Reference Pricing on Pharmaceutical Pricing Policy
VerifiedAdded on 2023/06/04
|17
|4693
|345
AI Summary
This proposal aims to ensure reference pricing is used in the overall pharmaceutical policy pricing to determine the price of generics thereby making it affordable to patients by reducing expenditure. The study will review pharmaceutical policies of five countries and collect data through worldwide web, interviews and questionnaires. The report will answer research questions on challenges encountered by health insurers and firms, effects of reference policy on the pharmaceutical market, approach used in determining the value of new medicine, and role of reference pricing in determining the prices to be used.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

REFERENCE PRICING ON PHARMACEUTICAL PRICING
POLICY.
1 .BACKGROUND
Pharmaceutical policy deals with provision of medicine and health care
facilities within a medical institution. Vaccines, drugs and natural health products
are mainly used for the treatement of diseases and ailments and these are produced
by the pharmaceutical companies and informed by health researches. After a health
research has been done on causes of diseases, the results are then taken to
pharmaceutical companies for drug development in relation to the discovered
disease. In many countries drug prices are regulated to reasonable prices so that
can be afforded by most people. Drug manufacturers submit a proposed price of
the drug to the relevant authority based on which the actual price of the
pharmaceutical product is determined. Pharmaceutical pricing and reimbursement
policies have contributed immensely to development of health policies and
influced the healthcare delivery strategies (Vogler, 2012; Paris & Docteur, 2007).
Price regulation differs from country to country, some countries use very similar
instruments such as international benchmarking (according to what other countries
pay), therapeutic referencing (pricing according to what competitors are charging)
and economic evaluation which are often used to determine pricing or
reimbursement of pharmaceutical products (Vogler, 2012; Acosta et al., 2014;
Branstetter, Chatterjee & Higgins, 2016).
Pricing policies can have variety of effects within a country. First effect of
reference pricing is a change in the market of the drug due to regulation which
leads to significant price reductions on both branded drugs as well as generic drugs
(Lanjouw, 2005; Branstetter, Chatterjee & Higgins, 2016). The second effect of
reference pricing is an impact of the price of medicine on the ability to avail other
healthcare services. According to authors, when large amount of money is used to
acquire drugs and medicines, the funds that are put aside for important healthcare
services such as prenatal care or non healthcare services such as developmental
services can get more restricted (Vogler, 2012; Acosta et al., 2014; Lanjouw, 2005;
Danzon & Epstein, 2008).
POLICY.
1 .BACKGROUND
Pharmaceutical policy deals with provision of medicine and health care
facilities within a medical institution. Vaccines, drugs and natural health products
are mainly used for the treatement of diseases and ailments and these are produced
by the pharmaceutical companies and informed by health researches. After a health
research has been done on causes of diseases, the results are then taken to
pharmaceutical companies for drug development in relation to the discovered
disease. In many countries drug prices are regulated to reasonable prices so that
can be afforded by most people. Drug manufacturers submit a proposed price of
the drug to the relevant authority based on which the actual price of the
pharmaceutical product is determined. Pharmaceutical pricing and reimbursement
policies have contributed immensely to development of health policies and
influced the healthcare delivery strategies (Vogler, 2012; Paris & Docteur, 2007).
Price regulation differs from country to country, some countries use very similar
instruments such as international benchmarking (according to what other countries
pay), therapeutic referencing (pricing according to what competitors are charging)
and economic evaluation which are often used to determine pricing or
reimbursement of pharmaceutical products (Vogler, 2012; Acosta et al., 2014;
Branstetter, Chatterjee & Higgins, 2016).
Pricing policies can have variety of effects within a country. First effect of
reference pricing is a change in the market of the drug due to regulation which
leads to significant price reductions on both branded drugs as well as generic drugs
(Lanjouw, 2005; Branstetter, Chatterjee & Higgins, 2016). The second effect of
reference pricing is an impact of the price of medicine on the ability to avail other
healthcare services. According to authors, when large amount of money is used to
acquire drugs and medicines, the funds that are put aside for important healthcare
services such as prenatal care or non healthcare services such as developmental
services can get more restricted (Vogler, 2012; Acosta et al., 2014; Lanjouw, 2005;
Danzon & Epstein, 2008).
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

López-Casasnovas and Puig-Junoy (2000) stated that health insurers and
pharmacists are now looking for alternative means on how to reduce costs of
purchasing medicine. They utilize various approaches to reduce the cost of
medicines such as reference pricing. In this process, they group together different
medicines that perform the same function and put a reference price to it (Danzon
and Furukawa 2008). If patient does not choose one of the drugs listed in the
group, then he or she will have to pay the difference in the amount (between the
price on the reference list and the price of the medicine purchased by the patient).
Theoretical result is also important due to the consistency in the findings thus it
provides an explanation to several previous empirical findings on reference
pricing. López-Casasnovas and Puig-Junoy (2000) further states that there is
enoughevidence which suggests reference pricing successfully reduces medicine
prices and overall expenditures of the pharmacy in the short-term . Prices are more
or less affordable where generics and substitutes have more market control and
power as compared to the other medicines. There has not been a negative effect
among patients who exchanged one drug for a generic one as per many studies.
However, it has been pointed out by some authors that there may also be negative
effect of referencing pricing on pharmaceutical products, as it could lead to lack of
incentives for pharmaceutical innovation (Lanjouw, 2005). Additionally there is
also uncertainity of the results of this research since very few studies have been
done on this topic.
Galizzi, Ghislandi and Miraldo (2011) stated that reference pricing has led to
reductions in prices of medicines which has made pharmacists to increase
reference medicine prescriptions which has ultimately led to a decrease in
prescriptions for more expensive medicines since most patients opt for medications
that are more affordable, thereby mainly opting for reference medicines. Moreover,
there is also decreased expenditure by insurers for purchasing medicines since they
mostly use generics which is cheaper than branded medicines (Walker, 1989).
There is also use of external reference pricing, or international price
comparison to determine the price of pharmaceutical products.This is used to
control prices of medicines charged by pharmaceuticals that are in a way protected
by intellectual property righs and may have legally monopolized the business.
These pharmaceutical companies may deal with patent drugs which is used in
different countries. Kanavos and Reinhardt (2003) stated that reference prices are
pharmacists are now looking for alternative means on how to reduce costs of
purchasing medicine. They utilize various approaches to reduce the cost of
medicines such as reference pricing. In this process, they group together different
medicines that perform the same function and put a reference price to it (Danzon
and Furukawa 2008). If patient does not choose one of the drugs listed in the
group, then he or she will have to pay the difference in the amount (between the
price on the reference list and the price of the medicine purchased by the patient).
Theoretical result is also important due to the consistency in the findings thus it
provides an explanation to several previous empirical findings on reference
pricing. López-Casasnovas and Puig-Junoy (2000) further states that there is
enoughevidence which suggests reference pricing successfully reduces medicine
prices and overall expenditures of the pharmacy in the short-term . Prices are more
or less affordable where generics and substitutes have more market control and
power as compared to the other medicines. There has not been a negative effect
among patients who exchanged one drug for a generic one as per many studies.
However, it has been pointed out by some authors that there may also be negative
effect of referencing pricing on pharmaceutical products, as it could lead to lack of
incentives for pharmaceutical innovation (Lanjouw, 2005). Additionally there is
also uncertainity of the results of this research since very few studies have been
done on this topic.
Galizzi, Ghislandi and Miraldo (2011) stated that reference pricing has led to
reductions in prices of medicines which has made pharmacists to increase
reference medicine prescriptions which has ultimately led to a decrease in
prescriptions for more expensive medicines since most patients opt for medications
that are more affordable, thereby mainly opting for reference medicines. Moreover,
there is also decreased expenditure by insurers for purchasing medicines since they
mostly use generics which is cheaper than branded medicines (Walker, 1989).
