6IM511 The Business Consultancy Project Research Ethics Form
VerifiedAdded on 2023/01/18
|8
|1596
|76
Homework Assignment
AI Summary
This homework assignment is a research ethics form completed by a student for the Business Consultancy Project (6IM511) at the University of Derby. The form outlines the student's research plan, focusing on a situational analysis of DENTAL CLINICS LTD, a private business in the UK. The student aims to identify key issues using questionnaires administered to employees. The form details research objectives, methods, the expected sample, and addresses ethical considerations such as informed consent, confidentiality, data protection, and potential risks to participants. It includes sections on debriefing, withdrawal from the study, and adherence to GDPR regulations, ensuring that the research complies with the University's Research Ethics Policy. The student confirms understanding of their obligations and the rights of participants, and that they will not begin data collection until the form is approved by their seminar tutor. The form was submitted by 1st February.
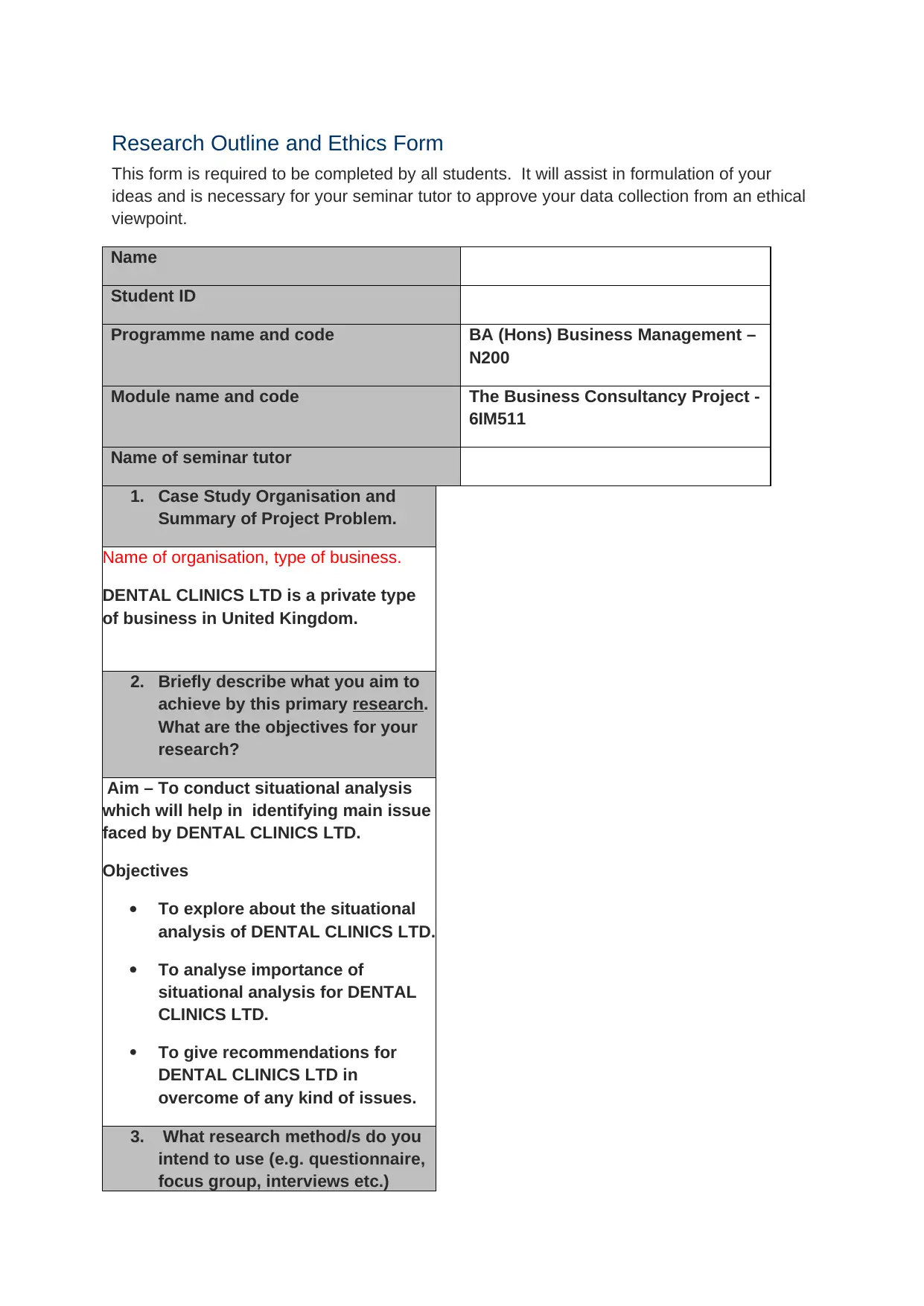
Research Outline and Ethics Form
This form is required to be completed by all students. It will assist in formulation of your
ideas and is necessary for your seminar tutor to approve your data collection from an ethical
viewpoint.
