RXMAN Hospital: Drug Use Evaluation of Enoxaparin (Jan-Jun 2019)
VerifiedAdded on 2022/09/25
|20
|4073
|17
Report
AI Summary
This report presents a retrospective drug use evaluation (DUE) of enoxaparin at RXMAN Hospital, focusing on post-operative patients from January to June 2019. The study evaluated the appropriateness, safety, and compliance of enoxaparin use in patients undergoing ACLR, knee arthroscopy, and foot/ankle surgeries. Data was collected from 197 patients who received enoxaparin post-operatively. Key findings include a high rate of enoxaparin use in the specified surgical procedures, with 195 patients receiving 40mg doses. Compliance with the VTE risk assessment and prophylaxis recommendations was high, with 92% of patients with identified risk factors receiving prophylaxis. The report also highlights the types of surgeries, enoxaparin dosages, and adverse drug reactions. The study found no reported VTEs post-surgery. The report concludes by summarizing the efficacy, safety, and compliance of enoxaparin use within the hospital's guidelines, noting the limitations of the data and areas for improvement.

RXMAN Hospital
Drug Use Evaluation Drug Use Evaluation of Enoxaparin at Sports and Orthopedic Hospital
Background
Venous thromboembolism (VTE) is a condition whereby a clot forms in the deep veins such as
leg veins or arm veins (Heit, Spencer, & White, 2016). It can eventually travel to the lungs or any
other organ in the body especially the heart. Most common trigger of VTE is surgery.
Immobilization and hospitalization are other triggers. All patients admitted are assessed for risk
of VTE using the Padua prediction score for risk of VTE. If the score is less than 4 then the
patient is a low risk while greater than 4 shows high risk and enoxaparin prophylaxis is
recommended so long as the patient does not have any contraindication when performing
lower limb surgeries of ACLR, knee arthroscopy, and foot and ankle surgeries. The incidence of
ACL reconstruction rose to 74.6% in 2014 in the USA (Herzog et al. 2017). A study that was done
in Elite Collegiate Athletes reports a 27% prevalence rate of knee and ankles surgeries.
Objectives
Evaluation of appropriateness of the use of enoxaparin, safety, and compliance with current
guideline for post-operative patients for the period from January 2019 to June 2019
Methods
A retrospective chart prescription review conducted to include all post-operative patients who
were prescribed and discharged on enoxaparin between January 1, 2019 and June 30, 2018.
Clinical pharmacy team agreed on the data criteria. Data was collected using an excel data
collection form. All collected data was analyzed.
Inclusion Criteria
All post-op patients who received and was discharged on enoxaparin
Exclusion Criteria
All outpatient (OPD) enoxaparin prescriptions
Definitions
Drug use evaluation (DUE)—an ongoing, systematic, criteria-based program of medicine
evaluations that will help ensure appropriate medicine use. If therapy is determined to be
inappropriate, interventions with providers or patients will be necessary to optimize
pharmaceutical therapy.
Data Collection
From January 1, 2019 to June 30, 2019 there were 570 surgical procedures at our hospital.
During the same period there were 198 prescribed enoxaparin. One of these prescriptions was
an OPD prescription and was excluded because the patient chose conventional treatment over
Drug Use Evaluation Drug Use Evaluation of Enoxaparin at Sports and Orthopedic Hospital
Background
Venous thromboembolism (VTE) is a condition whereby a clot forms in the deep veins such as
leg veins or arm veins (Heit, Spencer, & White, 2016). It can eventually travel to the lungs or any
other organ in the body especially the heart. Most common trigger of VTE is surgery.
Immobilization and hospitalization are other triggers. All patients admitted are assessed for risk
of VTE using the Padua prediction score for risk of VTE. If the score is less than 4 then the
patient is a low risk while greater than 4 shows high risk and enoxaparin prophylaxis is
recommended so long as the patient does not have any contraindication when performing
lower limb surgeries of ACLR, knee arthroscopy, and foot and ankle surgeries. The incidence of
ACL reconstruction rose to 74.6% in 2014 in the USA (Herzog et al. 2017). A study that was done
in Elite Collegiate Athletes reports a 27% prevalence rate of knee and ankles surgeries.
Objectives
Evaluation of appropriateness of the use of enoxaparin, safety, and compliance with current
guideline for post-operative patients for the period from January 2019 to June 2019
Methods
A retrospective chart prescription review conducted to include all post-operative patients who
were prescribed and discharged on enoxaparin between January 1, 2019 and June 30, 2018.
Clinical pharmacy team agreed on the data criteria. Data was collected using an excel data
collection form. All collected data was analyzed.