There is also use of external reference pricing, or international price
comparison to determine the price of pharmaceutical products.This is used to
control prices of medicines charged by pharmaceuticals that are in a way protected
by intellectual property righs and may have legally monopolized the business.
These pharmaceutical companies may deal with patent drugs which is used in
different countries. Kanavos and Reinhardt (2003) stated that reference prices are

usually applied for medicines which are generally making a new entry in the
market (new pharmaceutical products) and is later revised. Since 2011, all new
medicine that is licenced are subject to early assessment for quality, which forms a
basis for determining the prices of the new generic product (Von der Schulenburg,
Vandoros & Kanavos, 2011). Different countries use different algorithms to
calculate the reference price, for example, in France only 8% of drugs introduced
between 2007 and 2011 had external references made. This lead to variations in
drug price within the countries thus making it difficult for the pricing details to
remain clear and thus difficult to determine the actual price of the drugs. Thus it
has been pointed out that there should be transparency in the prices for the pricing
strategy to work effectively (Vogler, 2012).
Giuliani, Selke and Garattini (1998) stated that the only exception of this
rule is in Germany where the agency responsible for determining the value of
pharmaceutical products is located within the ministry of health, which means the
ultimate decision on the price of a drug is determined is decided by an inter
department committee within the ministry of health that has representatives from
health industry and social policy equality ministries (Walker, 1989).
1.2 Aims and objectives.
This proposal aims to ensure reference pricing is used in the overall
pharmaceutical policy pricing to determine the price of generics thereby making it
affordable to patients by reducing expenditure ( López-Casasnovas and Puig-
Junoy, 2000; Aaserud et al., 2006; Galizzi et al., 2011). This is done by looking at
the pharmaceutical prices of different countries. Thus the main objectives of this
study includes:
1) To know how pharmaceuticals pricing policies are brought about, applied and
implemented.
2) How the value of medicine is determined and how are other medicines
expensive while generic are so affordable?
3) To know the Impact of reference pricing on health of patients and the economic
growth
4) To identify opportunities and obstacles faced by reference pricing strategy
market (new pharmaceutical products) and is later revised. Since 2011, all new
medicine that is licenced are subject to early assessment for quality, which forms a
basis for determining the prices of the new generic product (Von der Schulenburg,
Vandoros & Kanavos, 2011). Different countries use different algorithms to
calculate the reference price, for example, in France only 8% of drugs introduced
between 2007 and 2011 had external references made. This lead to variations in
drug price within the countries thus making it difficult for the pricing details to
remain clear and thus difficult to determine the actual price of the drugs. Thus it
has been pointed out that there should be transparency in the prices for the pricing
strategy to work effectively (Vogler, 2012).
Giuliani, Selke and Garattini (1998) stated that the only exception of this
rule is in Germany where the agency responsible for determining the value of
pharmaceutical products is located within the ministry of health, which means the
ultimate decision on the price of a drug is determined is decided by an inter
department committee within the ministry of health that has representatives from
health industry and social policy equality ministries (Walker, 1989).
1.2 Aims and objectives.
This proposal aims to ensure reference pricing is used in the overall
pharmaceutical policy pricing to determine the price of generics thereby making it
affordable to patients by reducing expenditure ( López-Casasnovas and Puig-
Junoy, 2000; Aaserud et al., 2006; Galizzi et al., 2011). This is done by looking at
the pharmaceutical prices of different countries. Thus the main objectives of this
study includes:
1) To know how pharmaceuticals pricing policies are brought about, applied and
implemented.
2) How the value of medicine is determined and how are other medicines
expensive while generic are so affordable?
3) To know the Impact of reference pricing on health of patients and the economic
growth
4) To identify opportunities and obstacles faced by reference pricing strategy

5) To help health insurers know the best way they can reduce cost incurred.
1.3 Research questions
1) What are the challenges encountered by health insurers and firms in the use of
reference pricing policy and its effect on them and their patients?
2) What are the effects of reference policy on the pharmaceutical market?
3) What approach is used in determining the value of a new medicine?
4) Role of reference pricing in the determination of drug prices to be used.
1.4 proposed action plan
This report will be based on five countries namely UK, Italy, France,
Germany and Australia. The study will review their pharmaceutical policies as
these countries drugs have a strong pharmaceutical market strength. Galizzi,
Ghislandi and Miraldo (2011) studied the role reference pricing has played in
determining the price of the generics. Key informants used would be the health
insurers, patients and pharmaceutical experts (Galizzi et al., 2011; Kanavos et al.,
2003). Data will be collected through world wide web, interviews and
questionnaires. The experts are then called and invited for the interviews and to
answer the questionnaires. The questionnaire was designed to collect data on effect
of reference pricing on pharmaceutical pricing policies, the methods of
determining the price of the drugs, how the value is determined and the effect of
the reference policy on pharmaceutical market and the challenges encountered by
health insurers and firms as they use reference pricing policy in the countries being
studied. Sample size estimates were, Australia 9.7 percent, Germany 17.5 percent,
UK at 8.9 percent, Italy at 13 percent and France at 17.9 percent (Kyle, 2007;
Aaserud et al., 2006).
1.5 re-aim framework
1.3 Research questions
1) What are the challenges encountered by health insurers and firms in the use of
reference pricing policy and its effect on them and their patients?
2) What are the effects of reference policy on the pharmaceutical market?
3) What approach is used in determining the value of a new medicine?
4) Role of reference pricing in the determination of drug prices to be used.
1.4 proposed action plan
This report will be based on five countries namely UK, Italy, France,
Germany and Australia. The study will review their pharmaceutical policies as
these countries drugs have a strong pharmaceutical market strength. Galizzi,
Ghislandi and Miraldo (2011) studied the role reference pricing has played in
determining the price of the generics. Key informants used would be the health
insurers, patients and pharmaceutical experts (Galizzi et al., 2011; Kanavos et al.,
2003). Data will be collected through world wide web, interviews and
questionnaires. The experts are then called and invited for the interviews and to
answer the questionnaires. The questionnaire was designed to collect data on effect
of reference pricing on pharmaceutical pricing policies, the methods of
determining the price of the drugs, how the value is determined and the effect of
the reference policy on pharmaceutical market and the challenges encountered by
health insurers and firms as they use reference pricing policy in the countries being
studied. Sample size estimates were, Australia 9.7 percent, Germany 17.5 percent,
UK at 8.9 percent, Italy at 13 percent and France at 17.9 percent (Kyle, 2007;
Aaserud et al., 2006).
1.5 re-aim framework
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

The five countries being studied here have different policies and drug
pricing (Lee et al., 2012). The major source of finance in these countries is the
health insurance since most of the citizens have the insurance and they pay it
monthly. Kyle (2007) stated that each country has a different pharmaceutical
pricing policy which creates a difference in prices being charged for drugs in each
of the mentioned countries in accordance to the policies adopted by the countries
under review using reference pricing to determine the price of the drugs. It should
however be also noted that prices of drugs not prescribed are not regulated by the
pricing policies (Danzon & Ketcham 2004; Russell & Wolff 2002).
2) proceed model.