Name
Student ID
Programme name and code BA (Hons) Business Management –
N200
Module name and code The Business Consultancy Project -
6IM511
Name of seminar tutor
1. Case Study Organisation and
Summary of Project Problem.
Name of organisation, type of business.
DENTAL CLINICS LTD is a private type
of business in United Kingdom.
2. Briefly describe what you aim to
achieve by this primary research.
What are the objectives for your
research?
Aim – To conduct situational analysis
which will help in identifying main issue
faced by DENTAL CLINICS LTD.
Objectives
To explore about the situational
analysis of DENTAL CLINICS LTD.
To analyse importance of
situational analysis for DENTAL
CLINICS LTD.
To give recommendations for
DENTAL CLINICS LTD in
overcome of any kind of issues.
3. What research method/s do you
intend to use (e.g. questionnaire,
focus group, interviews etc.)
This form is required to be completed by all students. It will assist in formulation of your
ideas and is necessary for your seminar tutor to approve your data collection from an ethical
viewpoint.
Name
Student ID
Programme name and code BA (Hons) Business Management –
N200
Module name and code The Business Consultancy Project -
6IM511
Name of seminar tutor
1. Case Study Organisation and
Summary of Project Problem.
Name of organisation, type of business.
DENTAL CLINICS LTD is a private type
of business in United Kingdom.
2. Briefly describe what you aim to
achieve by this primary research.
What are the objectives for your
research?
Aim – To conduct situational analysis
which will help in identifying main issue
faced by DENTAL CLINICS LTD.
Objectives
To explore about the situational
analysis of DENTAL CLINICS LTD.
To analyse importance of
situational analysis for DENTAL
CLINICS LTD.
To give recommendations for
DENTAL CLINICS LTD in
overcome of any kind of issues.