Inclusion Criteria
All post-op patients who received and was discharged on enoxaparin
Exclusion Criteria
All outpatient (OPD) enoxaparin prescriptions
Definitions
Drug use evaluation (DUE)—an ongoing, systematic, criteria-based program of medicine
evaluations that will help ensure appropriate medicine use. If therapy is determined to be
inappropriate, interventions with providers or patients will be necessary to optimize
pharmaceutical therapy.
Data Collection
From January 1, 2019 to June 30, 2019 there were 570 surgical procedures at our hospital.
During the same period there were 198 prescribed enoxaparin. One of these prescriptions was
an OPD prescription and was excluded because the patient chose conventional treatment over
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
surgery. From the 570 surgical procedures, 197 (35%) of the patients received enoxaparin post
op.
The hospital’s guideline:
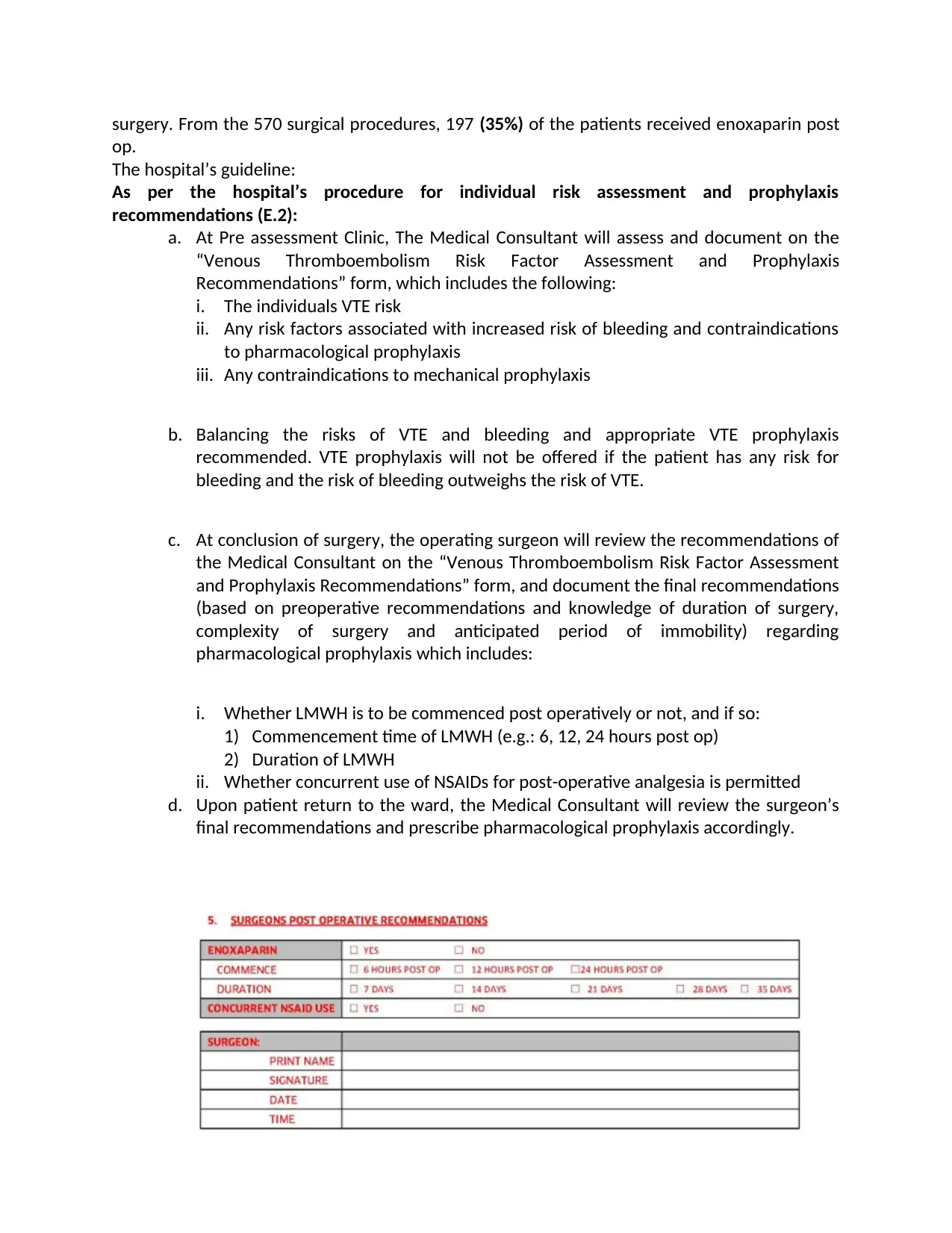
As per the hospital’s procedure for individual risk assessment and prophylaxis
recommendations (E.2):
a. At Pre assessment Clinic, The Medical Consultant will assess and document on the
“Venous Thromboembolism Risk Factor Assessment and Prophylaxis
Recommendations” form, which includes the following:
i. The individuals VTE risk
ii. Any risk factors associated with increased risk of bleeding and contraindications
to pharmacological prophylaxis
iii. Any contraindications to mechanical prophylaxis
b. Balancing the risks of VTE and bleeding and appropriate VTE prophylaxis
recommended. VTE prophylaxis will not be offered if the patient has any risk for
bleeding and the risk of bleeding outweighs the risk of VTE.