2.1 study design
The study used the following designs:
1) non randomized trial
2) interrupted time (IT) study
3)randomized trial
4) controlled before after (CBA)study
5) repeated measures (RM) study
The methods will be used to answer the research questions as follows:
1) What are the challenges encountered by health insurers and firms as they use
reference pricing policy and its effect on them and their patients?2,4,1
2) What are the effects of reference policy on the pharmaceutical market?4,1
3) What approach is used in determining the value of new medicine?3,4
4) Role of reference pricing in determining the prices to be used.1,5
2.2 Study population
Acosta et al. (2014) conducted a research on different pharmacies in their
countries and how they use reference pricing for different levels of drug
pricing (Lee et al., 2012). The major source of finance in these countries is the
health insurance since most of the citizens have the insurance and they pay it
monthly. Kyle (2007) stated that each country has a different pharmaceutical
pricing policy which creates a difference in prices being charged for drugs in each
of the mentioned countries in accordance to the policies adopted by the countries
under review using reference pricing to determine the price of the drugs. It should
however be also noted that prices of drugs not prescribed are not regulated by the
pricing policies (Danzon & Ketcham 2004; Russell & Wolff 2002).
2) proceed model.
2.1 study design
The study used the following designs:
1) non randomized trial
2) interrupted time (IT) study
3)randomized trial
4) controlled before after (CBA)study
5) repeated measures (RM) study
The methods will be used to answer the research questions as follows:
1) What are the challenges encountered by health insurers and firms as they use
reference pricing policy and its effect on them and their patients?2,4,1
2) What are the effects of reference policy on the pharmaceutical market?4,1
3) What approach is used in determining the value of new medicine?3,4
4) Role of reference pricing in determining the prices to be used.1,5
2.2 Study population
Acosta et al. (2014) conducted a research on different pharmacies in their
countries and how they use reference pricing for different levels of drug
groups.The country samples will include some first world countries (Brekke et al.
2009). The Department of Health have helped in deciding the countries to be
chosen by offering helpful guidance. Countries are selected in accordance to the
size of their pharmaceutical market (Danzon and Ketcham 2004).
Operational definition of generics: Acosta et al. (2014) conducted a study to
look at motivations for generic substitution at pharmaceutical level. We look at the
positive sales margin that they are forced to encounter that is lower than the
reference pricing when they sell generics together with the negative sales margin
of selling a drug that costs more than the reference pricing (Vogler, 2012). In
accordance tostudy population which includes both the pharmacies and the
patients, the following table shows the outline of methods and target population:
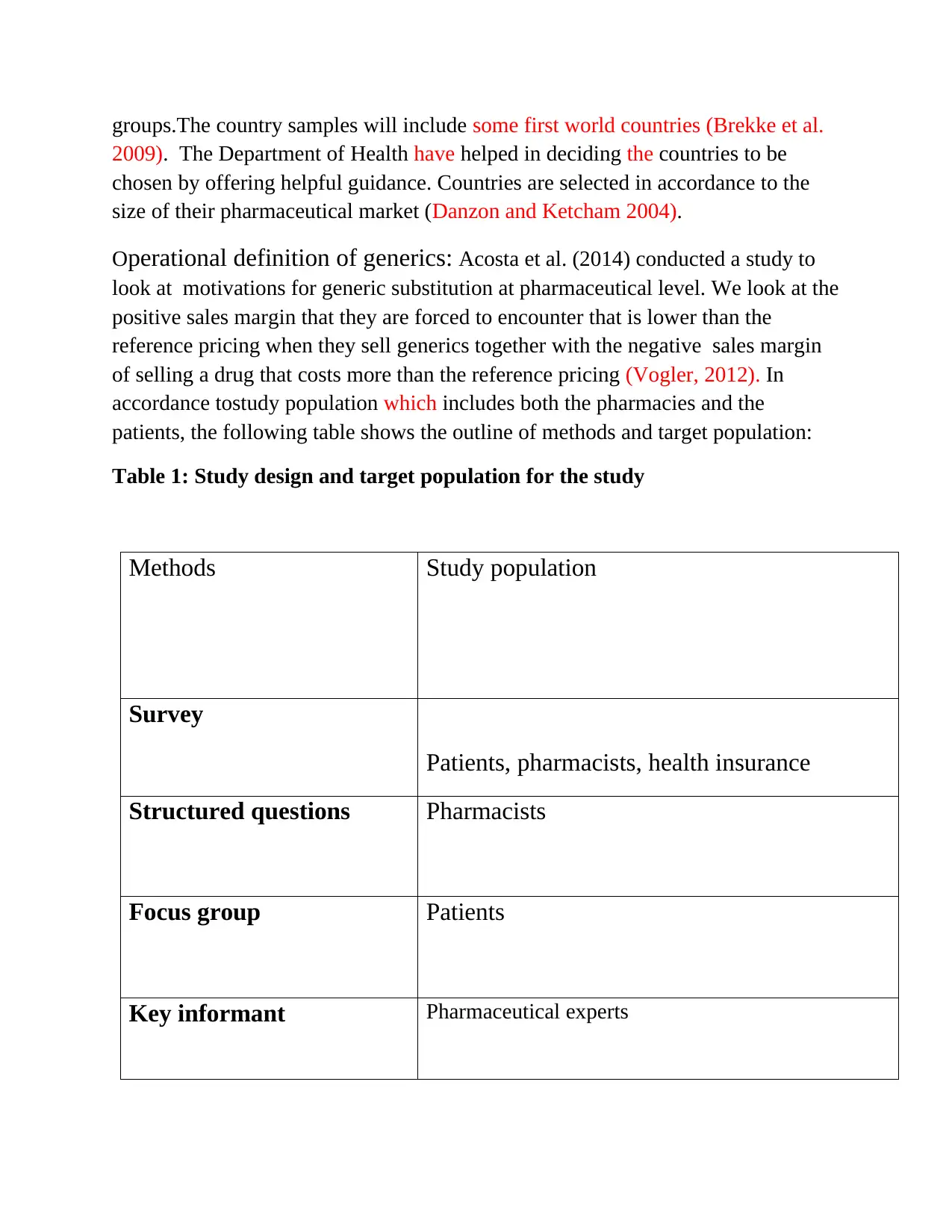
Table 1: Study design and target population for the study
Methods Study population
Survey
Patients, pharmacists, health insurance
Structured questions Pharmacists
Focus group Patients
Key informant Pharmaceutical experts
2009). The Department of Health have helped in deciding the countries to be
chosen by offering helpful guidance. Countries are selected in accordance to the
size of their pharmaceutical market (Danzon and Ketcham 2004).
Operational definition of generics: Acosta et al. (2014) conducted a study to
look at motivations for generic substitution at pharmaceutical level. We look at the
positive sales margin that they are forced to encounter that is lower than the
reference pricing when they sell generics together with the negative sales margin
of selling a drug that costs more than the reference pricing (Vogler, 2012). In
accordance tostudy population which includes both the pharmacies and the
patients, the following table shows the outline of methods and target population:
Table 1: Study design and target population for the study
Methods Study population
Survey
Patients, pharmacists, health insurance
Structured questions Pharmacists
Focus group Patients
Key informant Pharmaceutical experts
2.3 Sampling
The sample was taken from five countries namely Germany, Spain, UK,
Australia and France.
2.3.1 first world sample
The sample is determined according to the countries strength in the
pharmaceutical market. The major key informants were experts involved and who
contribute to the work done by OECD on pharmaceutical pricing policies
(Kanavos, 2001). They were officially invited on personal interviews and to
undertake well detailed questionnaires. These questionnaires helped collect data on
the methods used in pricing of pharmaceutical products under the countries being
reviewed, strategies being employed, approaches to value determination and the
role of external reference pricing.