3. What research method/s do you
intend to use (e.g. questionnaire,
focus group, interviews etc.)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
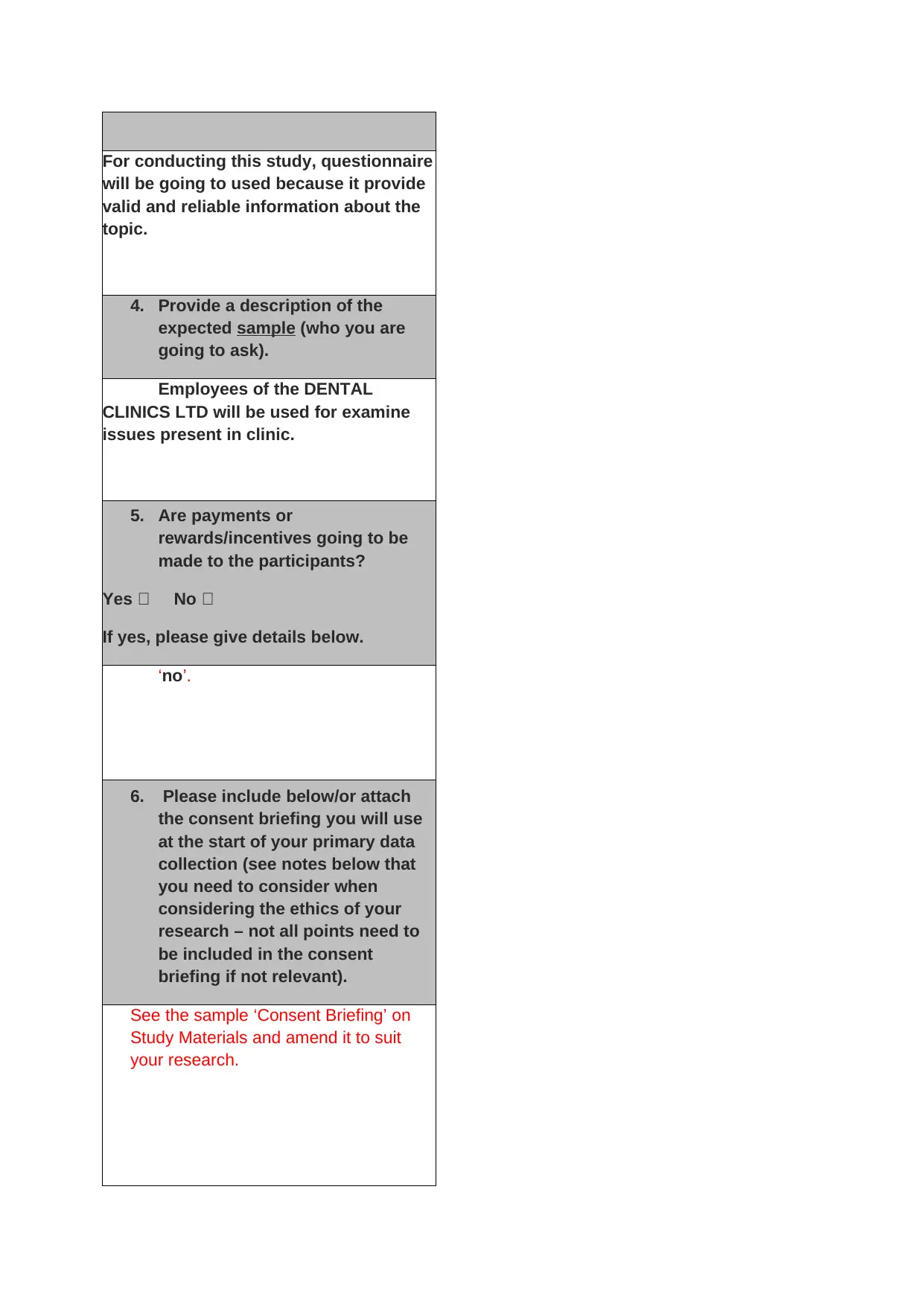
For conducting this study, questionnaire
will be going to used because it provide
valid and reliable information about the
topic.
4. Provide a description of the
expected sample (who you are
going to ask).
Employees of the DENTAL
CLINICS LTD will be used for examine
issues present in clinic.
5. Are payments or
rewards/incentives going to be
made to the participants?
Yes No
If yes, please give details below.
‘no’.
6. Please include below/or attach
the consent briefing you will use
at the start of your primary data
collection (see notes below that
you need to consider when
considering the ethics of your
research – not all points need to
be included in the consent
briefing if not relevant).
See the sample ‘Consent Briefing’ on
Study Materials and amend it to suit
your research.
will be going to used because it provide
valid and reliable information about the
topic.
4. Provide a description of the
expected sample (who you are
going to ask).
Employees of the DENTAL
CLINICS LTD will be used for examine
issues present in clinic.
5. Are payments or
rewards/incentives going to be
made to the participants?
Yes No
If yes, please give details below.