c. At conclusion of surgery, the operating surgeon will review the recommendations of
the Medical Consultant on the “Venous Thromboembolism Risk Factor Assessment
and Prophylaxis Recommendations” form, and document the final recommendations
(based on preoperative recommendations and knowledge of duration of surgery,
complexity of surgery and anticipated period of immobility) regarding
pharmacological prophylaxis which includes:
i. Whether LMWH is to be commenced post operatively or not, and if so:
1) Commencement time of LMWH (e.g.: 6, 12, 24 hours post op)
2) Duration of LMWH
ii. Whether concurrent use of NSAIDs for post-operative analgesia is permitted
d. Upon patient return to the ward, the Medical Consultant will review the surgeon’s
final recommendations and prescribe pharmacological prophylaxis accordingly.
op.
The hospital’s guideline:
As per the hospital’s procedure for individual risk assessment and prophylaxis
recommendations (E.2):
a. At Pre assessment Clinic, The Medical Consultant will assess and document on the
“Venous Thromboembolism Risk Factor Assessment and Prophylaxis
Recommendations” form, which includes the following:
i. The individuals VTE risk
ii. Any risk factors associated with increased risk of bleeding and contraindications
to pharmacological prophylaxis
iii. Any contraindications to mechanical prophylaxis
b. Balancing the risks of VTE and bleeding and appropriate VTE prophylaxis
recommended. VTE prophylaxis will not be offered if the patient has any risk for
bleeding and the risk of bleeding outweighs the risk of VTE.
c. At conclusion of surgery, the operating surgeon will review the recommendations of
the Medical Consultant on the “Venous Thromboembolism Risk Factor Assessment
and Prophylaxis Recommendations” form, and document the final recommendations
(based on preoperative recommendations and knowledge of duration of surgery,
complexity of surgery and anticipated period of immobility) regarding
pharmacological prophylaxis which includes:
i. Whether LMWH is to be commenced post operatively or not, and if so:
1) Commencement time of LMWH (e.g.: 6, 12, 24 hours post op)
2) Duration of LMWH
ii. Whether concurrent use of NSAIDs for post-operative analgesia is permitted
d. Upon patient return to the ward, the Medical Consultant will review the surgeon’s
final recommendations and prescribe pharmacological prophylaxis accordingly.
Results
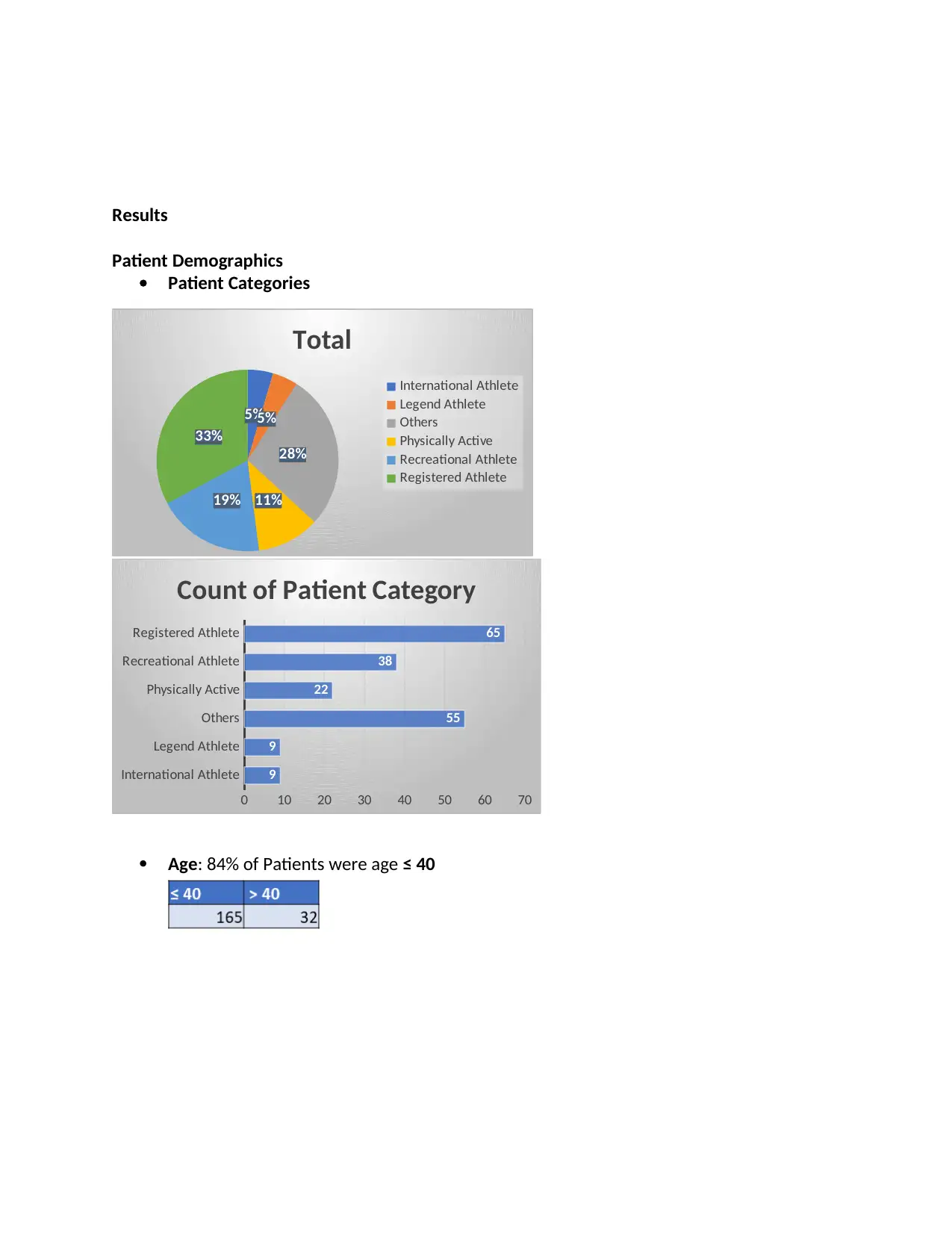
Patient Demographics
Patient Categories
5%5%
28%
11%19%
33%
Total
International Athlete
Legend Athlete
Others
Physically Active
Recreational Athlete