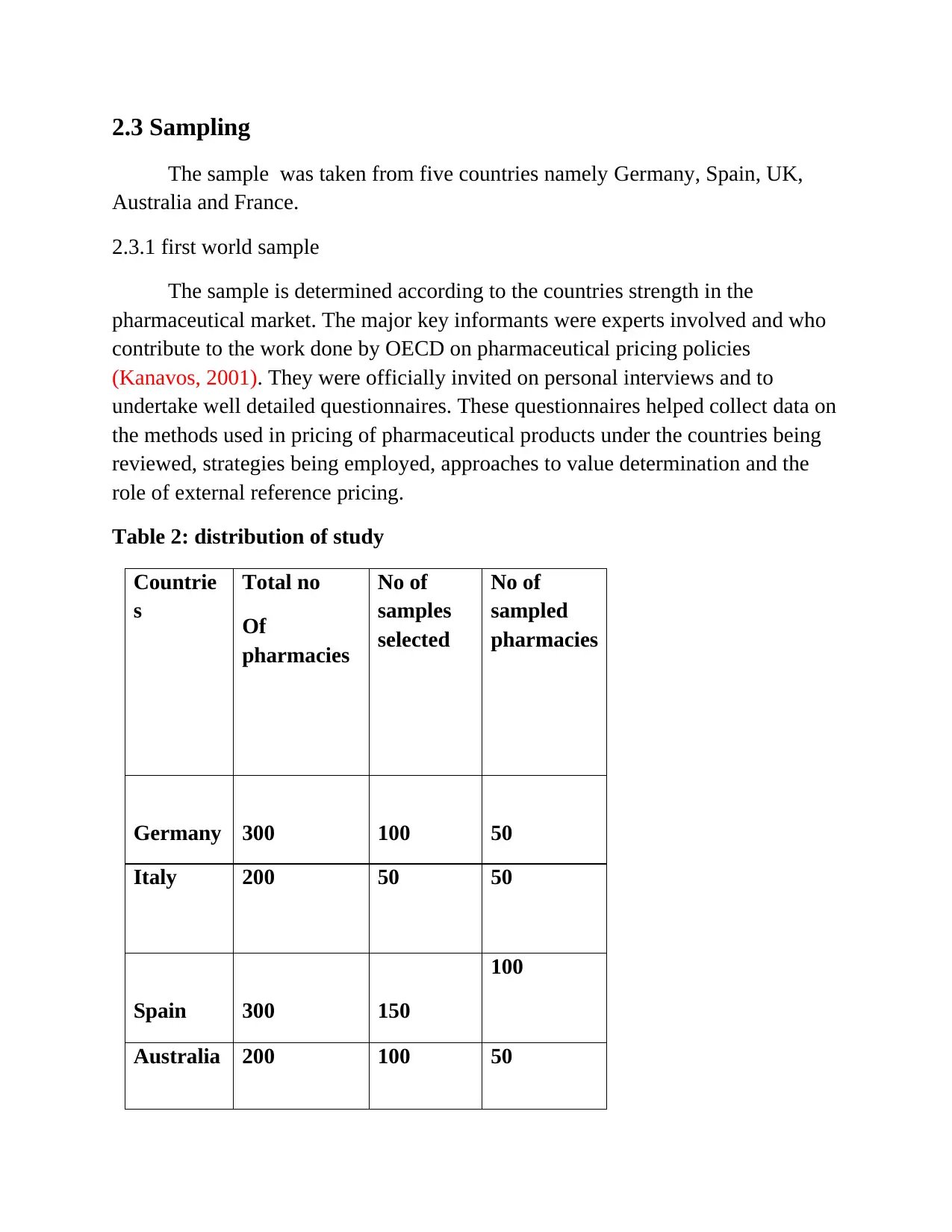
Table 2: distribution of study
Countrie
s
Total no
Of
pharmacies
No of
samples
selected
No of
sampled
pharmacies
Germany 300 100 50
Italy 200 50 50
Spain 300 150
100
Australia 200 100 50
The sample was taken from five countries namely Germany, Spain, UK,
Australia and France.
2.3.1 first world sample
The sample is determined according to the countries strength in the
pharmaceutical market. The major key informants were experts involved and who
contribute to the work done by OECD on pharmaceutical pricing policies
(Kanavos, 2001). They were officially invited on personal interviews and to
undertake well detailed questionnaires. These questionnaires helped collect data on
the methods used in pricing of pharmaceutical products under the countries being
reviewed, strategies being employed, approaches to value determination and the
role of external reference pricing.
Table 2: distribution of study
Countrie
s
Total no
Of
pharmacies
No of
samples
selected
No of
sampled
pharmacies
Germany 300 100 50
Italy 200 50 50
Spain 300 150
100
Australia 200 100 50
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
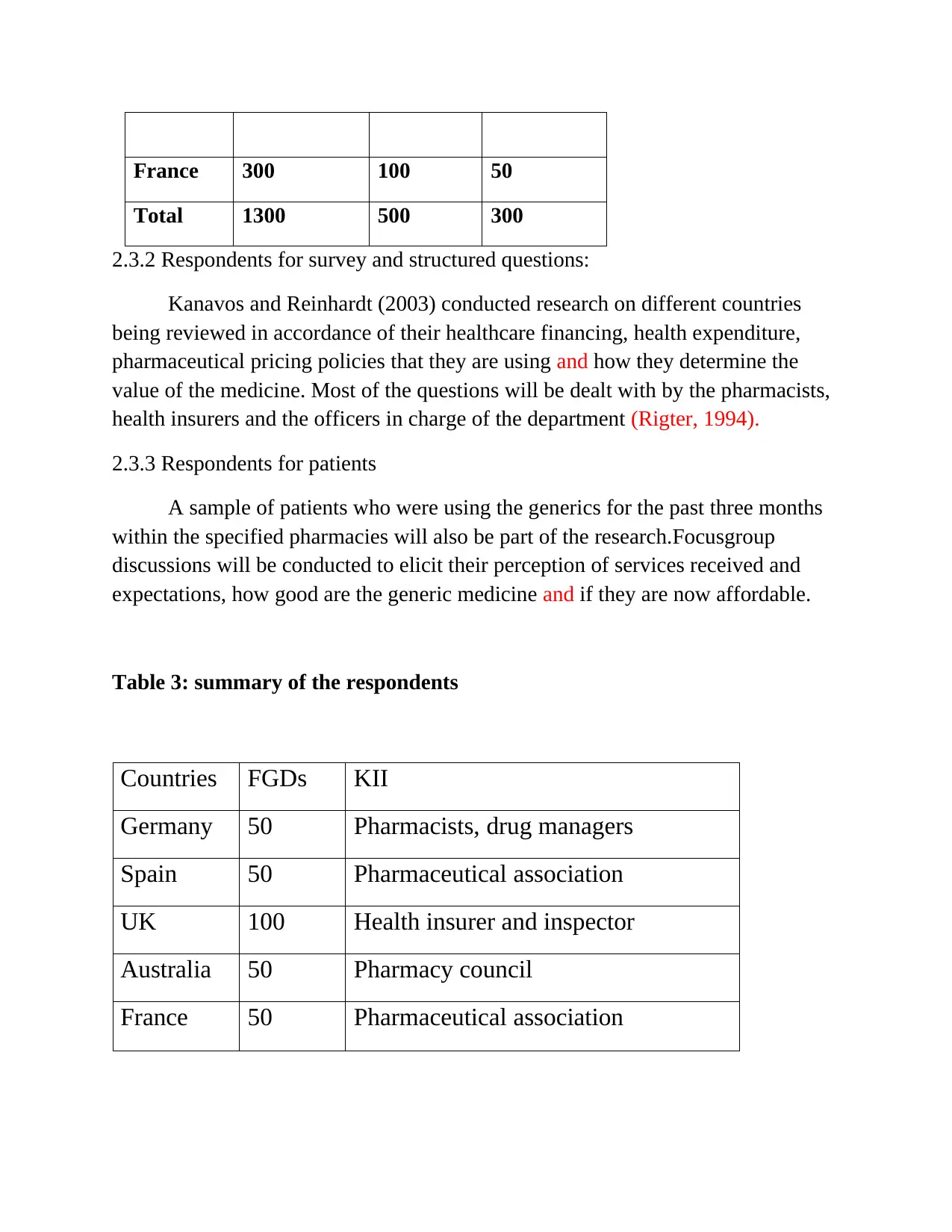
France 300 100 50
Total 1300 500 300
2.3.2 Respondents for survey and structured questions:
Kanavos and Reinhardt (2003) conducted research on different countries
being reviewed in accordance of their healthcare financing, health expenditure,
pharmaceutical pricing policies that they are using and how they determine the
value of the medicine. Most of the questions will be dealt with by the pharmacists,
health insurers and the officers in charge of the department (Rigter, 1994).