‘no’.
6. Please include below/or attach
the consent briefing you will use
at the start of your primary data
collection (see notes below that
you need to consider when
considering the ethics of your
research – not all points need to
be included in the consent
briefing if not relevant).
See the sample ‘Consent Briefing’ on
Study Materials and amend it to suit
your research.

a. Consent
Informed consent must be obtained for all
participants before they take part in your
project. The form should clearly state what
they will be doing; drawing attention to
anything they could conceivably object to
subsequently. It should be in language that
the person signing it will understand. It
should also state that they can withdraw
from the study at any time and the
measures you are taking to ensure the
confidentiality of data. If children are
recruited from schools you will require the
permission, depending on the school, of the
head teacher, and of parents. Children over
14 years should also sign an individual
consent form themselves. If conducting
research on children you will normally also
require DBS clearance. You will need to
check with the school if they require you to
obtain one of these. It is usually necessary
if working alone with children, however,
some schools may request you have DBS
clearance for any type of research you want
to conduct within the school. Research to
be carried out in any institution (prison,
hospital, etc.) will require permission from
the appropriate authority.
a. Covert or Deceptive Research
Research involving any form of deception
can be particularly problematical, and you
should provide a full explanation of why a
covert or deceptive approach is necessary,
why there are no acceptable alternative
approaches not involving deception, and
Informed consent must be obtained for all
participants before they take part in your
project. The form should clearly state what
they will be doing; drawing attention to
anything they could conceivably object to
subsequently. It should be in language that
the person signing it will understand. It
should also state that they can withdraw
from the study at any time and the
measures you are taking to ensure the
confidentiality of data. If children are
recruited from schools you will require the
permission, depending on the school, of the
head teacher, and of parents. Children over
14 years should also sign an individual
consent form themselves. If conducting
research on children you will normally also
require DBS clearance. You will need to
check with the school if they require you to
obtain one of these. It is usually necessary
if working alone with children, however,
some schools may request you have DBS
clearance for any type of research you want
to conduct within the school. Research to
be carried out in any institution (prison,
hospital, etc.) will require permission from
the appropriate authority.
a. Covert or Deceptive Research
Research involving any form of deception
can be particularly problematical, and you
should provide a full explanation of why a
covert or deceptive approach is necessary,
why there are no acceptable alternative
approaches not involving deception, and
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

the scientific justification for deception.
b. Debriefing
Debriefing is a process of reflection once
the research intervention is complete, for
example at the end of an interview session.
How will participants be debriefed (written
or spoken feedback)? If they will not be
debriefed, give reasons. Please attach the
written debrief or transcript for the oral
debrief. This can be particularly important if
covert or deceptive research methods are
used.
c. Withdrawal from the Investigation
Participants should be told explicitly that
they are free to leave the study at any time
without jeopardy. It is important that you
clarify exactly how and when this will be
explained to participants. Participants also
have the right to withdraw their data in
retrospect, after you have received it. You
will need to clarify how they will do this and
at what point they will not be able to
withdraw (i.e. after the data has been
analysed and disseminated).
d. Confidentiality and Data
Protection
You must comply with GDPR (General Data
Protection Regulation) and the University's
Good Practice
https://www.derby.ac.uk/research/about-
our-research/ethics/research-ethics/policy-
and-guidance/ This means, amongst other
things:
It is very important that the
Participant Information Sheet
includes information on what the
research is for, who will conduct the
research, how the personal
information will be used, who will
have access to the information and
how long the information will be kept
for. This is known as a 'fair
processing statement.'
b. Debriefing
Debriefing is a process of reflection once
the research intervention is complete, for
example at the end of an interview session.