Registered Athlete
International Athlete
Legend Athlete
Others
Physically Active
Recreational Athlete
Registered Athlete
0 10 20 30 40 50 60 70
9
9
55
22
38
65
Count of Patient Category
Age: 84% of Patients were age ≤ 40
Patient Demographics
Patient Categories
5%5%
28%
11%19%
33%
Total
International Athlete
Legend Athlete
Others
Physically Active
Recreational Athlete
Registered Athlete
International Athlete
Legend Athlete
Others
Physically Active
Recreational Athlete
Registered Athlete
0 10 20 30 40 50 60 70
9
9
55
22
38
65
Count of Patient Category
Age: 84% of Patients were age ≤ 40
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
≤ 40 > 40
165
32
Age
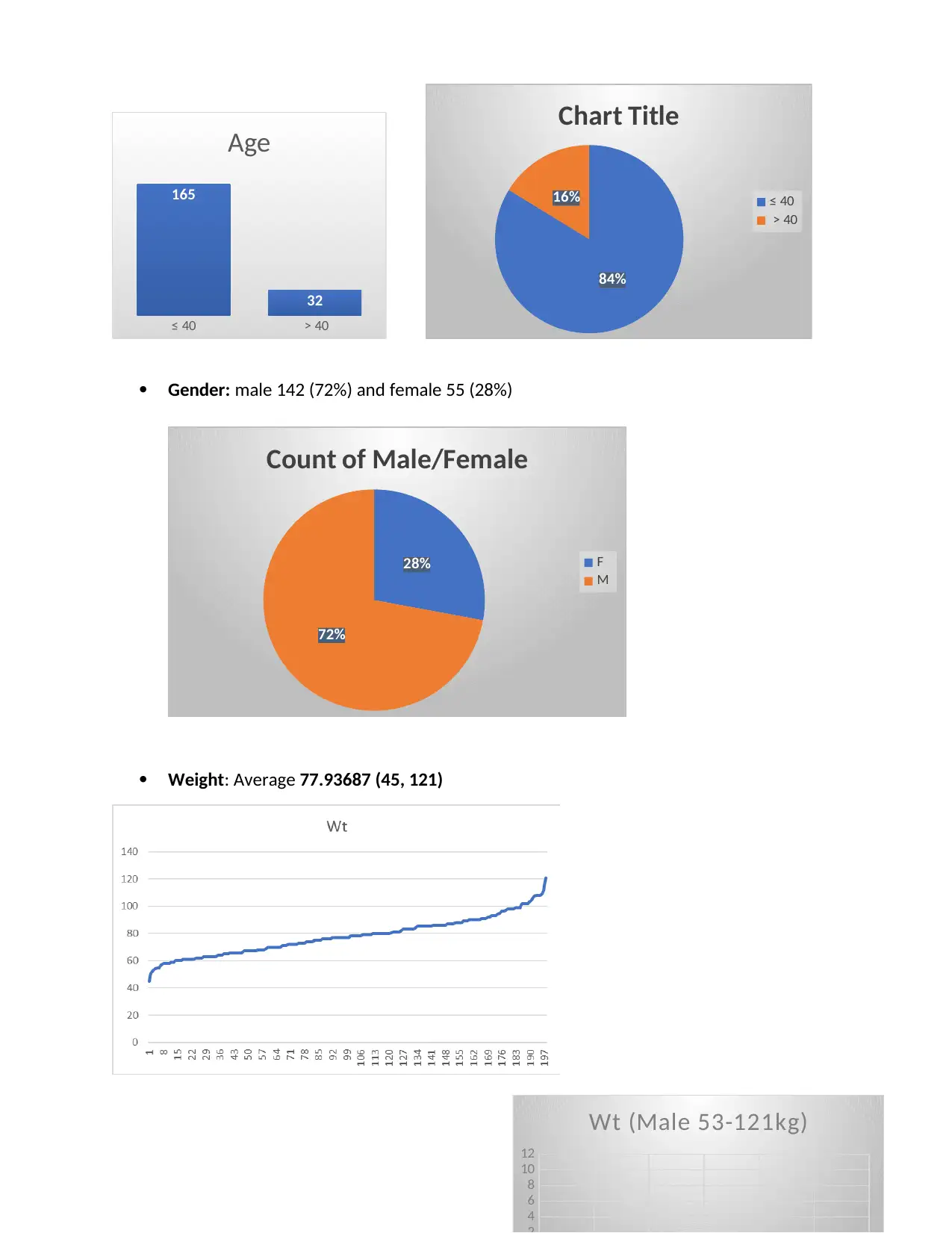
Gender: male 142 (72%) and female 55 (28%)
28%
72%
Count of Male/Female
F
M
Weight: Average 77.93687 (45, 121)
4
6
8
10
12
Wt (Male 53-121kg)
84%
16%
Chart Title
≤ 40
> 40
165
32
Age
Gender: male 142 (72%) and female 55 (28%)
28%
72%
Count of Male/Female
F
M
Weight: Average 77.93687 (45, 121)
4
6
8
10
12
Wt (Male 53-121kg)
84%
16%
Chart Title
≤ 40
> 40
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
0 2 4 6 8 10 12
0
2
4
6
8
10
12
Wt (Females 45-99kg)
Labs:
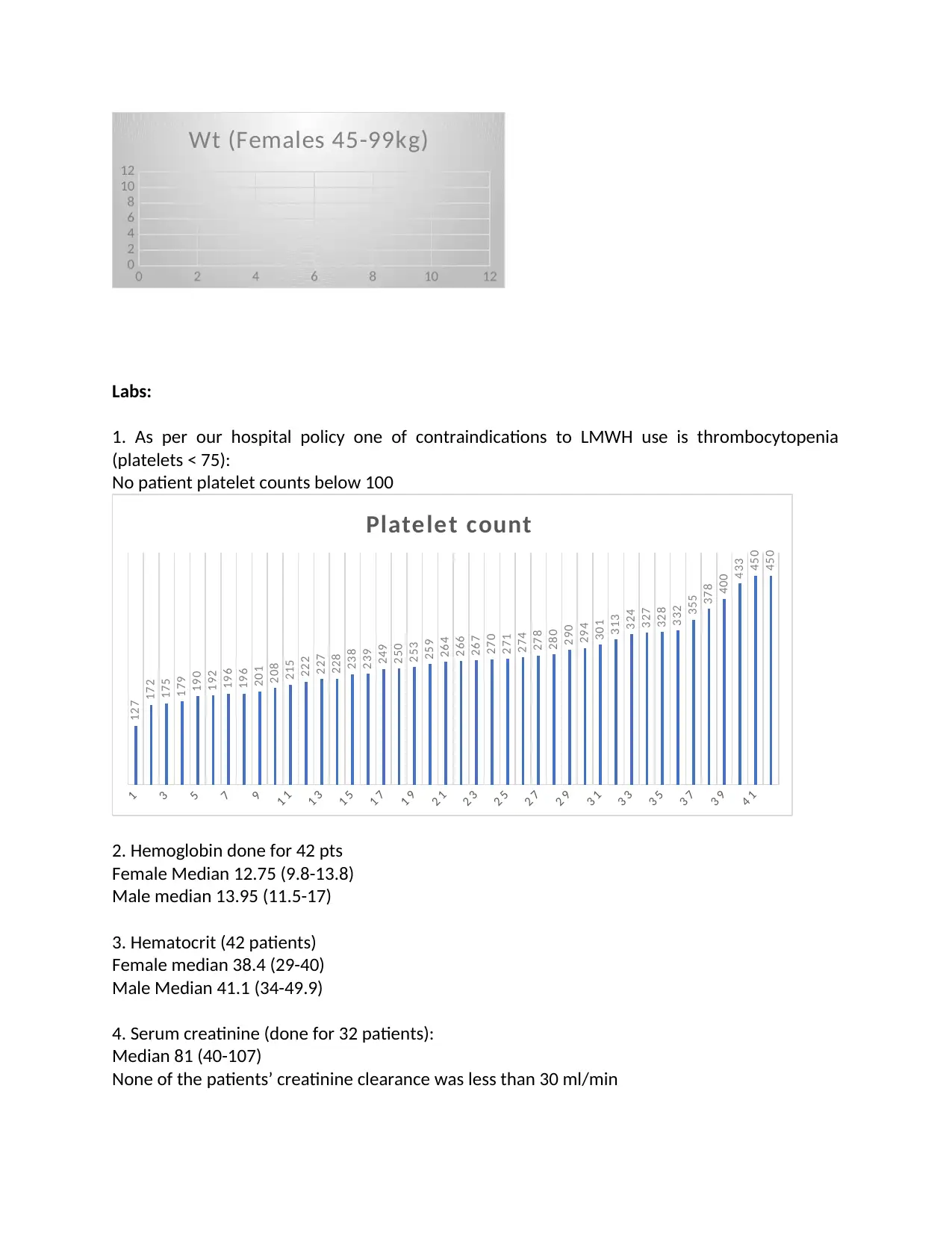
1. As per our hospital policy one of contraindications to LMWH use is thrombocytopenia
