor (ICHM) placed in the right ventricular
outflow tract or right ventricular septum. This sensor (Chroni-
cle) detects heart rate, body temperature, patient activity, right
ventricular systolic and diastolic pressures, and changes in
those pressures over time. After implantation, patients were
randomized to an intervention group in which physicians could
review the ICHM information on a weekly basis (n = 134) or
to a control group in which that data were not available (n =
140). After 6 months, ICHM data were made available for both
groups of patients. The primary outcome was the frequency
of HF-related events (hospitalizations, emergency department
visits, urgent outpatient visits) at 6 months of follow-up. Dur-
ing that follow-up period, 84 HF events occurred in 44 pa-
tients in the intervention group and 113 events occurred in 60
patients in the control group. This 21% relative risk reduction
failed to reach statistical significance (P = 0.33). All-cause hos-
pitalizations and mortality were not reported.
The Reducing Decompensation Events Utilizing Intra-
cardiac Pressures in Patients with Chronic Heart Failure (RE-
DUCEhf) randomized 400 patients who had the right ven-
tricular pressure monitor (Chronicle) implanted to a treatment
group in which pressure data were available (n = 202) or to
a control group where pressure data were not made available
(n = 198) [67]. The primary outcome was a composite of HF
hospitalizations, emergency department visits, or urgent clinic
visits over a 12-month follow-up. The intervention failed to
have any effect on these outcomes with 91 events occurring
in 43 patients in the treatment group compared to 90 events in
43 patients in the control group (P = 0.98). Mortality was not
reported.
There have been no randomized, controlled studies using
left atrial pressure monitoring. The Hemodynamically Guided
Home Self-Therapy in Severe Heart Failure Patients (HO-
MEOSTASIS) followed 40 NYHA class III/IV patients for a
median of 25 months after implantation of a left atrial pressure
monitor (HeartPOD, St. Jude Medical, St. Paul, MN, USA)
[68]. Patients had significant improvements in functional class,
reductions in left ventricular pressures, and fewer substantial
increases in left atrial pressure. These favorable outcomes most
likely resulted from more efficient use of diuretic and vasodila-
tor therapy in response to changes in left atrial pressure.
Discussion
The Heart Failure Society of America and the European Soci-
ety of Cardiology Heart Failure Association recommend en-
rollment in DMPs for patients with HF who have been recently
hospitalized or for high-risk HF patients [69, 70]. High-risk
patients include those with renal dysfunction, diabetes melli-
tus, chronic obstructive pulmonary disease, New York Heart
Association (NYHA) class III or IV symptoms, frequent hospi-
talizations for any reason, multiple comorbidities, a history of
depression, cognitive impairment, inadequate social or home
support, poor health literacy, or a history of non-adherence to
treatment recommendations.
The recommended elements of an HF DMP are summa-
rized in Table 6. Although comprehensive discharge planning
with post-discharge support has been shown to reduce read-
mission rates in HF patients, substantial numbers of patients
continue to be readmitted [71, 72]. Recent changes in health-
care policy and the HRRP have increased the importance of
reducing hospital readmissions in patients discharged with an
HF diagnosis [73]. The challenges associated with impacting
HF readmissions are enormous. With more than half of read-
missions for reasons other than HF, DMPs specifically directed
at HF alone would be expected to fail to reduce readmissions
in a large number of patients.
The results of our systematic review, limited to rand-
omized, controlled trials, found substantial heterogeneity in
the results of all of the available types of HF DMPs. The vast
majority of the published studies were not adequately powered
to demonstrate reductions in clinical endpoints. Programs that
utilized face-to-face interventions either in outpatient clinics
or at patients’ homes were able to demonstrate significant re-
ductions in HF hospitalizations in just four of 13 studies re-
porting that outcome [12, 14, 23, 27]. Home visits appear to be
less effective in reducing all-cause hospitalizations compared
to outpatient visits. Neither approach had a consistent impact
on mortality compared to usual care. There is only a single
randomized comparison of these two treatment interventions
[19]. As this study failed to show a significant difference be-
tween the interventions, it is impossible to reach valid conclu-
sions concerning their relative effectiveness. It is plausible to
Table 6. HFSA Recommended Elements of Heart Failure Disease Management Programs [69]
1 Comprehensive education and counseling individualized to the patient and patients’ environment
2 Promotion of self-care behaviors including potentially self-titration of diuretic dosing (with family member/healthcare provider assistance)
3 Emphasis on behavioral strategies to ensure adequate compliance
4 Adequate follow-up after hospital discharge or clinical instability (preferably within the first 7 days after event)
5 Optimization of oral therapy especially evidence-based therapy
6 Increased access to healthcare providers
7 Early attention to signs and symptoms of fluid overload
8 Assistance with financial and social concerns
HFSA: Heart Failure Society of America.
Gorthi et al Cardiol Res. 2014;5(5):126-138
with HF [66, 67]. The Chronicle Offers Management to Pa-
tients with Advanced Signs and Symptoms of Heart Failure
(COMPASS-HF) [66]. This study randomized 274 NYHA
class III/IV HF patients who had an implantable continuous
hemodynamic monitor (ICHM) placed in the right ventricular
outflow tract or right ventricular septum. This sensor (Chroni-
cle) detects heart rate, body temperature, patient activity, right
ventricular systolic and diastolic pressures, and changes in
those pressures over time. After implantation, patients were
randomized to an intervention group in which physicians could
review the ICHM information on a weekly basis (n = 134) or
to a control group in which that data were not available (n =
140). After 6 months, ICHM data were made available for both
groups of patients. The primary outcome was the frequency
of HF-related events (hospitalizations, emergency department
visits, urgent outpatient visits) at 6 months of follow-up. Dur-
ing that follow-up period, 84 HF events occurred in 44 pa-
tients in the intervention group and 113 events occurred in 60
patients in the control group. This 21% relative risk reduction
failed to reach statistical significance (P = 0.33). All-cause hos-
pitalizations and mortality were not reported.
The Reducing Decompensation Events Utilizing Intra-
cardiac Pressures in Patients with Chronic Heart Failure (RE-
DUCEhf) randomized 400 patients who had the right ven-
tricular pressure monitor (Chronicle) implanted to a treatment
group in which pressure data were available (n = 202) or to
a control group where pressure data were not made available
(n = 198) [67]. The primary outcome was a composite of HF
hospitalizations, emergency department visits, or urgent clinic
visits over a 12-month follow-up. The intervention failed to
have any effect on these outcomes with 91 events occurring
in 43 patients in the treatment group compared to 90 events in
43 patients in the control group (P = 0.98). Mortality was not
reported.
There have been no randomized, controlled studies using
left atrial pressure monitoring. The Hemodynamically Guided
Home Self-Therapy in Severe Heart Failure Patients (HO-
MEOSTASIS) followed 40 NYHA class III/IV patients for a
median of 25 months after implantation of a left atrial pressure
monitor (HeartPOD, St. Jude Medical, St. Paul, MN, USA)
[68]. Patients had significant improvements in functional class,
reductions in left ventricular pressures, and fewer substantial
increases in left atrial pressure. These favorable outcomes most
likely resulted from more efficient use of diuretic and vasodila-
tor therapy in response to changes in left atrial pressure.