How will participants be debriefed (written
or spoken feedback)? If they will not be
debriefed, give reasons. Please attach the
written debrief or transcript for the oral
debrief. This can be particularly important if
covert or deceptive research methods are
used.
c. Withdrawal from the Investigation
Participants should be told explicitly that
they are free to leave the study at any time
without jeopardy. It is important that you
clarify exactly how and when this will be
explained to participants. Participants also
have the right to withdraw their data in
retrospect, after you have received it. You
will need to clarify how they will do this and
at what point they will not be able to
withdraw (i.e. after the data has been
analysed and disseminated).
d. Confidentiality and Data
Protection
You must comply with GDPR (General Data
Protection Regulation) and the University's
Good Practice
https://www.derby.ac.uk/research/about-
our-research/ethics/research-ethics/policy-
and-guidance/ This means, amongst other
things:
It is very important that the
Participant Information Sheet
includes information on what the
research is for, who will conduct the
research, how the personal
information will be used, who will
have access to the information and
how long the information will be kept
for. This is known as a 'fair
processing statement.'
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

You must not do anything with the
personal information you collect over
and above that for which you have
consent.
You can only make audio or visual
recordings of participants with their
consent (this should be stated on
the Participant Information sheet)
Identifiable personal information
should only be conveyed to others
with the participant's permission,
although this is ill advised and
should not be necessary within the
expectations of this module..
You must store data securely.
Consent forms and data should be
stored separately and securely.
You should only collect data that is
relevant to the study being
undertaken.
Data may be kept indefinitely
providing its sole use is for research
purposes and meets the following
conditions:
The data is not being used to take
decisions in respect of any living
individual.
The data is not being used in any
which is, or is likely to, cause
damage and/or distress to any living
individual.
You should always protect a
participant's anonymity unless they
have given their permission to be
identified (if they do so, this should
be stated on the Informed Consent
Form).
All data should be returned to
participants or destroyed if consent
is not given after the fact, or if a
participant withdraws.
e. Protection of participants
Are the participants at risk of physical,
psychological or emotional harm greater
than encountered ordinary life? If yes,
personal information you collect over
and above that for which you have