2.3.3 Respondents for patients
A sample of patients who were using the generics for the past three months
within the specified pharmacies will also be part of the research.Focusgroup
discussions will be conducted to elicit their perception of services received and
expectations, how good are the generic medicine and if they are now affordable.
Table 3: summary of the respondents
Countries FGDs KII
Germany 50 Pharmacists, drug managers
Spain 50 Pharmaceutical association
UK 100 Health insurer and inspector
Australia 50 Pharmacy council
France 50 Pharmaceutical association
Total 1300 500 300
2.3.2 Respondents for survey and structured questions:
Kanavos and Reinhardt (2003) conducted research on different countries
being reviewed in accordance of their healthcare financing, health expenditure,
pharmaceutical pricing policies that they are using and how they determine the
value of the medicine. Most of the questions will be dealt with by the pharmacists,
health insurers and the officers in charge of the department (Rigter, 1994).
2.3.3 Respondents for patients
A sample of patients who were using the generics for the past three months
within the specified pharmacies will also be part of the research.Focusgroup
discussions will be conducted to elicit their perception of services received and
expectations, how good are the generic medicine and if they are now affordable.
Table 3: summary of the respondents
Countries FGDs KII
Germany 50 Pharmacists, drug managers
Spain 50 Pharmaceutical association
UK 100 Health insurer and inspector
Australia 50 Pharmacy council
France 50 Pharmaceutical association

Total 300
2.4data collection
Data collection activities involved in the review of publications, journals,
online literature as identified in the world wideweb through search engines like
Google (López-Casasnovas & Puig-Junoy, 2000).
Tools that were used for data collection will include survey questionnaires
and direct personal interviews, will be developed by adopting tools used by
pubMed which is a repository of several pharmaceutical journals. A list of key
essential generic drugs will be used as a reference point for common diseases
based on the essential drugs list as per the government approval, which will be
prepared for the purpose of this study. This reference list will be used to check
whether the key essential drugs for common diseases are available, whether their
price is clearly mentioned, as well as the quality, manufacturing date, the expiry
date, packaging and storing of the drug which will be assessed during the checklist
guidelines (Lewis, 2015; Fusch & Ness, 2015).
The Research Assistants, will then help in the survey after undergoing
training. The everyday field activities during research of the teams involved will
be inspected by the chosen team leaders of every team and the assigned
supervisors (Caretta & Cheptum 2017). The entire survey exercise will be
controlled, supervised, inspected and managed by field supervisors,who will make
frequent and impromptu field visits and provide help and guidance when needed.
The field exercise and activityand the consequent data entry is expected to be
completed within 45 working days.
2.5 ethical clearance
The study will be submitted to the ethical review board of pharmaceutical
pricing policies and the university for ethics approval. Data from the pharmacists
and patients will be taken after their consent before the interview is conducted
(Mayan, 2016).
2.4data collection
Data collection activities involved in the review of publications, journals,
online literature as identified in the world wideweb through search engines like
Google (López-Casasnovas & Puig-Junoy, 2000).
Tools that were used for data collection will include survey questionnaires
and direct personal interviews, will be developed by adopting tools used by
pubMed which is a repository of several pharmaceutical journals. A list of key
essential generic drugs will be used as a reference point for common diseases
based on the essential drugs list as per the government approval, which will be
prepared for the purpose of this study. This reference list will be used to check
whether the key essential drugs for common diseases are available, whether their
price is clearly mentioned, as well as the quality, manufacturing date, the expiry
date, packaging and storing of the drug which will be assessed during the checklist
guidelines (Lewis, 2015; Fusch & Ness, 2015).
The Research Assistants, will then help in the survey after undergoing
training. The everyday field activities during research of the teams involved will
be inspected by the chosen team leaders of every team and the assigned
supervisors (Caretta & Cheptum 2017). The entire survey exercise will be
controlled, supervised, inspected and managed by field supervisors,who will make
frequent and impromptu field visits and provide help and guidance when needed.
The field exercise and activityand the consequent data entry is expected to be
completed within 45 working days.
2.5 ethical clearance
The study will be submitted to the ethical review board of pharmaceutical
pricing policies and the university for ethics approval. Data from the pharmacists
and patients will be taken after their consent before the interview is conducted
(Mayan, 2016).

2.6 Quality assurance of data
In order to ensure the data collected is accurate and its quality is
maintained,the research assistants will have to cross examine each and every
questionnaire. They will have to record the interviews to ensure there is no loss of
information. In case of inconsistencies, the research team will have to re interview
together with field supervisors to ensure quality is upheld (Dingwall et al., 2017).
After data collection is completed there will be a debriefing between the research
analysts and field supervisors to record their feedbacks and findings.
2.7.Data management and analysis
Survey data will be entered through Microsoft excel. After all the
questionnaires have been collected, the data will then be put in excel for survey.
Upon collection of all surveyed questionnaire, data will be entered into the MS
Access database. Data management will then be done. The data will be quality
controlled by re entering Ten percent of the total data. The project coordinator will
closely coordinate with the field supervisors regarding any issues related to data
entry. The research team develops a codebook which will be used strictly during
the data entry. The project coordinator and the PI also will do overall supervision
of the data entry and data management which ensures the data collected has been
upheld in terms of quality and its validity (Nassaji 2015).
Upon completion of data entry, the data will be transferred into SPSS for
Windows where the data will be analyzed. The demographic will be presented in
form of means, frequency and standard deviation.
2.8 strategies to evaluate impact
I will assess if the promotion of reference pricing has worked by evaluating
the data collected. If most of the patients are now using generics that are more
affordable then it shows that the promotion has worked. I would evaluate by
making sure the research has reached the appropriate target and that the answers
given are accurate according to the questions asked in interviews and
questionnaires. I will ensure that the method of implementation is the most cost
effective and easily adaptable to pharmacists and can be implemented in the
pharmaceutical policies with no problem (Lewis, 2015).
In order to ensure the data collected is accurate and its quality is
maintained,the research assistants will have to cross examine each and every
questionnaire. They will have to record the interviews to ensure there is no loss of
information. In case of inconsistencies, the research team will have to re interview
together with field supervisors to ensure quality is upheld (Dingwall et al., 2017).
After data collection is completed there will be a debriefing between the research
analysts and field supervisors to record their feedbacks and findings.
2.7.Data management and analysis
Survey data will be entered through Microsoft excel. After all the
questionnaires have been collected, the data will then be put in excel for survey.
Upon collection of all surveyed questionnaire, data will be entered into the MS
Access database. Data management will then be done. The data will be quality
controlled by re entering Ten percent of the total data. The project coordinator will
closely coordinate with the field supervisors regarding any issues related to data
entry. The research team develops a codebook which will be used strictly during
the data entry. The project coordinator and the PI also will do overall supervision
of the data entry and data management which ensures the data collected has been
upheld in terms of quality and its validity (Nassaji 2015).
Upon completion of data entry, the data will be transferred into SPSS for
Windows where the data will be analyzed. The demographic will be presented in
form of means, frequency and standard deviation.