Discussion
The Heart Failure Society of America and the European Soci-
ety of Cardiology Heart Failure Association recommend en-
rollment in DMPs for patients with HF who have been recently
hospitalized or for high-risk HF patients [69, 70]. High-risk
patients include those with renal dysfunction, diabetes melli-
tus, chronic obstructive pulmonary disease, New York Heart
Association (NYHA) class III or IV symptoms, frequent hospi-
talizations for any reason, multiple comorbidities, a history of
depression, cognitive impairment, inadequate social or home
support, poor health literacy, or a history of non-adherence to
treatment recommendations.
The recommended elements of an HF DMP are summa-
rized in Table 6. Although comprehensive discharge planning
with post-discharge support has been shown to reduce read-
mission rates in HF patients, substantial numbers of patients
continue to be readmitted [71, 72]. Recent changes in health-
care policy and the HRRP have increased the importance of
reducing hospital readmissions in patients discharged with an
HF diagnosis [73]. The challenges associated with impacting
HF readmissions are enormous. With more than half of read-
missions for reasons other than HF, DMPs specifically directed
at HF alone would be expected to fail to reduce readmissions
in a large number of patients.
The results of our systematic review, limited to rand-
omized, controlled trials, found substantial heterogeneity in
the results of all of the available types of HF DMPs. The vast
majority of the published studies were not adequately powered
to demonstrate reductions in clinical endpoints. Programs that
utilized face-to-face interventions either in outpatient clinics
or at patients’ homes were able to demonstrate significant re-
ductions in HF hospitalizations in just four of 13 studies re-
porting that outcome [12, 14, 23, 27]. Home visits appear to be
less effective in reducing all-cause hospitalizations compared
to outpatient visits. Neither approach had a consistent impact
on mortality compared to usual care. There is only a single
randomized comparison of these two treatment interventions
[19]. As this study failed to show a significant difference be-
tween the interventions, it is impossible to reach valid conclu-
sions concerning their relative effectiveness. It is plausible to
Table 6. HFSA Recommended Elements of Heart Failure Disease Management Programs [69]
1 Comprehensive education and counseling individualized to the patient and patients’ environment
2 Promotion of self-care behaviors including potentially self-titration of diuretic dosing (with family member/healthcare provider assistance)
3 Emphasis on behavioral strategies to ensure adequate compliance
4 Adequate follow-up after hospital discharge or clinical instability (preferably within the first 7 days after event)
5 Optimization of oral therapy especially evidence-based therapy
6 Increased access to healthcare providers
7 Early attention to signs and symptoms of fluid overload
8 Assistance with financial and social concerns
HFSA: Heart Failure Society of America.

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org134
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
consider that office-based interventions may be able to provide
a wider range of diagnostic and treatment options (i.e. chest
X-ray, echocardiograms, etc.) that may have accounted for the
more consistent impact of this intervention on all-cause hospi-
talization. Neither of these interventions would be considered
inexpensive.
The results of the COACH and HART studies indicate
that intensive face-to-face interventions are no better than less
costly and less time-intensive interventions in patients with
mild-to-moderate HF [18, 28]. The study populations were
similar in that the vast majority of patients had NYHA class
II/III HF and were receiving evidence-based therapies (ACEI
83-85% and beta-blockers 66-70%). Patients in COACH were
probably a higher risk population as only patients discharged
from the hospital following an admission for HF were enrolled
while patients in HART were recruited from both the hospi-
tal and outpatient clinic. Neither study was able to determine
why the more intensive intervention failed to produce benefit
particularly when compared to earlier, smaller studies. One ex-
planation may be that a higher proportion of control patients in
COACH and HART were receiving evidence-based therapies
and that current levels of expertise provided through “usual
care” are substantially improved compared to patients treated
with “usual care” in the early 1990s. Another possible expla-
nation is that more intensive face-to-face interventions used
in COACH and HART are actually not effective. Earlier stud-
ies demonstrating benefit of face-to-face healthcare provider
and patient interactions included smaller numbers of patients
generally treated at a single site [12]. These studies may have
overestimated the benefit of such interactions. The conclusions
of the investigators of both the COACH and HART are that
HF DMPs should not be abandoned, but that further research
is needed to better define what elements of such programs are
effective and how they should be implemented. It would be
erroneous to assume that one type of DMP will fit all types of
HF patients or all healthcare systems across the three phase
terrain of readmissions.
DMPs relying on telephone or non-invasive telemonitor-
ing have the advantage of being able to reach large numbers
of patients who live in geographically distant areas or who
have other reasons for limited travel. In addition, structured
telephone support should be a relatively inexpensive treatment
option. Non-invasive telemonitoring is associated with greater
expense and requires a certain degree of health-literacy on the
part of patients who must interact with the system that trans-
mits patient information to the healthcare provider.
The results of structured telephone support and non-inva-
sive telemonitoring have also been heterogeneous. Only four
of the 13 studies evaluating structured telephone support were
able to demonstrate reductions in HF hospitalizations [29-31,
37]. All-cause hospitalizations were reduced in one structured
telephone support study while all-cause mortality was reduced
in two of these studies [35, 36, 39]. The largest of the struc-
tured telephone support studies was able to demonstrate a sta-
tistically significant reduction in the primary composite out-
come of all-cause mortality plus HF hospitalizations primarily
due to a significant reduction in HF hospitalizations [37].
Of the 14 randomized, controlled trials of non-invasive
telemonitoring, only 10 reported on rates of HF readmissions.
In these 10 studies, HF hospitalizations were reduced in only
three [44, 50, 51]. All-cause hospitalization was reported in
all 14 studies with three reporting significant reductions [44,
46, 50]. Two of the 12 studies reporting mortality were able to
demonstrate significant reductions in all-cause mortality [36,
43].
The Cochrane Library meta-analysis of 27 randomized
controlled trials of structured telephone support compared
with non-invasive telemonitoring found significant reductions
in HF hospitalization for both interventions [55]. In addition,
this meta-analysis found a significant reduction in all-cause
mortality with telemonitoring and trend to a significant reduc-
tion with structured telephone support. However, the results
of meta-analyses are generally only considered to be hypoth-
esis generating. In addition, both randomized comparisons of
structured telephone support and non-invasive telemonitoring
failed to demonstrate one intervention to be superior to the
other or to less intensive interventions [36, 40].
The value of invasive hemodynamic monitoring as a part
of an integrated disease management strategy for HF patients
remains an area of intense research interest. Of the avail-
able published invasive hemodynamic monitoring studies, the
CHAMPION study generated the most interest due to the fa-
vorable reduction in HF hospitalization [64]. With the recent
FDA approval of the CardioMEMS HF System, the clinical
utility of this device will be closely followed to determine if it
performs as well in general clinical use as it did in the CHAM-
PION study.
The largest volume of published data is with intrathorac-
ic impedance monitoring typically used in combination with
a variety of other device derived parameters. This approach
is limited to patients who qualify for insertion of an ICD or
CRT-D. In two of the three controlled trials using intrathoracic
impedance monitoring, the primary outcome was time to clini-
cal decision or a reduction in urgent emergency department or
clinic visits [62, 63]. Although potentially clinically relevant,
achievement of this primary outcome appears to be irrelevant
considering that neither of those trials was able to demonstrate
reductions in hospitalizations or mortality. It should also be
noted that only one of the intrathoracic impedance studies was
adequately powered to evaluate clinical events which were not
favorably impacted [62].