(platelets < 75):
No patient platelet counts below 100
1
3
5
7
9
1 1
1 3
1 5
1 7
1 9
2 1
2 3
2 5
2 7
2 9
3 1
3 3
3 5
3 7
3 9
4 1
127
172
175
179
190
192
196
196
201
208
215
222
227
228
238
239
249
250
253
259
264
266
267
270
271
274
278
280
290
294
301
313
324
327
328
332
355
378
400
433
450
450
Platelet count
2. Hemoglobin done for 42 pts
Female Median 12.75 (9.8-13.8)
Male median 13.95 (11.5-17)
3. Hematocrit (42 patients)
Female median 38.4 (29-40)
Male Median 41.1 (34-49.9)
4. Serum creatinine (done for 32 patients):
Median 81 (40-107)
None of the patients’ creatinine clearance was less than 30 ml/min
0 2 4 6 8 10 12
0
2
4
6
8
10
12
Wt (Females 45-99kg)
Labs:
1. As per our hospital policy one of contraindications to LMWH use is thrombocytopenia
(platelets < 75):
No patient platelet counts below 100
1
3
5
7
9
1 1
1 3
1 5
1 7
1 9
2 1
2 3
2 5
2 7
2 9
3 1
3 3
3 5
3 7
3 9
4 1
127
172
175
179
190
192
196
196
201
208
215
222
227
228
238
239
249
250
253
259
264
266
267
270
271
274
278
280
290
294
301
313
324
327
328
332
355
378
400
433
450
450
Platelet count
2. Hemoglobin done for 42 pts
Female Median 12.75 (9.8-13.8)
Male median 13.95 (11.5-17)
3. Hematocrit (42 patients)
Female median 38.4 (29-40)
Male Median 41.1 (34-49.9)
4. Serum creatinine (done for 32 patients):
Median 81 (40-107)