consent.
You can only make audio or visual
recordings of participants with their
consent (this should be stated on
the Participant Information sheet)
Identifiable personal information
should only be conveyed to others
with the participant's permission,
although this is ill advised and
should not be necessary within the
expectations of this module..
You must store data securely.
Consent forms and data should be
stored separately and securely.
You should only collect data that is
relevant to the study being
undertaken.
Data may be kept indefinitely
providing its sole use is for research
purposes and meets the following
conditions:
The data is not being used to take
decisions in respect of any living
individual.
The data is not being used in any
which is, or is likely to, cause
damage and/or distress to any living
individual.
You should always protect a
participant's anonymity unless they
have given their permission to be
identified (if they do so, this should
be stated on the Informed Consent
Form).
All data should be returned to
participants or destroyed if consent
is not given after the fact, or if a
participant withdraws.
e. Protection of participants
Are the participants at risk of physical,
psychological or emotional harm greater
than encountered ordinary life? If yes,
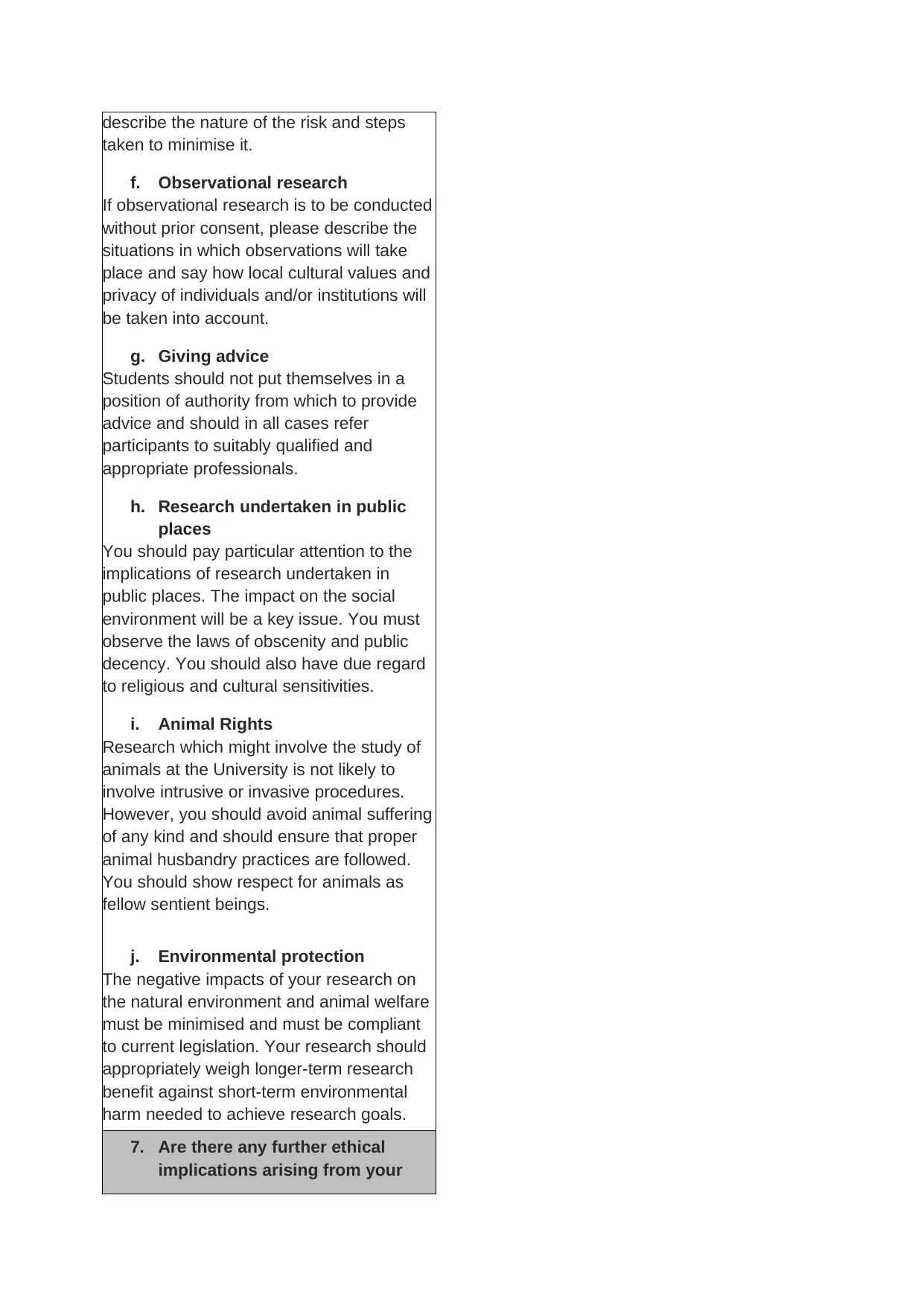
describe the nature of the risk and steps
taken to minimise it.
f. Observational research
If observational research is to be conducted
without prior consent, please describe the
situations in which observations will take
place and say how local cultural values and
privacy of individuals and/or institutions will
be taken into account.
g. Giving advice
Students should not put themselves in a
position of authority from which to provide
advice and should in all cases refer
participants to suitably qualified and
appropriate professionals.
h. Research undertaken in public
places
You should pay particular attention to the
implications of research undertaken in
public places. The impact on the social
environment will be a key issue. You must
observe the laws of obscenity and public
decency. You should also have due regard
to religious and cultural sensitivities.
i. Animal Rights
Research which might involve the study of
animals at the University is not likely to
involve intrusive or invasive procedures.
However, you should avoid animal suffering
of any kind and should ensure that proper
animal husbandry practices are followed.
You should show respect for animals as
fellow sentient beings.
j. Environmental protection
The negative impacts of your research on
the natural environment and animal welfare
must be minimised and must be compliant
to current legislation. Your research should
appropriately weigh longer-term research
benefit against short-term environmental
harm needed to achieve research goals.