Both of the studies assessing right ventricular pressure
indices also failed to reduce urgent HF-related healthcare con-
tacts including hospitalization [66, 67]. Neither study reported
the impact of monitoring on mortality. Both of these studies
were underpowered for clinical events. The REDUCEhf was
stopped prematurely by the manufacturer due to a high rate of
lead failures in other studies in which that particular lead was
used [67]. As a result, only 400 of a planned 1,350 patients
were actually enrolled in the trial.
Although right ventricular pressure (CHRONICLE) and
pulmonary artery pressure (CardioMEMS HF System) moni-
toring require implantation of a special sensor that does not
offer the other therapeutic features of an ICD/CRT-D, there is
no waiting period for pressure monitoring to start. With ICD/
CRT-D therapy, intrathoracic impedance monitoring requires a
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
consider that office-based interventions may be able to provide
a wider range of diagnostic and treatment options (i.e. chest
X-ray, echocardiograms, etc.) that may have accounted for the
more consistent impact of this intervention on all-cause hospi-
talization. Neither of these interventions would be considered
inexpensive.
The results of the COACH and HART studies indicate
that intensive face-to-face interventions are no better than less
costly and less time-intensive interventions in patients with
mild-to-moderate HF [18, 28]. The study populations were
similar in that the vast majority of patients had NYHA class
II/III HF and were receiving evidence-based therapies (ACEI
83-85% and beta-blockers 66-70%). Patients in COACH were
probably a higher risk population as only patients discharged
from the hospital following an admission for HF were enrolled
while patients in HART were recruited from both the hospi-
tal and outpatient clinic. Neither study was able to determine
why the more intensive intervention failed to produce benefit
particularly when compared to earlier, smaller studies. One ex-
planation may be that a higher proportion of control patients in
COACH and HART were receiving evidence-based therapies
and that current levels of expertise provided through “usual
care” are substantially improved compared to patients treated
with “usual care” in the early 1990s. Another possible expla-
nation is that more intensive face-to-face interventions used
in COACH and HART are actually not effective. Earlier stud-
ies demonstrating benefit of face-to-face healthcare provider
and patient interactions included smaller numbers of patients
generally treated at a single site [12]. These studies may have
overestimated the benefit of such interactions. The conclusions
of the investigators of both the COACH and HART are that
HF DMPs should not be abandoned, but that further research
is needed to better define what elements of such programs are
effective and how they should be implemented. It would be
erroneous to assume that one type of DMP will fit all types of
HF patients or all healthcare systems across the three phase
terrain of readmissions.
DMPs relying on telephone or non-invasive telemonitor-
ing have the advantage of being able to reach large numbers
of patients who live in geographically distant areas or who
have other reasons for limited travel. In addition, structured
telephone support should be a relatively inexpensive treatment
option. Non-invasive telemonitoring is associated with greater
expense and requires a certain degree of health-literacy on the
part of patients who must interact with the system that trans-
mits patient information to the healthcare provider.
The results of structured telephone support and non-inva-
sive telemonitoring have also been heterogeneous. Only four
of the 13 studies evaluating structured telephone support were
able to demonstrate reductions in HF hospitalizations [29-31,
37]. All-cause hospitalizations were reduced in one structured
telephone support study while all-cause mortality was reduced
in two of these studies [35, 36, 39]. The largest of the struc-
tured telephone support studies was able to demonstrate a sta-
tistically significant reduction in the primary composite out-
come of all-cause mortality plus HF hospitalizations primarily
due to a significant reduction in HF hospitalizations [37].
Of the 14 randomized, controlled trials of non-invasive
telemonitoring, only 10 reported on rates of HF readmissions.
In these 10 studies, HF hospitalizations were reduced in only
three [44, 50, 51]. All-cause hospitalization was reported in
all 14 studies with three reporting significant reductions [44,
46, 50]. Two of the 12 studies reporting mortality were able to
demonstrate significant reductions in all-cause mortality [36,
43].
The Cochrane Library meta-analysis of 27 randomized
controlled trials of structured telephone support compared
with non-invasive telemonitoring found significant reductions
in HF hospitalization for both interventions [55]. In addition,
this meta-analysis found a significant reduction in all-cause
mortality with telemonitoring and trend to a significant reduc-
tion with structured telephone support. However, the results
of meta-analyses are generally only considered to be hypoth-
esis generating. In addition, both randomized comparisons of
structured telephone support and non-invasive telemonitoring
failed to demonstrate one intervention to be superior to the
other or to less intensive interventions [36, 40].
The value of invasive hemodynamic monitoring as a part
of an integrated disease management strategy for HF patients
remains an area of intense research interest. Of the avail-
able published invasive hemodynamic monitoring studies, the
CHAMPION study generated the most interest due to the fa-
vorable reduction in HF hospitalization [64]. With the recent
FDA approval of the CardioMEMS HF System, the clinical
utility of this device will be closely followed to determine if it
performs as well in general clinical use as it did in the CHAM-
PION study.
The largest volume of published data is with intrathorac-
ic impedance monitoring typically used in combination with
a variety of other device derived parameters. This approach
is limited to patients who qualify for insertion of an ICD or
CRT-D. In two of the three controlled trials using intrathoracic
impedance monitoring, the primary outcome was time to clini-
cal decision or a reduction in urgent emergency department or
clinic visits [62, 63]. Although potentially clinically relevant,
achievement of this primary outcome appears to be irrelevant
considering that neither of those trials was able to demonstrate
reductions in hospitalizations or mortality. It should also be
noted that only one of the intrathoracic impedance studies was
adequately powered to evaluate clinical events which were not
favorably impacted [62].
Both of the studies assessing right ventricular pressure
indices also failed to reduce urgent HF-related healthcare con-
tacts including hospitalization [66, 67]. Neither study reported
the impact of monitoring on mortality. Both of these studies
were underpowered for clinical events. The REDUCEhf was
stopped prematurely by the manufacturer due to a high rate of
lead failures in other studies in which that particular lead was
used [67]. As a result, only 400 of a planned 1,350 patients
were actually enrolled in the trial.
Although right ventricular pressure (CHRONICLE) and
pulmonary artery pressure (CardioMEMS HF System) moni-
toring require implantation of a special sensor that does not
offer the other therapeutic features of an ICD/CRT-D, there is
no waiting period for pressure monitoring to start. With ICD/
CRT-D therapy, intrathoracic impedance monitoring requires a
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org 135
Gorthi et al Cardiol Res. 2014;5(5):126-138
waiting period of about 30 days before monitoring is consid-
ered reliable. Additional studies with the other types of inva-
sive hemodynamic monitoring will be required before they can
be considered a standard of care for the patient with severe HF.
Summary and Conclusion
The currently available evidence supporting the efficacy of
HF DMPs based on our systematic review restricted to rand-
omized, controlled trials, demonstrated highly inconsistent re-
sults. This should not be interpreted to indicate that HF DMPs
are not potentially effective. Rather, our data suggest that one
approach applied to a broad spectrum of different patient types
may not be effective. HF DMPs should be flexible enough to
be individualized to meet the needs of the specific patient. An
effective HF DMP remains as much an art as it does science.
Financial Disclosures
Dr. Hilleman is a member of the speaker’s bureau for Bristol-
Myers-Squibb and Malinckrodt. Drs. Gorthi, Hunter, Mooss,
and Alla have no financial disclosures.