2.8 strategies to evaluate impact
I will assess if the promotion of reference pricing has worked by evaluating
the data collected. If most of the patients are now using generics that are more
affordable then it shows that the promotion has worked. I would evaluate by
making sure the research has reached the appropriate target and that the answers
given are accurate according to the questions asked in interviews and
questionnaires. I will ensure that the method of implementation is the most cost
effective and easily adaptable to pharmacists and can be implemented in the
pharmaceutical policies with no problem (Lewis, 2015).
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

2.9 anticipated outcomes and significance.
Reference pricing is very important since most people who suffer from
diseases may not have a good insurance cover. This may prevent them from
accessing the best drugs that would help them. Kanavos and Reinhardt (2003)
stated that reference pricing brings together the generics or substitutes of the
original expensive drug, it then decides on one price that the generics will be sold
at which is lower than the original expensive drug but still performs the same
function. This makes the drug to be very affordable and can therefore be used by
all patients.
The use of reference pricing is significant in pharmaceutical pricing policy,
since it helps in reduction of death rates. It has also made health care accessible to
many people which has improved the living standards of people (Acosta et al.
2014). This intervention is important since it will lead to better access of medicine
which will lead to a better working nation.
10.deliverables and results dissemination plans.
The study will consist of a project report and a workshop organized to
disseminate the study findings. This will be used to analyze the results from the
research and to ensure the right thing has been done. The results will then be
recorded to be used in the final report.
3.0Proposed timeline
activities year-one year- 2 year- 3 year- 4
Approval of the
budget, development
of the proposal,
signing of the
documents especially
MOU
Reference pricing is very important since most people who suffer from
diseases may not have a good insurance cover. This may prevent them from
accessing the best drugs that would help them. Kanavos and Reinhardt (2003)
stated that reference pricing brings together the generics or substitutes of the
original expensive drug, it then decides on one price that the generics will be sold
at which is lower than the original expensive drug but still performs the same
function. This makes the drug to be very affordable and can therefore be used by
all patients.
The use of reference pricing is significant in pharmaceutical pricing policy,
since it helps in reduction of death rates. It has also made health care accessible to
many people which has improved the living standards of people (Acosta et al.
2014). This intervention is important since it will lead to better access of medicine
which will lead to a better working nation.
10.deliverables and results dissemination plans.
The study will consist of a project report and a workshop organized to
disseminate the study findings. This will be used to analyze the results from the
research and to ensure the right thing has been done. The results will then be
recorded to be used in the final report.
3.0Proposed timeline
activities year-one year- 2 year- 3 year- 4
Approval of the
budget, development
of the proposal,
signing of the
documents especially
MOU
Recruitment of
enumerators,Training
of the field
researchers ,
allocation of research
specialists,collection
of data.
Collection of field
data
Data analysis and
drafting of the final
report
Submission of the
Final report and
dissemination
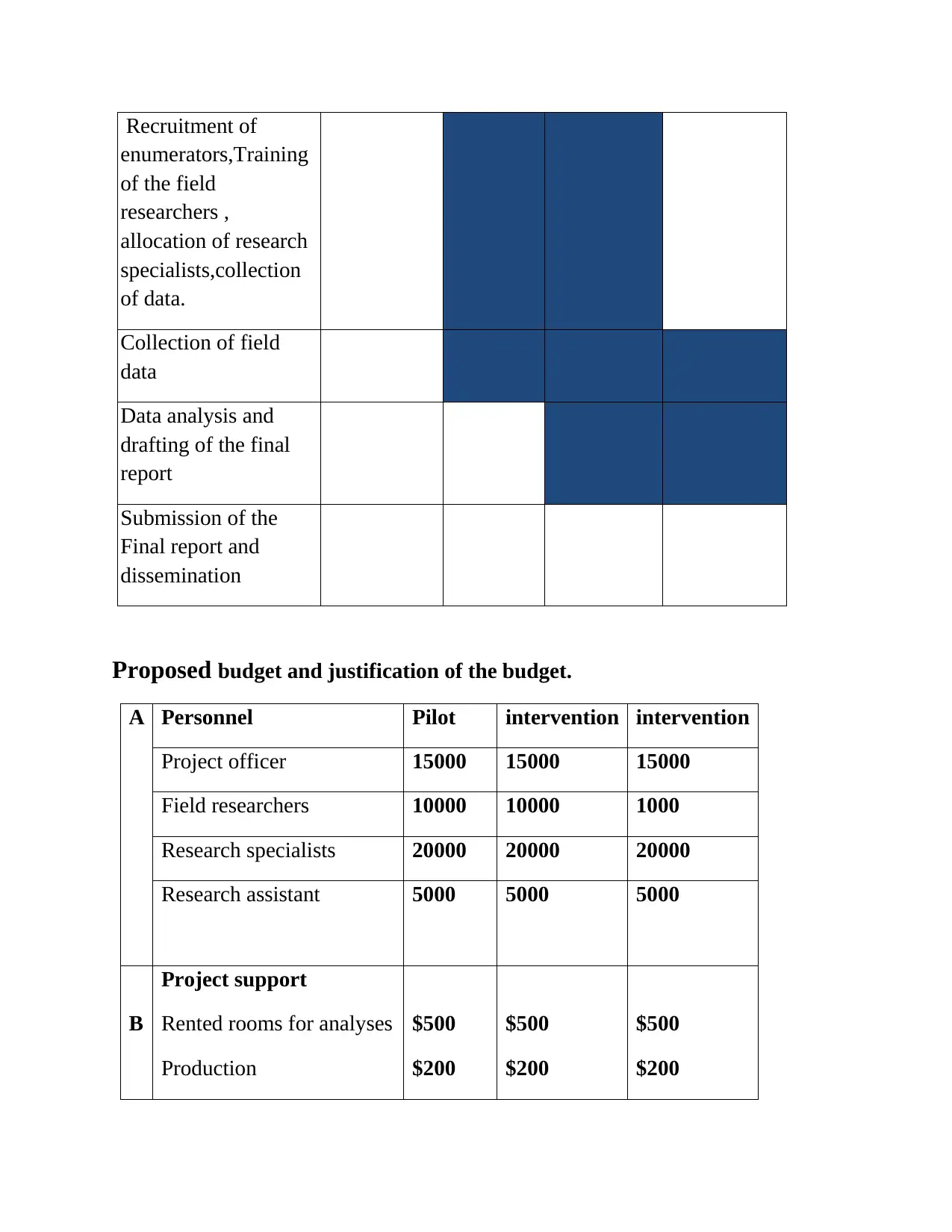
Proposed budget and justification of the budget.
A Personnel Pilot intervention intervention
Project officer 15000 15000 15000
Field researchers 10000 10000 1000
Research specialists 20000 20000 20000
Research assistant 5000 5000 5000
B
Project support
Rented rooms for analyses
Production
$500
$200
$500
$200
$500
$200
enumerators,Training
of the field
researchers ,
allocation of research
specialists,collection
of data.
Collection of field
data
Data analysis and
drafting of the final
report
Submission of the
Final report and
dissemination
Proposed budget and justification of the budget.