7. Are there any further ethical
implications arising from your
taken to minimise it.
f. Observational research
If observational research is to be conducted
without prior consent, please describe the
situations in which observations will take
place and say how local cultural values and
privacy of individuals and/or institutions will
be taken into account.
g. Giving advice
Students should not put themselves in a
position of authority from which to provide
advice and should in all cases refer
participants to suitably qualified and
appropriate professionals.
h. Research undertaken in public
places
You should pay particular attention to the
implications of research undertaken in
public places. The impact on the social
environment will be a key issue. You must
observe the laws of obscenity and public
decency. You should also have due regard
to religious and cultural sensitivities.
i. Animal Rights
Research which might involve the study of
animals at the University is not likely to
involve intrusive or invasive procedures.
However, you should avoid animal suffering
of any kind and should ensure that proper
animal husbandry practices are followed.
You should show respect for animals as
fellow sentient beings.
j. Environmental protection
The negative impacts of your research on
the natural environment and animal welfare
must be minimised and must be compliant
to current legislation. Your research should
appropriately weigh longer-term research
benefit against short-term environmental
harm needed to achieve research goals.
7. Are there any further ethical
implications arising from your
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
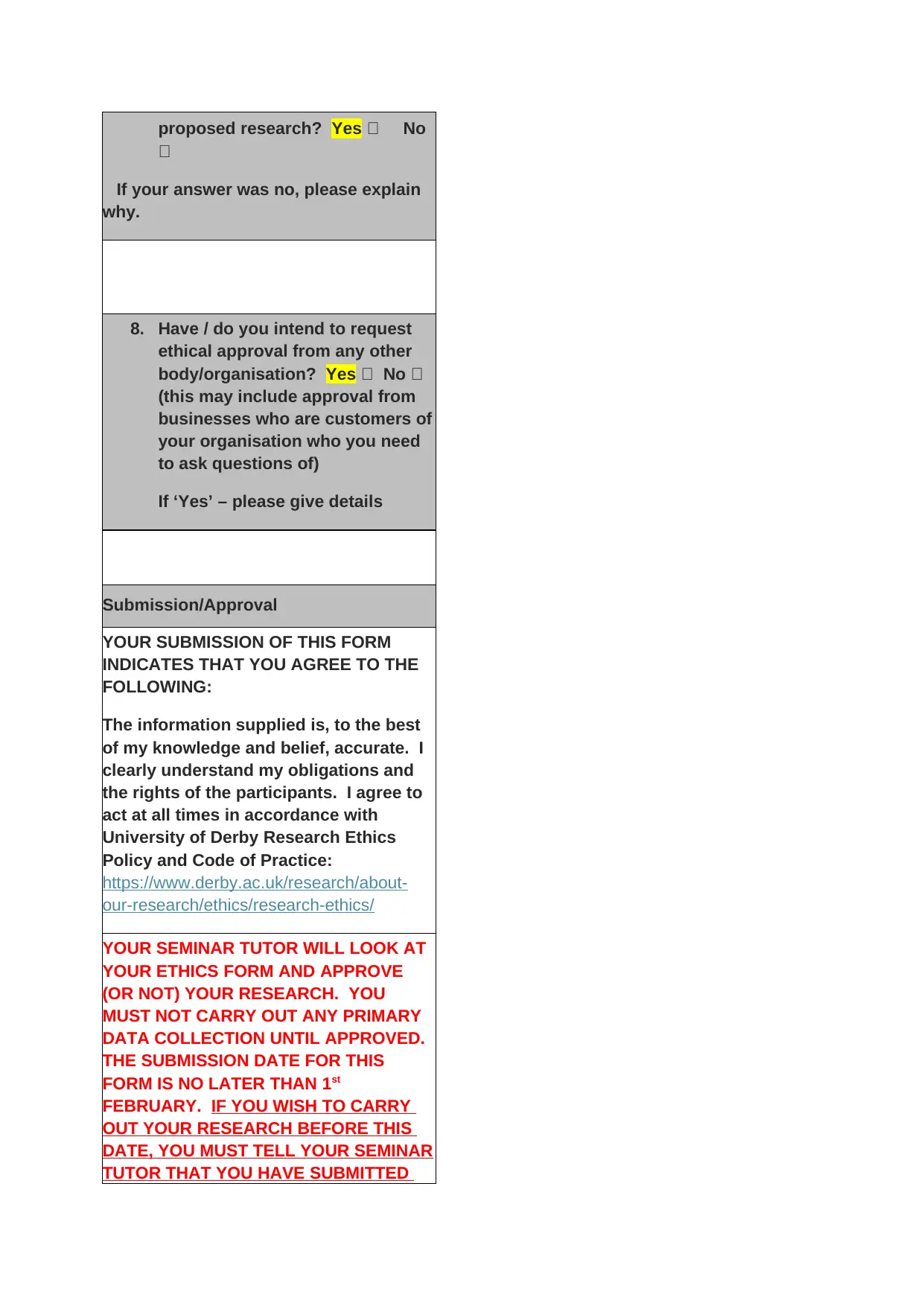
proposed research? Yes No
If your answer was no, please explain
why.
8. Have / do you intend to request
ethical approval from any other
body/organisation? Yes No
(this may include approval from
businesses who are customers of
your organisation who you need
to ask questions of)