References
1. Krumholz HM, Merrill AR, Schone EM, Schreiner GC,
Chen J, Bradley EH, Wang Y, et al. Patterns of hospital
performance in acute myocardial infarction and heart fail-
ure 30-day mortality and readmission. Circ Cardiovasc
Qual Outcomes. 2009;2(5):407-413.
2. Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X,
Levy D, Lee DS. Lifetime analysis of hospitalizations
and survival of patients newly admitted with heart failure.
Circ Heart Fail. 2012;5(4):414-421.
3. Desai AS. The three-phase terrain of heart failure read-
missions. Circ Heart Fail. 2012;5(4):398-400.
4. Centers for Medicare & Medicaid Services. Readmis-
sions Reduction Program. Available at: http://www.
cms.gov/Medicare/Medicaid-Fee-for-Service-Payment/
AcuteinpatientPPS/Readmissions-Reduction-Program.
html. Accessed September 13, 2013.
5. U.S. Department of Health and Human Services. Hospital
Quality Overview. Available at: http://www.hospitalcom-
pare.hhs.gov/staticpages/for-consumers/for-consumers.
aspx. Accessed September 13, 2013.
6. Rau J. Effort to curb Medicare spending begins with
crackdown on hospital readmissions. Available at: http://
www.kaiserhealthnews.org/Stories/2012/November/27/
medicare-spending-hospital-readmissions.aspx?p=1. Ac-
cessed September 13, 2013.
7. Kocher RP, Adashi EY. Hospital readmissions and the Af-
fordable Care Act: paying for coordinated quality care.
JAMA. 2011;306(16):1794-1795.
8. Mehrotra A, McNeil BJ, Landon BE. Congestive heart
failure disease management in Medicare-managed care.
Am Heart J. 2007;154(6):1153-1159.
9. Bradley EH, Curry L, Horwitz LI, Sipsma H, Thomp-
son JW, Elma M, Walsh MN, et al. Contemporary evi-
dence about hospital strategies for reducing 30-day
readmissions: a national study. J Am Coll Cardiol.
2012;60(7):607-614.
10. Higgins JPT, Green S (Editors). Cochrane Handbook for
Systematic Reviews of Interventions. Version 5.10 (up-
dated March 2011). The Cochrane Collaboration, 2011.
Available from www.cochrane-handbook.org.
11. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson
GD, Rennie D, Moher D, et al. Meta-analysis of observa-
tional studies in epidemiology: a proposal for reporting.
Meta-analysis Of Observational Studies in Epidemiology
(MOOSE) group. JAMA. 2000;283(15):2008-2012.
12. Rich MW, Beckham V, Wittenberg C, Leven CL, Freed-
land KE, Carney RM. A multidisciplinary intervention
to prevent the readmission of elderly patients with con-
gestive heart failure. N Engl J Med. 1995;333(18):1190-
1195.
13. Jaarsma T, Halfens R, Huijer Abu-Saad H, Dracup K,
Gorgels T, van Ree J, Stappers J. Effects of education and
support on self-care and resource utilization in patients
with heart failure. Eur Heart J. 1999;20(9):673-682.
14. Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA,
Murdoch DR, Petrie MC, et al. Randomised controlled
trial of specialist nurse intervention in heart failure. BMJ.
2001;323(7315):715-718.
15. Harrison MB, Browne GB, Roberts J, Tugwell P, Gaf-
ni A, Graham ID. Quality of life of individuals with
heart failure: a randomized trial of the effectiveness of
two models of hospital-to-home transition. Med Care.
2002;40(4):271-282.
16. Stewart S, Horowitz JD. Home-based intervention in con-
gestive heart failure: long-term implications on readmis-
sion and survival. Circulation. 2002;105(24):2861-2866.
17. Holland R, Brooksby I, Lenaghan E, Ashton K, Hay
L, Smith R, Shepstone L, et al. Effectiveness of vis-
its from community pharmacists for patients with heart
failure: HeartMed randomised controlled trial. BMJ.
2007;334(7603):1098.
18. Jaarsma T, van der Wal MH, Lesman-Leegte I, Lut-
tik ML, Hogenhuis J, Veeger NJ, Sanderman R, et al.
Effect of moderate or intensive disease management
program on outcome in patients with heart failure: Co-
ordinating Study Evaluating Outcomes of Advising and
Counseling in Heart Failure (COACH). Arch Intern Med.
2008;168(3):316-324.
19. Stewart S, Carrington MJ, Marwick TH, Davidson PM,
Macdonald P, Horowitz JD, Krum H, et al. Impact of
home versus clinic-based management of chronic heart
failure: the WHICH? (Which Heart Failure Intervention
Is Most Cost-Effective & Consumer Friendly in Reduc-
ing Hospital Care) multicenter, randomized trial. J Am
Coll Cardiol. 2012;60(14):1239-1248.
20. Cline CM, Israelsson BY, Willenheimer RB, Broms
K, Erhardt LR. Cost effective management pro-
gramme for heart failure reduces hospitalisation. Heart.
Gorthi et al Cardiol Res. 2014;5(5):126-138
waiting period of about 30 days before monitoring is consid-
ered reliable. Additional studies with the other types of inva-
sive hemodynamic monitoring will be required before they can
be considered a standard of care for the patient with severe HF.
Summary and Conclusion
The currently available evidence supporting the efficacy of
HF DMPs based on our systematic review restricted to rand-
omized, controlled trials, demonstrated highly inconsistent re-
sults. This should not be interpreted to indicate that HF DMPs
are not potentially effective. Rather, our data suggest that one
approach applied to a broad spectrum of different patient types
may not be effective. HF DMPs should be flexible enough to
be individualized to meet the needs of the specific patient. An
effective HF DMP remains as much an art as it does science.
Financial Disclosures
Dr. Hilleman is a member of the speaker’s bureau for Bristol-
Myers-Squibb and Malinckrodt. Drs. Gorthi, Hunter, Mooss,
and Alla have no financial disclosures.
References
1. Krumholz HM, Merrill AR, Schone EM, Schreiner GC,
Chen J, Bradley EH, Wang Y, et al. Patterns of hospital
performance in acute myocardial infarction and heart fail-
ure 30-day mortality and readmission. Circ Cardiovasc
Qual Outcomes. 2009;2(5):407-413.
2. Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X,
Levy D, Lee DS. Lifetime analysis of hospitalizations
and survival of patients newly admitted with heart failure.
Circ Heart Fail. 2012;5(4):414-421.
3. Desai AS. The three-phase terrain of heart failure read-
missions. Circ Heart Fail. 2012;5(4):398-400.
4. Centers for Medicare & Medicaid Services. Readmis-
sions Reduction Program. Available at: http://www.
cms.gov/Medicare/Medicaid-Fee-for-Service-Payment/
AcuteinpatientPPS/Readmissions-Reduction-Program.
html. Accessed September 13, 2013.
5. U.S. Department of Health and Human Services. Hospital
Quality Overview. Available at: http://www.hospitalcom-
pare.hhs.gov/staticpages/for-consumers/for-consumers.
aspx. Accessed September 13, 2013.
6. Rau J. Effort to curb Medicare spending begins with
crackdown on hospital readmissions. Available at: http://
www.kaiserhealthnews.org/Stories/2012/November/27/
medicare-spending-hospital-readmissions.aspx?p=1. Ac-
cessed September 13, 2013.