A Personnel Pilot intervention intervention
Project officer 15000 15000 15000
Field researchers 10000 10000 1000
Research specialists 20000 20000 20000
Research assistant 5000 5000 5000
B
Project support
Rented rooms for analyses
Production
$500
$200
$500
$200
$500
$200
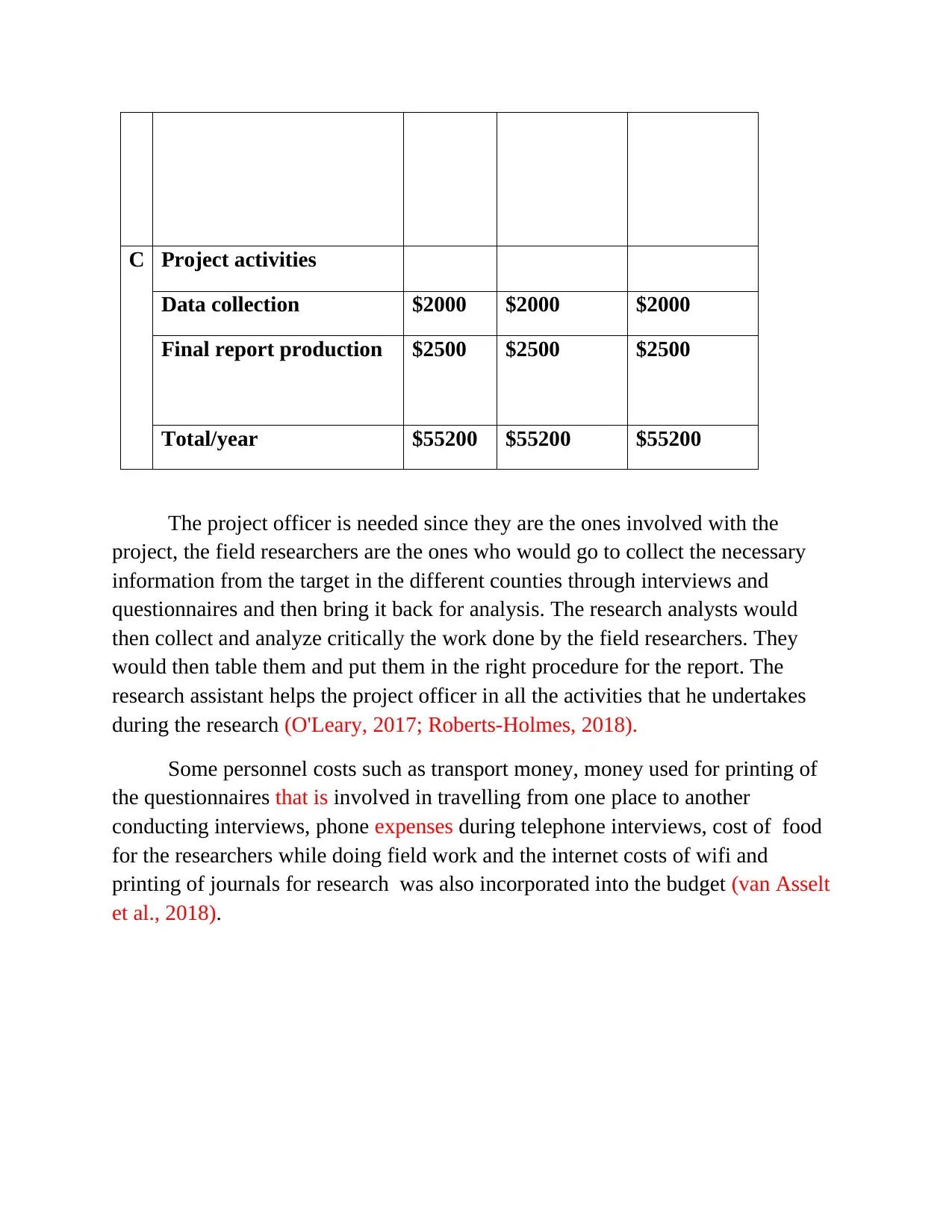
C Project activities
Data collection $2000 $2000 $2000
Final report production $2500 $2500 $2500
Total/year $55200 $55200 $55200
The project officer is needed since they are the ones involved with the
project, the field researchers are the ones who would go to collect the necessary
information from the target in the different counties through interviews and
questionnaires and then bring it back for analysis. The research analysts would
then collect and analyze critically the work done by the field researchers. They
would then table them and put them in the right procedure for the report. The
research assistant helps the project officer in all the activities that he undertakes
during the research (O'Leary, 2017; Roberts-Holmes, 2018).
Some personnel costs such as transport money, money used for printing of
the questionnaires that is involved in travelling from one place to another
conducting interviews, phone expenses during telephone interviews, cost of food
for the researchers while doing field work and the internet costs of wifi and
printing of journals for research was also incorporated into the budget (van Asselt
et al., 2018).
Data collection $2000 $2000 $2000
Final report production $2500 $2500 $2500
Total/year $55200 $55200 $55200
The project officer is needed since they are the ones involved with the
project, the field researchers are the ones who would go to collect the necessary
information from the target in the different counties through interviews and
questionnaires and then bring it back for analysis. The research analysts would
then collect and analyze critically the work done by the field researchers. They
would then table them and put them in the right procedure for the report. The
research assistant helps the project officer in all the activities that he undertakes
during the research (O'Leary, 2017; Roberts-Holmes, 2018).
Some personnel costs such as transport money, money used for printing of
the questionnaires that is involved in travelling from one place to another
conducting interviews, phone expenses during telephone interviews, cost of food
for the researchers while doing field work and the internet costs of wifi and
printing of journals for research was also incorporated into the budget (van Asselt
et al., 2018).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Aaserud, M., Austvoll‐Dahlgren, A., Kösters, J. P., Oxman, A. D., Ramsay, C., &
Sturm, H. (2006). Pharmaceutical policies: effects of reference pricing, other
pricing, and purchasing policies. Cochrane Database of Systematic Reviews,
(2).
Acosta, A., Ciapponi, A., Aaserud, M., Vietto, V., Austvoll-Dahlgren, A., Kösters,
J. P., ...& Oxman, A. D. (2014). Pharmaceutical policies: effects of reference
pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev,
10(10
Branstetter, L., Chatterjee, C., & Higgins, M. J. (2016). Regulation and welfare:
evidence from paragraph IV generic entry in the pharmaceutical industry.
The RAND Journal of Economics, 47(4), 857-890
Brekke, K. R., Grasdal, A. L., & Holmås, T. H. (2009). Regulation and pricing of
pharmaceuticals: Reference pricing or price cap regulation?.European
Economic Review, 53(2),
Caretta, M. A., & Cheptum, F. J. (2017). Leaving the field:(de‐) linked lives of the
researcher and research assistant. Area, 49(4), 415-420.
Danzon, P. M., & Epstein, A. J. (2008).Effects of regulation on drug launch and
pricing in interdependent markets (No. w14041). National Bureau of
Economic Research.
Danzon, P. M., & Furukawa, M. F. (2008).International prices and availability of
pharmaceuticals in 2005.Health affairs, 27(1), 221-233.
Danzon, P. M., & Ketcham, J. D. (2004, January). Reference pricing of
pharmaceuticals for Medicare: evidence from Germany, The Netherlands,
and New Zealand. In Forum for health economics & policy (Vol. 7, No. 1).
De Gruyter.
Dingwall, R., Iphofen, R., Lewis, J., Oates, J., & Emmerich, N. (2017). Towards
common principles for social science research ethics: A discussion
document for the Academy of Social Sciences. In Finding common ground:
Aaserud, M., Austvoll‐Dahlgren, A., Kösters, J. P., Oxman, A. D., Ramsay, C., &
Sturm, H. (2006). Pharmaceutical policies: effects of reference pricing, other
pricing, and purchasing policies. Cochrane Database of Systematic Reviews,
(2).
Acosta, A., Ciapponi, A., Aaserud, M., Vietto, V., Austvoll-Dahlgren, A., Kösters,
J. P., ...& Oxman, A. D. (2014). Pharmaceutical policies: effects of reference
pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev,
10(10
Branstetter, L., Chatterjee, C., & Higgins, M. J. (2016). Regulation and welfare:
evidence from paragraph IV generic entry in the pharmaceutical industry.