If ‘Yes’ – please give details
Submission/Approval
YOUR SUBMISSION OF THIS FORM
INDICATES THAT YOU AGREE TO THE
FOLLOWING:
The information supplied is, to the best
of my knowledge and belief, accurate. I
clearly understand my obligations and
the rights of the participants. I agree to
act at all times in accordance with
University of Derby Research Ethics
Policy and Code of Practice:
https://www.derby.ac.uk/research/about-
our-research/ethics/research-ethics/
YOUR SEMINAR TUTOR WILL LOOK AT
YOUR ETHICS FORM AND APPROVE
(OR NOT) YOUR RESEARCH. YOU
MUST NOT CARRY OUT ANY PRIMARY
DATA COLLECTION UNTIL APPROVED.
THE SUBMISSION DATE FOR THIS
FORM IS NO LATER THAN 1st
FEBRUARY. IF YOU WISH TO CARRY
OUT YOUR RESEARCH BEFORE THIS
DATE, YOU MUST TELL YOUR SEMINAR
TUTOR THAT YOU HAVE SUBMITTED
If your answer was no, please explain
why.
8. Have / do you intend to request
ethical approval from any other
body/organisation? Yes No
(this may include approval from
businesses who are customers of
your organisation who you need
to ask questions of)
If ‘Yes’ – please give details
Submission/Approval
YOUR SUBMISSION OF THIS FORM
INDICATES THAT YOU AGREE TO THE
FOLLOWING:
The information supplied is, to the best
of my knowledge and belief, accurate. I
clearly understand my obligations and
the rights of the participants. I agree to
act at all times in accordance with
University of Derby Research Ethics
Policy and Code of Practice:
https://www.derby.ac.uk/research/about-
our-research/ethics/research-ethics/
YOUR SEMINAR TUTOR WILL LOOK AT
YOUR ETHICS FORM AND APPROVE
(OR NOT) YOUR RESEARCH. YOU
MUST NOT CARRY OUT ANY PRIMARY
DATA COLLECTION UNTIL APPROVED.
THE SUBMISSION DATE FOR THIS
FORM IS NO LATER THAN 1st
FEBRUARY. IF YOU WISH TO CARRY
OUT YOUR RESEARCH BEFORE THIS
DATE, YOU MUST TELL YOUR SEMINAR
TUTOR THAT YOU HAVE SUBMITTED
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

THIS FORM EARLY.
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