7. Kocher RP, Adashi EY. Hospital readmissions and the Af-
fordable Care Act: paying for coordinated quality care.
JAMA. 2011;306(16):1794-1795.
8. Mehrotra A, McNeil BJ, Landon BE. Congestive heart
failure disease management in Medicare-managed care.
Am Heart J. 2007;154(6):1153-1159.
9. Bradley EH, Curry L, Horwitz LI, Sipsma H, Thomp-
son JW, Elma M, Walsh MN, et al. Contemporary evi-
dence about hospital strategies for reducing 30-day
readmissions: a national study. J Am Coll Cardiol.
2012;60(7):607-614.
10. Higgins JPT, Green S (Editors). Cochrane Handbook for
Systematic Reviews of Interventions. Version 5.10 (up-
dated March 2011). The Cochrane Collaboration, 2011.
Available from www.cochrane-handbook.org.
11. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson
GD, Rennie D, Moher D, et al. Meta-analysis of observa-
tional studies in epidemiology: a proposal for reporting.
Meta-analysis Of Observational Studies in Epidemiology
(MOOSE) group. JAMA. 2000;283(15):2008-2012.
12. Rich MW, Beckham V, Wittenberg C, Leven CL, Freed-
land KE, Carney RM. A multidisciplinary intervention
to prevent the readmission of elderly patients with con-
gestive heart failure. N Engl J Med. 1995;333(18):1190-
1195.
13. Jaarsma T, Halfens R, Huijer Abu-Saad H, Dracup K,
Gorgels T, van Ree J, Stappers J. Effects of education and
support on self-care and resource utilization in patients
with heart failure. Eur Heart J. 1999;20(9):673-682.
14. Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA,
Murdoch DR, Petrie MC, et al. Randomised controlled
trial of specialist nurse intervention in heart failure. BMJ.
2001;323(7315):715-718.
15. Harrison MB, Browne GB, Roberts J, Tugwell P, Gaf-
ni A, Graham ID. Quality of life of individuals with
heart failure: a randomized trial of the effectiveness of
two models of hospital-to-home transition. Med Care.
2002;40(4):271-282.
16. Stewart S, Horowitz JD. Home-based intervention in con-
gestive heart failure: long-term implications on readmis-
sion and survival. Circulation. 2002;105(24):2861-2866.
17. Holland R, Brooksby I, Lenaghan E, Ashton K, Hay
L, Smith R, Shepstone L, et al. Effectiveness of vis-
its from community pharmacists for patients with heart
failure: HeartMed randomised controlled trial. BMJ.
2007;334(7603):1098.
18. Jaarsma T, van der Wal MH, Lesman-Leegte I, Lut-
tik ML, Hogenhuis J, Veeger NJ, Sanderman R, et al.
Effect of moderate or intensive disease management
program on outcome in patients with heart failure: Co-
ordinating Study Evaluating Outcomes of Advising and
Counseling in Heart Failure (COACH). Arch Intern Med.
2008;168(3):316-324.
19. Stewart S, Carrington MJ, Marwick TH, Davidson PM,
Macdonald P, Horowitz JD, Krum H, et al. Impact of
home versus clinic-based management of chronic heart
failure: the WHICH? (Which Heart Failure Intervention
Is Most Cost-Effective & Consumer Friendly in Reduc-
ing Hospital Care) multicenter, randomized trial. J Am
Coll Cardiol. 2012;60(14):1239-1248.
20. Cline CM, Israelsson BY, Willenheimer RB, Broms
K, Erhardt LR. Cost effective management pro-
gramme for heart failure reduces hospitalisation. Heart.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org136
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
1998;80(5):442-446.
21. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B,
Fagerberg B. Feasibility of a nurse-monitored, outpatient-
care programme for elderly patients with moderate-to-se-
vere, chronic heart failure. Eur Heart J. 1998;19(8):1254-
1260.
22. Kasper EK, Gerstenblith G, Hefter G, Van Anden E,
Brinker JA, Thiemann DR, Terrin M, et al. A randomized
trial of the efficacy of multidisciplinary care in heart fail-
ure outpatients at high risk of hospital readmission. J Am
Coll Cardiol. 2002;39(3):471-480.
23. Doughty RN, Wright SP, Pearl A, Walsh HJ, Muncas-
ter S, Whalley GA, Gamble G, et al. Randomized, con-
trolled trial of integrated heart failure management: The
Auckland Heart Failure Management Study. Eur Heart J.
2002;23(2):139-146.
24. Ledwidge M, Barry M, Cahill J, Ryan E, Maurer B, Ryder
M, Travers B, et al. Is multidisciplinary care of heart fail-
ure cost-beneficial when combined with optimal medical
care? Eur J Heart Fail. 2003;5(3):381-389.
25. Capomolla S, Febo O, Ceresa M, Caporotondi A, Guaz-
zotti G, La Rovere M, Ferrari M, et al. Cost/utility ratio
in chronic heart failure: comparison between heart fail-
ure management program delivered by day-hospital and
usual care. J Am Coll Cardiol. 2002;40(7):1259-1266.
26. Stromberg A, Martensson J, Fridlund B, Levin LA, Karls-
son JE, Dahlstrom U. Nurse-led heart failure clinics im-
prove survival and self-care behaviour in patients with
heart failure: results from a prospective, randomised trial.
Eur Heart J. 2003;24(11):1014-1023.
27. de la Porte PW, Lok DJ, van Veldhuisen DJ, van Wijn-
gaarden J, Cornel JH, Zuithoff NP, Badings E, et al. Add-
ed value of a physician-and-nurse-directed heart failure
clinic: results from the Deventer-Alkmaar heart failure
study. Heart. 2007;93(7):819-825.
28. Powell LH, Calvin JE, Jr., Richardson D, Janssen I,
Mendes de Leon CF, Flynn KJ, Grady KL, et al. Self-
management counseling in patients with heart failure: the
heart failure adherence and retention randomized behav-
ioral trial. JAMA. 2010;304(12):1331-1338.
29. Gattis WA, Hasselblad V, Whellan DJ, O'Connor CM. Re-
duction in heart failure events by the addition of a clinical
pharmacist to the heart failure management team: results
of the Pharmacist in Heart Failure Assessment Recom-
mendation and Monitoring (PHARM) Study. Arch Intern
Med. 1999;159(16):1939-1945.
30. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Un-
ger A. Effect of a standardized nurse case-management
telephone intervention on resource use in patients with
chronic heart failure. Arch Intern Med. 2002;162(6):705-
712.
31. Krumholz HM, Amatruda J, Smith GL, Mattera JA, Rou-
manis SA, Radford MJ, Crombie P, et al. Randomized
trial of an education and support intervention to prevent
readmission of patients with heart failure. J Am Coll Car-
diol. 2002;39(1):83-89.
32. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P.
Case management in a heterogeneous congestive heart
failure population: a randomized controlled trial. Arch
Intern Med. 2003;163(7):809-817.
33. Tsuyuki RT, Fradette M, Johnson JA, Bungard TJ, Eu-
rich DT, Ashton T, Gordon W, et al. A multicenter disease
management program for hospitalized patients with heart
failure. J Card Fail. 2004;10(6):473-480.
34. DeBusk RF, Miller NH, Parker KM, Bandura A, Kraemer
HC, Cher DJ, West JA, et al. Care management for low-
risk patients with heart failure: a randomized, controlled
trial. Ann Intern Med. 2004;141(8):606-613.