The RAND Journal of Economics, 47(4), 857-890
Brekke, K. R., Grasdal, A. L., & Holmås, T. H. (2009). Regulation and pricing of
pharmaceuticals: Reference pricing or price cap regulation?.European
Economic Review, 53(2),
Caretta, M. A., & Cheptum, F. J. (2017). Leaving the field:(de‐) linked lives of the
researcher and research assistant. Area, 49(4), 415-420.
Danzon, P. M., & Epstein, A. J. (2008).Effects of regulation on drug launch and
pricing in interdependent markets (No. w14041). National Bureau of
Economic Research.
Danzon, P. M., & Furukawa, M. F. (2008).International prices and availability of
pharmaceuticals in 2005.Health affairs, 27(1), 221-233.
Danzon, P. M., & Ketcham, J. D. (2004, January). Reference pricing of
pharmaceuticals for Medicare: evidence from Germany, The Netherlands,
and New Zealand. In Forum for health economics & policy (Vol. 7, No. 1).
De Gruyter.
Dingwall, R., Iphofen, R., Lewis, J., Oates, J., & Emmerich, N. (2017). Towards
common principles for social science research ethics: A discussion
document for the Academy of Social Sciences. In Finding common ground:

Consensus in research ethics across the social sciences (pp. 111-123).
Emerald Publishing Limited.
Fusch, P. I., & Ness, L. R. (2015). Are we there yet? Data saturation in qualitative
research. The qualitative report, 20(9), 1408-1416.
Galizzi, Matteo Maria, Simone Ghislandi, and Marisa Miraldo."Effects of
reference pricing in pharmaceutical markets."Pharmacoeconomics 29, no. 1
(2011): 17-33.
Giuliani, G., Selke, G., & Garattini, L. (1998).The German experience in reference
pricing.Health policy, 44(1), 73-85.
Kanavos, P. (2001). Overview of pharmaceutical pricing and reimbursement
regulation
Kanavos, P., & Reinhardt, U. (2003). Reference pricing for drugs: is it compatible
with US health care?.Health Affairs, 22(3), 16-30
Kyle, M. K. (2007). Pharmaceutical price controls and entry strategies. The Review
of Economics and Statistics, 89(1), 88-99
Lanjouw, J. O. (2005). Patents, price controls and access to new drugs: how policy
affects global market entry (No. w11321). Cambridge (MA): National
Bureau of Economic Research
Lee, J. L., Fischer, M. A., Shrank, W. H., Polinski, J. M., & Choudhry, N. K.
(2012). A systematic review of reference pricing: implications for US
prescription drug spending.
Lewis, S. (2015). Qualitative inquiry and research design: Choosing among five
approaches. Health promotion practice, 16(4), 473-475.
López-Casasnovas, G., & Puig-Junoy, J. (2000). Review of the literature on
reference pricing. Health policy, 54(2), 87-123.
Mayan, M. J. (2016). Ethics Boards, Risk, and Qualitative Research. In Essentials
of Qualitative Inquiry (pp. 125-131). Routledge.
Emerald Publishing Limited.
Fusch, P. I., & Ness, L. R. (2015). Are we there yet? Data saturation in qualitative
research. The qualitative report, 20(9), 1408-1416.
Galizzi, Matteo Maria, Simone Ghislandi, and Marisa Miraldo."Effects of
reference pricing in pharmaceutical markets."Pharmacoeconomics 29, no. 1
(2011): 17-33.
Giuliani, G., Selke, G., & Garattini, L. (1998).The German experience in reference
pricing.Health policy, 44(1), 73-85.
Kanavos, P. (2001). Overview of pharmaceutical pricing and reimbursement
regulation
Kanavos, P., & Reinhardt, U. (2003). Reference pricing for drugs: is it compatible
with US health care?.Health Affairs, 22(3), 16-30
Kyle, M. K. (2007). Pharmaceutical price controls and entry strategies. The Review
of Economics and Statistics, 89(1), 88-99
Lanjouw, J. O. (2005). Patents, price controls and access to new drugs: how policy
affects global market entry (No. w11321). Cambridge (MA): National
Bureau of Economic Research
Lee, J. L., Fischer, M. A., Shrank, W. H., Polinski, J. M., & Choudhry, N. K.
(2012). A systematic review of reference pricing: implications for US
prescription drug spending.
Lewis, S. (2015). Qualitative inquiry and research design: Choosing among five
approaches. Health promotion practice, 16(4), 473-475.
López-Casasnovas, G., & Puig-Junoy, J. (2000). Review of the literature on
reference pricing. Health policy, 54(2), 87-123.
Mayan, M. J. (2016). Ethics Boards, Risk, and Qualitative Research. In Essentials
of Qualitative Inquiry (pp. 125-131). Routledge.

Nassaji, H. (2015). Qualitative and descriptive research: Data type versus data
analysis.
O'Leary, Z. (2017). The essential guide to doing your research project. Sage.
Paris, V., & Docteur, É. (2007).Pharmaceutical pricing and reimbursement policies
in Canada.
Rigter, H. (1994). Recent public policies in the Netherlands to control
pharmaceutical pricing and reimbursement.Pharmacoeconomics, 6(1), 15-21
Roberts-Holmes, G. (2018). Doing your early years research project: A step by
step guide. Sage.
Russell, L. B., & Wolff, N. (2002).The impact of drug pricing policies on the
health of the elderly.American journal of preventive medicine, 22(3), 151-
155
van Asselt, T., Ramaekers, B., Ramos, I. C., Joore, M., Al, M., Lesman-Leegte,
I., ... & Feenstra, T. (2018). Research Costs Investigated: A Study Into the
Budgets of Dutch Publicly Funded Drug-Related Research.
PharmacoEconomics, 36(1), 105-113.
Vogler, S. (2012). The impact of pharmaceutical pricing and reimbursement
policies on generics uptake: implementation of policy options on generics in
29 European countries–an overview. Generics and Biosimilars Initiative
Journal, 1(2), 44-51.
Von der Schulenburg, F., Vandoros, S., & Kanavos, P. (2011). The effects of drug
market regulation on pharmaceutical prices in Europe: overview and
evidence from the market of ACE inhibitors. Health economics review, 1(1),
18.
Walker, W. O. (1989). Drug control in the Americas (p. 1). Albuquerque:
University of New Mexico Press.
analysis.
O'Leary, Z. (2017). The essential guide to doing your research project. Sage.
Paris, V., & Docteur, É. (2007).Pharmaceutical pricing and reimbursement policies
in Canada.
Rigter, H. (1994). Recent public policies in the Netherlands to control
pharmaceutical pricing and reimbursement.Pharmacoeconomics, 6(1), 15-21
Roberts-Holmes, G. (2018). Doing your early years research project: A step by
step guide. Sage.
Russell, L. B., & Wolff, N. (2002).The impact of drug pricing policies on the
health of the elderly.American journal of preventive medicine, 22(3), 151-
155
van Asselt, T., Ramaekers, B., Ramos, I. C., Joore, M., Al, M., Lesman-Leegte,
I., ... & Feenstra, T. (2018). Research Costs Investigated: A Study Into the
Budgets of Dutch Publicly Funded Drug-Related Research.
PharmacoEconomics, 36(1), 105-113.
Vogler, S. (2012). The impact of pharmaceutical pricing and reimbursement
policies on generics uptake: implementation of policy options on generics in
29 European countries–an overview. Generics and Biosimilars Initiative
Journal, 1(2), 44-51.
Von der Schulenburg, F., Vandoros, S., & Kanavos, P. (2011). The effects of drug
market regulation on pharmaceutical prices in Europe: overview and
evidence from the market of ACE inhibitors. Health economics review, 1(1),
18.
Walker, W. O. (1989). Drug control in the Americas (p. 1). Albuquerque:
University of New Mexico Press.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1 out of 17
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