35. Galbreath AD, Krasuski RA, Smith B, Stajduhar KC,
Kwan MD, Ellis R, Freeman GL. Long-term healthcare
and cost outcomes of disease management in a large, ran-
domized, community-based population with heart failure.
Circulation. 2004;110(23):3518-3526.
36. Cleland JG, Louis AA, Rigby AS, Janssens U, Balk
AH. Noninvasive home telemonitoring for patients with
heart failure at high risk of recurrent admission and
death: the Trans-European Network-Home-Care Man-
agement System (TEN-HMS) study. J Am Coll Cardiol.
2005;45(10):1654-1664.
37. Randomised trial of telephone intervention in chronic
heart failure: DIAL trial. BMJ. 2005;331(7514):425.
38. Riegel B, Carlson B, Glaser D, Romero T. Randomized
controlled trial of telephone case management in His-
panics of Mexican origin with heart failure. J Card Fail.
2006;12(3):211-219.
39. Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA,
Wang JJ, Chassin MR. Effects of nurse management on
the quality of heart failure care in minority communities:
a randomized trial. Ann Intern Med. 2006;145(4):273-
283.
40. Mortara A, Pinna GD, Johnson P, Maestri R, Capomolla S,
La Rovere MT, Ponikowski P, et al. Home telemonitoring
in heart failure patients: the HHH study (Home or Hos-
pital in Heart Failure). Eur J Heart Fail. 2009;11(3):312-
318.
41. DeWalt DA, Schillinger D, Ruo B, Bibbins-Domingo K,
Baker DW, Holmes GM, Weinberger M, et al. Multisite
randomized trial of a single-session versus multisession
literacy-sensitive self-care intervention for patients with
heart failure. Circulation. 2012;125(23):2854-2862.
42. Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart
S. Telemonitoring or structured telephone support pro-
grammes for patients with chronic heart failure: systemat-
ic review and meta-analysis. BMJ. 2007;334(7600):942.
43. Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE,
Smith AL, Rodriguez R, et al. Randomized trial of a daily
electronic home monitoring system in patients with ad-
vanced heart failure: the Weight Monitoring in Heart Fail-
ure (WHARF) trial. Am Heart J. 2003;146(4):705-712.
44. Capomolla S, Pinna GD, La Rovere MT, Maestri R, Ce-
resa M, Ferrari M, et al. Heart failure case disease man-
agement program: a pilot study of home telemonitoring
versus usual care. Eur Heart J 2004; 6 (Suppl F): F91-F98.
45. Balk AH, Davidse W, Dommelen P, Klaassen E, Calis-
kan K, van der Burgh P, Leenders CM. Tele-guidance of
chronic heart failure patients enhances knowledge about
Reducing Heart Failure Hospital Readmissions Cardiol Res. 2014;5(5):126-138
1998;80(5):442-446.
21. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B,
Fagerberg B. Feasibility of a nurse-monitored, outpatient-
care programme for elderly patients with moderate-to-se-
vere, chronic heart failure. Eur Heart J. 1998;19(8):1254-
1260.
22. Kasper EK, Gerstenblith G, Hefter G, Van Anden E,
Brinker JA, Thiemann DR, Terrin M, et al. A randomized
trial of the efficacy of multidisciplinary care in heart fail-
ure outpatients at high risk of hospital readmission. J Am
Coll Cardiol. 2002;39(3):471-480.
23. Doughty RN, Wright SP, Pearl A, Walsh HJ, Muncas-
ter S, Whalley GA, Gamble G, et al. Randomized, con-
trolled trial of integrated heart failure management: The
Auckland Heart Failure Management Study. Eur Heart J.
2002;23(2):139-146.
24. Ledwidge M, Barry M, Cahill J, Ryan E, Maurer B, Ryder
M, Travers B, et al. Is multidisciplinary care of heart fail-
ure cost-beneficial when combined with optimal medical
care? Eur J Heart Fail. 2003;5(3):381-389.
25. Capomolla S, Febo O, Ceresa M, Caporotondi A, Guaz-
zotti G, La Rovere M, Ferrari M, et al. Cost/utility ratio
in chronic heart failure: comparison between heart fail-
ure management program delivered by day-hospital and
usual care. J Am Coll Cardiol. 2002;40(7):1259-1266.
26. Stromberg A, Martensson J, Fridlund B, Levin LA, Karls-
son JE, Dahlstrom U. Nurse-led heart failure clinics im-
prove survival and self-care behaviour in patients with
heart failure: results from a prospective, randomised trial.
Eur Heart J. 2003;24(11):1014-1023.
27. de la Porte PW, Lok DJ, van Veldhuisen DJ, van Wijn-
gaarden J, Cornel JH, Zuithoff NP, Badings E, et al. Add-
ed value of a physician-and-nurse-directed heart failure
clinic: results from the Deventer-Alkmaar heart failure
study. Heart. 2007;93(7):819-825.
28. Powell LH, Calvin JE, Jr., Richardson D, Janssen I,
Mendes de Leon CF, Flynn KJ, Grady KL, et al. Self-
management counseling in patients with heart failure: the
heart failure adherence and retention randomized behav-
ioral trial. JAMA. 2010;304(12):1331-1338.
29. Gattis WA, Hasselblad V, Whellan DJ, O'Connor CM. Re-
duction in heart failure events by the addition of a clinical
pharmacist to the heart failure management team: results
of the Pharmacist in Heart Failure Assessment Recom-
mendation and Monitoring (PHARM) Study. Arch Intern
Med. 1999;159(16):1939-1945.
30. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Un-
ger A. Effect of a standardized nurse case-management
telephone intervention on resource use in patients with
chronic heart failure. Arch Intern Med. 2002;162(6):705-
712.
31. Krumholz HM, Amatruda J, Smith GL, Mattera JA, Rou-
manis SA, Radford MJ, Crombie P, et al. Randomized
trial of an education and support intervention to prevent
readmission of patients with heart failure. J Am Coll Car-
diol. 2002;39(1):83-89.
32. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P.
Case management in a heterogeneous congestive heart
failure population: a randomized controlled trial. Arch
Intern Med. 2003;163(7):809-817.
33. Tsuyuki RT, Fradette M, Johnson JA, Bungard TJ, Eu-
rich DT, Ashton T, Gordon W, et al. A multicenter disease
management program for hospitalized patients with heart
failure. J Card Fail. 2004;10(6):473-480.
34. DeBusk RF, Miller NH, Parker KM, Bandura A, Kraemer
HC, Cher DJ, West JA, et al. Care management for low-
risk patients with heart failure: a randomized, controlled
trial. Ann Intern Med. 2004;141(8):606-613.
35. Galbreath AD, Krasuski RA, Smith B, Stajduhar KC,
Kwan MD, Ellis R, Freeman GL. Long-term healthcare
and cost outcomes of disease management in a large, ran-
domized, community-based population with heart failure.
Circulation. 2004;110(23):3518-3526.
36. Cleland JG, Louis AA, Rigby AS, Janssens U, Balk
AH. Noninvasive home telemonitoring for patients with
heart failure at high risk of recurrent admission and
death: the Trans-European Network-Home-Care Man-
agement System (TEN-HMS) study. J Am Coll Cardiol.
2005;45(10):1654-1664.
37. Randomised trial of telephone intervention in chronic
heart failure: DIAL trial. BMJ. 2005;331(7514):425.
38. Riegel B, Carlson B, Glaser D, Romero T. Randomized
controlled trial of telephone case management in His-
panics of Mexican origin with heart failure. J Card Fail.
2006;12(3):211-219.
39. Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA,
Wang JJ, Chassin MR. Effects of nurse management on
the quality of heart failure care in minority communities:
a randomized trial. Ann Intern Med. 2006;145(4):273-
283.
40. Mortara A, Pinna GD, Johnson P, Maestri R, Capomolla S,
La Rovere MT, Ponikowski P, et al. Home telemonitoring
in heart failure patients: the HHH study (Home or Hos-
pital in Heart Failure). Eur J Heart Fail. 2009;11(3):312-
318.
41. DeWalt DA, Schillinger D, Ruo B, Bibbins-Domingo K,
Baker DW, Holmes GM, Weinberger M, et al. Multisite
randomized trial of a single-session versus multisession
literacy-sensitive self-care intervention for patients with
heart failure. Circulation. 2012;125(23):2854-2862.
42. Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart
S. Telemonitoring or structured telephone support pro-
grammes for patients with chronic heart failure: systemat-
ic review and meta-analysis. BMJ. 2007;334(7600):942.
43. Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE,
Smith AL, Rodriguez R, et al. Randomized trial of a daily
electronic home monitoring system in patients with ad-
vanced heart failure: the Weight Monitoring in Heart Fail-
ure (WHARF) trial. Am Heart J. 2003;146(4):705-712.
44. Capomolla S, Pinna GD, La Rovere MT, Maestri R, Ce-
resa M, Ferrari M, et al. Heart failure case disease man-
agement program: a pilot study of home telemonitoring
versus usual care. Eur Heart J 2004; 6 (Suppl F): F91-F98.
45. Balk AH, Davidse W, Dommelen P, Klaassen E, Calis-
kan K, van der Burgh P, Leenders CM. Tele-guidance of
chronic heart failure patients enhances knowledge about

Articles © The authors | Journal compilation © Cardiol Res and Elmer Press Inc™ | www.cardiologyres.org 137
Gorthi et al Cardiol Res. 2014;5(5):126-138
the disease. A multi-centre, randomised controlled study.
Eur J Heart Fail. 2008;10(11):1136-1142.
46. Antonicelli R, Testarmata P, Spazzafumo L, Gagliardi
C, Bilo G, Valentini M, Olivieri F, et al. Impact of te-
lemonitoring at home on the management of elderly pa-
tients with congestive heart failure. J Telemed Telecare.
2008;14(6):300-305.
47. Soran OZ, Pina IL, Lamas GA, Kelsey SF, Selzer F, Pi-
lotte J, Lave JR, et al. A randomized clinical trial of the
clinical effects of enhanced heart failure monitoring using
a computer-based telephonic monitoring system in older
minorities and women. J Card Fail. 2008;14(9):711-717.
48. Woodend AK, Sherrard H, Fraser M, Stuewe L, Cheung
T, Struthers C. Telehome monitoring in patients with car-
diac disease who are at high risk of readmission. Heart
Lung. 2008;37(1):36-45.
49. Dar O, Riley J, Chapman C, Dubrey SW, Morris S, Rosen
SD, Roughton M, et al. A randomized trial of home te-
lemonitoring in a typical elderly heart failure population
in North West London: results of the Home-HF study. Eur
J Heart Fail. 2009;11(3):319-325.
50. Giordano A, Scalvini S, Zanelli E, Corra U, Longobardi
GL, Ricci VA, Baiardi P, et al. Multicenter randomised
trial on home-based telemanagement to prevent hospital
readmission of patients with chronic heart failure. Int J
Cardiol. 2009;131(2):192-199.
51. Weintraub A, Gregory D, Patel AR, Levine D, Venesy
D, Perry K, Delano C, et al. A multicenter randomized
controlled evaluation of automated home monitoring and
telephonic disease management in patients recently hos-
pitalized for congestive heart failure: the SPAN-CHF II
trial. J Card Fail. 2010;16(4):285-292.
52. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J,
Lin Z, Phillips CO, et al. Telemonitoring in patients with
heart failure. N Engl J Med. 2010;363(24):2301-2309.
53. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl
K, Bohm M, Boll H, et al. Impact of remote telemedical
management on mortality and hospitalizations in ambula-
tory patients with chronic heart failure: the telemedical
interventional monitoring in heart failure study. Circula-
tion. 2011;123(17):1873-1880.
54. Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C,
Ross HJ. Mobile phone-based telemonitoring for heart
failure management: a randomized controlled trial. J Med
Internet Res. 2012;14(1):e31.
55. Inglis SC, Clark RA, McAlister FA, Ball J, Lewinter
C, Cullington D, Stewart S, et al. Structured telephone
support or telemonitoring programmes for patients with
chronic heart failure. Cochrane Database Syst Rev.
2010;(8):CD007228.
56. Smith SA, Abraham WT. Implantable cardiovascular sen-
sors and computers: interventional heart failure strate-
gies. Curr Cardiol Rep. 2012;14(5):611-618.
57. Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO,
Christensen J, et al. Intrathoracic impedance monitoring
in patients with heart failure: correlation with fluid status
and feasibility of early warning preceding hospitalization.
Circulation. 2005;112(6):841-848.
58. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford
I, Cowie MR, Jondeau G, Kautzner J, et al. Intratho-
racic impedance monitoring, audible patient alerts,
and outcome in patients with heart failure. Circulation.
2011;124(16):1719-1726.
59. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse
B, Yu CM, Cowie MR. Sensitivity and positive predic-
tive value of implantable intrathoracic impedance moni-
toring as a predictor of heart failure hospitalizations: the
SENSE-HF trial. Eur Heart J. 2011;32(18):2266-2273.
60. Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar
S, Porter CB, Pavri BB, et al. Combined heart failure
device diagnostics identify patients at higher risk of
subsequent heart failure hospitalizations: results from
PARTNERS HF (Program to Access and Review Trend-
ing Information and Evaluate Correlation to Symptoms
in Patients With Heart Failure) study. J Am Coll Cardiol.
2010;55(17):1803-1810.
61. Small RS, Wickemeyer W, Germany R, Hoppe B, An-
drulli J, Brady PA, Labeau M, et al. Changes in intratho-
racic impedance are associated with subsequent risk of
hospitalizations for acute decompensated heart failure:
clinical utility of implanted device monitoring without a
patient alert. J Card Fail. 2009;15(6):475-481.
62. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH.
The CONNECT (Clinical Evaluation of Remote Notifica-
tion to Reduce Time to Clinical Decision) trial: the value
of wireless remote monitoring with automatic clinician
alerts. J Am Coll Cardiol. 2011;57(10):1181-1189.
63. Landolina M, Perego GB, Lunati M, Curnis A, Guen-
zati G, Vicentini A, Parati G, et al. Remote monitoring
reduces healthcare use and improves quality of care in
heart failure patients with implantable defibrillators: the
evolution of management strategies of heart failure pa-
tients with implantable defibrillators (EVOLVO) study.
Circulation. 2012;125(24):2985-2992.
64. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Cos-
tanzo MR, Stevenson LW, Strickland W, et al. Wireless
pulmonary artery haemodynamic monitoring in chron-
ic heart failure: a randomised controlled trial. Lancet.
2011;377(9766):658-666.
65. Loh JP, Barbash IM, Waksman R. Overview of the
2011 Food and Drug Administration Circulatory Sys-
tem Devices Panel of the Medical Devices Advisory
Committee Meeting on the CardioMEMS Champion
Heart Failure Monitoring System. J Am Coll Cardiol.
2013;61(15):1571-1576.
66. Bourge RC, Abraham WT, Adamson PB, Aaron MF,
Aranda JM, Jr., Magalski A, Zile MR, et al. Rand-
omized controlled trial of an implantable continuous
hemodynamic monitor in patients with advanced heart
failure: the COMPASS-HF study. J Am Coll Cardiol.
2008;51(11):1073-1079.
67. Adamson PB, Gold MR, Bennett T, Bourge RC, Steven-
son LW, Trupp R, Stromberg K, et al. Continuous hemo-
dynamic monitoring in patients with mild to moderate
heart failure: results of The Reducing Decompensation
Events Utilizing Intracardiac Pressures in Patients With
Gorthi et al Cardiol Res. 2014;5(5):126-138
the disease. A multi-centre, randomised controlled study.
Eur J Heart Fail. 2008;10(11):1136-1142.
46. Antonicelli R, Testarmata P, Spazzafumo L, Gagliardi
C, Bilo G, Valentini M, Olivieri F, et al. Impact of te-
lemonitoring at home on the management of elderly pa-
tients with congestive heart failure. J Telemed Telecare.
2008;14(6):300-305.
47. Soran OZ, Pina IL, Lamas GA, Kelsey SF, Selzer F, Pi-
lotte J, Lave JR, et al. A randomized clinical trial of the
clinical effects of enhanced heart failure monitoring using
a computer-based telephonic monitoring system in older
minorities and women. J Card Fail. 2008;14(9):711-717.
48. Woodend AK, Sherrard H, Fraser M, Stuewe L, Cheung
T, Struthers C. Telehome monitoring in patients with car-
diac disease who are at high risk of readmission. Heart
Lung. 2008;37(1):36-45.
49. Dar O, Riley J, Chapman C, Dubrey SW, Morris S, Rosen
SD, Roughton M, et al. A randomized trial of home te-
lemonitoring in a typical elderly heart failure population
in North West London: results of the Home-HF study. Eur
J Heart Fail. 2009;11(3):319-325.
50. Giordano A, Scalvini S, Zanelli E, Corra U, Longobardi
GL, Ricci VA, Baiardi P, et al. Multicenter randomised
trial on home-based telemanagement to prevent hospital
readmission of patients with chronic heart failure. Int J
Cardiol. 2009;131(2):192-199.
51. Weintraub A, Gregory D, Patel AR, Levine D, Venesy
D, Perry K, Delano C, et al. A multicenter randomized
controlled evaluation of automated home monitoring and
telephonic disease management in patients recently hos-
pitalized for congestive heart failure: the SPAN-CHF II
trial. J Card Fail. 2010;16(4):285-292.
52. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J,
Lin Z, Phillips CO, et al. Telemonitoring in patients with
heart failure. N Engl J Med. 2010;363(24):2301-2309.
53. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl
K, Bohm M, Boll H, et al. Impact of remote telemedical
management on mortality and hospitalizations in ambula-
tory patients with chronic heart failure: the telemedical
interventional monitoring in heart failure study. Circula-
tion. 2011;123(17):1873-1880.
54. Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C,
Ross HJ. Mobile phone-based telemonitoring for heart
failure management: a randomized controlled trial. J Med
Internet Res. 2012;14(1):e31.
55. Inglis SC, Clark RA, McAlister FA, Ball J, Lewinter
C, Cullington D, Stewart S, et al. Structured telephone
support or telemonitoring programmes for patients with
chronic heart failure. Cochrane Database Syst Rev.
2010;(8):CD007228.
56. Smith SA, Abraham WT. Implantable cardiovascular sen-
sors and computers: interventional heart failure strate-
gies. Curr Cardiol Rep. 2012;14(5):611-618.
57. Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO,
Christensen J, et al. Intrathoracic impedance monitoring
in patients with heart failure: correlation with fluid status
and feasibility of early warning preceding hospitalization.
Circulation. 2005;112(6):841-848.
58. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford
I, Cowie MR, Jondeau G, Kautzner J, et al. Intratho-
racic impedance monitoring, audible patient alerts,
and outcome in patients with heart failure. Circulation.
2011;124(16):1719-1726.
59. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse
B, Yu CM, Cowie MR. Sensitivity and positive predic-
tive value of implantable intrathoracic impedance moni-
toring as a predictor of heart failure hospitalizations: the
SENSE-HF trial. Eur Heart J. 2011;32(18):2266-2273.
60. Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar
S, Porter CB, Pavri BB, et al. Combined heart failure
device diagnostics identify patients at higher risk of
subsequent heart failure hospitalizations: results from
PARTNERS HF (Program to Access and Review Trend-
ing Information and Evaluate Correlation to Symptoms
in Patients With Heart Failure) study. J Am Coll Cardiol.
2010;55(17):1803-1810.
61. Small RS, Wickemeyer W, Germany R, Hoppe B, An-
drulli J, Brady PA, Labeau M, et al. Changes in intratho-
racic impedance are associated with subsequent risk of
hospitalizations for acute decompensated heart failure:
clinical utility of implanted device monitoring without a
patient alert. J Card Fail. 2009;15(6):475-481.
62. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH.
The CONNECT (Clinical Evaluation of Remote Notifica-
tion to Reduce Time to Clinical Decision) trial: the value
of wireless remote monitoring with automatic clinician
alerts. J Am Coll Cardiol. 2011;57(10):1181-1189.
63. Landolina M, Perego GB, Lunati M, Curnis A, Guen-
zati G, Vicentini A, Parati G, et al. Remote monitoring
reduces healthcare use and improves quality of care in
heart failure patients with implantable defibrillators: the
evolution of management strategies of heart failure pa-
tients with implantable defibrillators (EVOLVO) study.
Circulation. 2012;125(24):2985-2992.
64. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Cos-
tanzo MR, Stevenson LW, Strickland W, et al. Wireless
pulmonary artery haemodynamic monitoring in chron-
ic heart failure: a randomised controlled trial. Lancet.
2011;377(9766):658-666.
65. Loh JP, Barbash IM, Waksman R. Overview of the
2011 Food and Drug Administration Circulatory Sys-
tem Devices Panel of the Medical Devices Advisory
Committee Meeting on the CardioMEMS Champion
Heart Failure Monitoring System. J Am Coll Cardiol.
2013;61(15):1571-1576.
66. Bourge RC, Abraham WT, Adamson PB, Aaron MF,
Aranda JM, Jr., Magalski A, Zile MR, et al. Rand-
omized controlled trial of an implantable continuous
hemodynamic monitor in patients with advanced heart
failure: the COMPASS-HF study. J Am Coll Cardiol.
2008;51(11):1073-1079.
67. Adamson PB, Gold MR, Bennett T, Bourge RC, Steven-
son LW, Trupp R, Stromberg K, et al. Continuous hemo-
dynamic monitoring in patients with mild to moderate
heart failure: results of The Reducing Decompensation
Events Utilizing Intracardiac Pressures in Patients With
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