None of the patients’ creatinine clearance was less than 30 ml/min
0 2 4 6 8 10 12
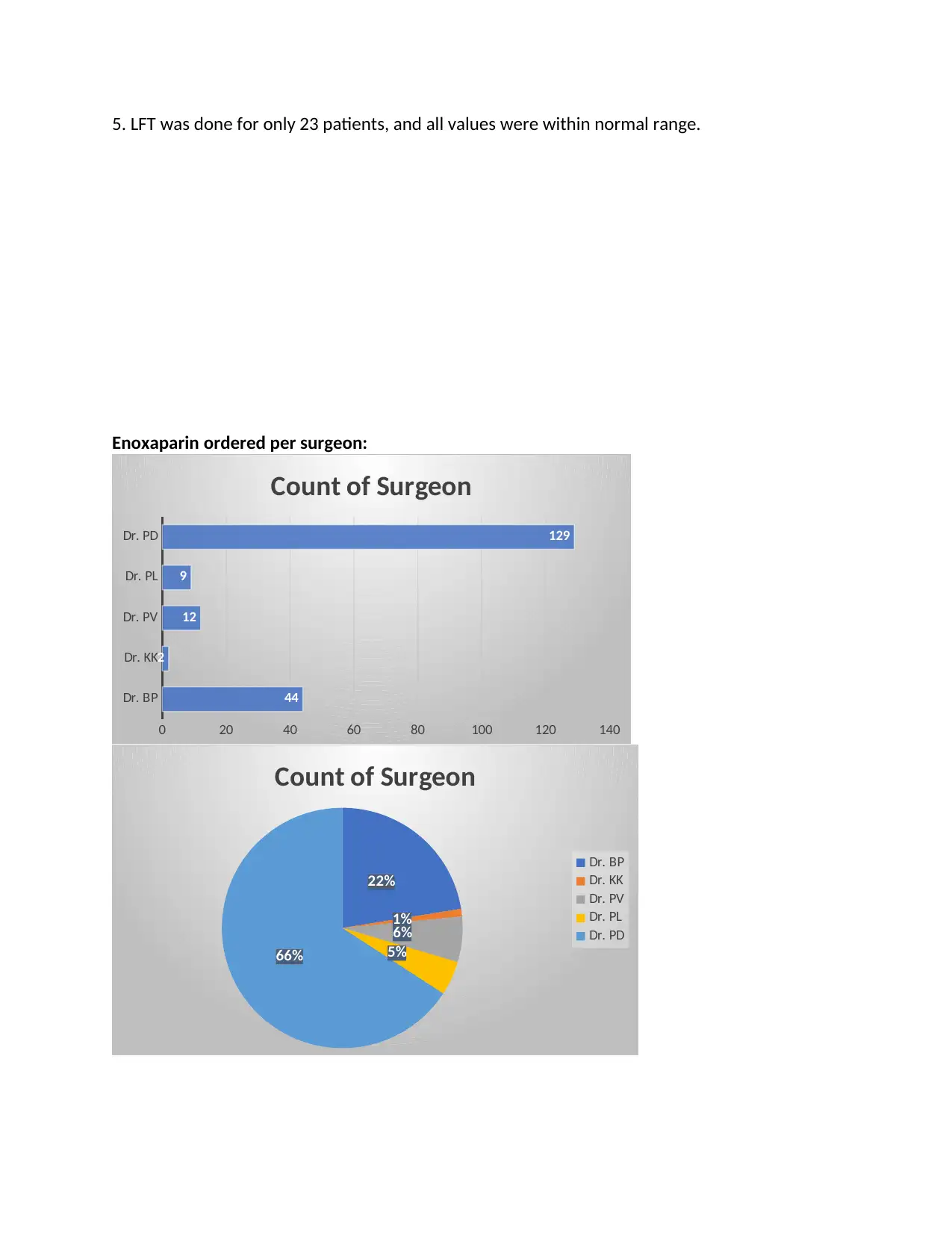
5. LFT was done for only 23 patients, and all values were within normal range.
Enoxaparin ordered per surgeon:
Dr. BP
Dr. KK
Dr. PV
Dr. PL
Dr. PD
0 20 40 60 80 100 120 140
44
2
12
9
129
Count of Surgeon
22%
1%
6%
5%66%
Count of Surgeon
Dr. BP
Dr. KK
Dr. PV
Dr. PL
Dr. PD
Enoxaparin ordered per surgeon:
Dr. BP
Dr. KK
Dr. PV
Dr. PL
Dr. PD
0 20 40 60 80 100 120 140
44
2
12
9
129
Count of Surgeon
22%
1%
6%
5%66%
Count of Surgeon
Dr. BP
Dr. KK
Dr. PV
Dr. PL
Dr. PD
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
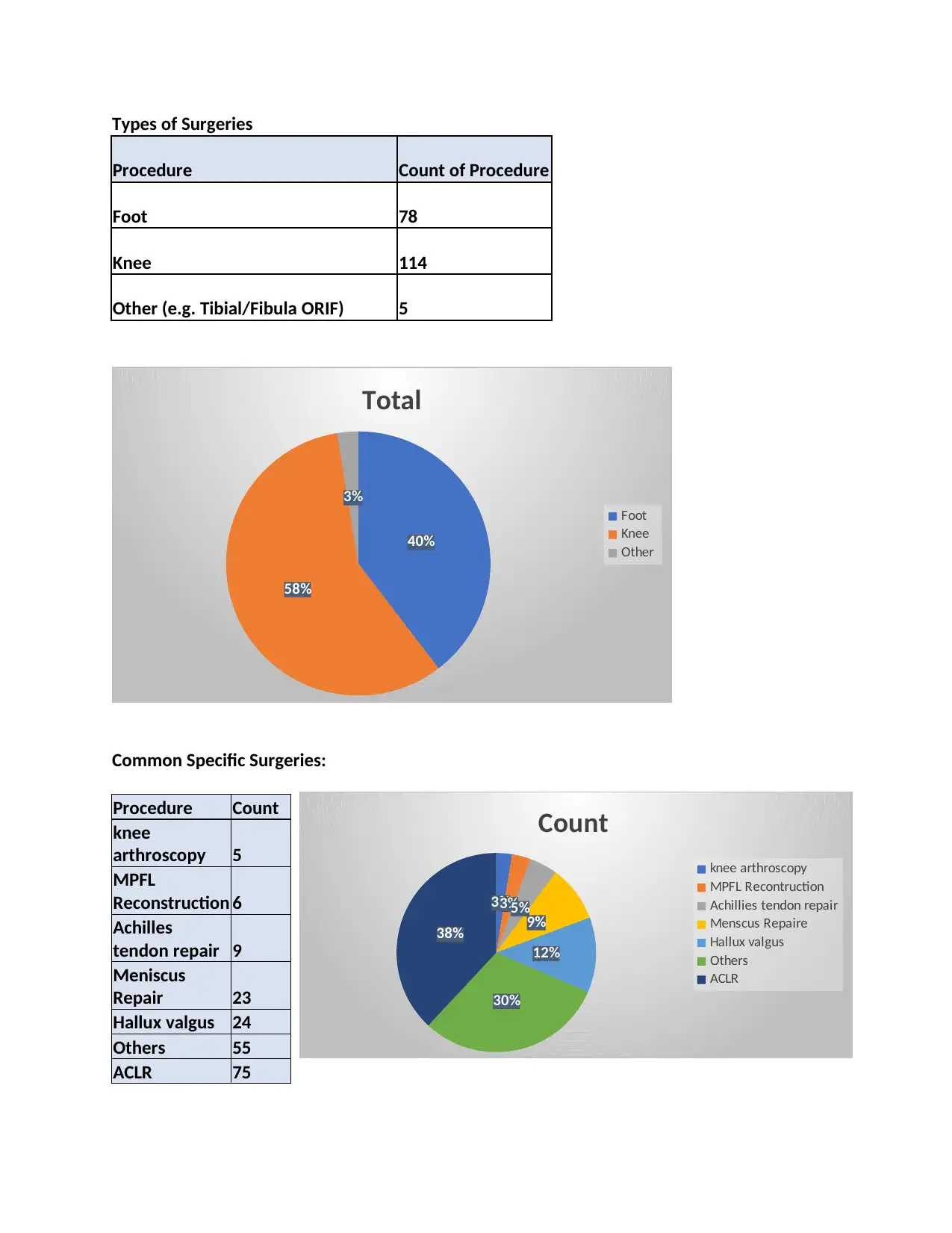
Types of Surgeries
Procedure Count of Procedure
Foot 78
Knee 114
Other (e.g. Tibial/Fibula ORIF) 5
40%
58%
3%
Total
Foot
Knee
Other
Common Specific Surgeries:
Procedure Count
knee
arthroscopy 5
MPFL
Reconstruction 6
Achilles
tendon repair 9
Meniscus
Repair 23
Hallux valgus 24
Others 55
ACLR 75
3%3%5%
9%
12%
30%
38%
Count
knee arthroscopy
MPFL Recontruction
Achillies tendon repair
Menscus Repaire
Hallux valgus
Others
ACLR
Procedure Count of Procedure
Foot 78
Knee 114
Other (e.g. Tibial/Fibula ORIF) 5
40%
58%
3%
Total
Foot
Knee
Other
Common Specific Surgeries:
Procedure Count
knee
arthroscopy 5
MPFL
Reconstruction 6
Achilles
tendon repair 9
Meniscus
Repair 23
Hallux valgus 24
Others 55
ACLR 75
3%3%5%
9%
12%
30%
38%
Count
knee arthroscopy
MPFL Recontruction
Achillies tendon repair
Menscus Repaire
Hallux valgus
Others
ACLR
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
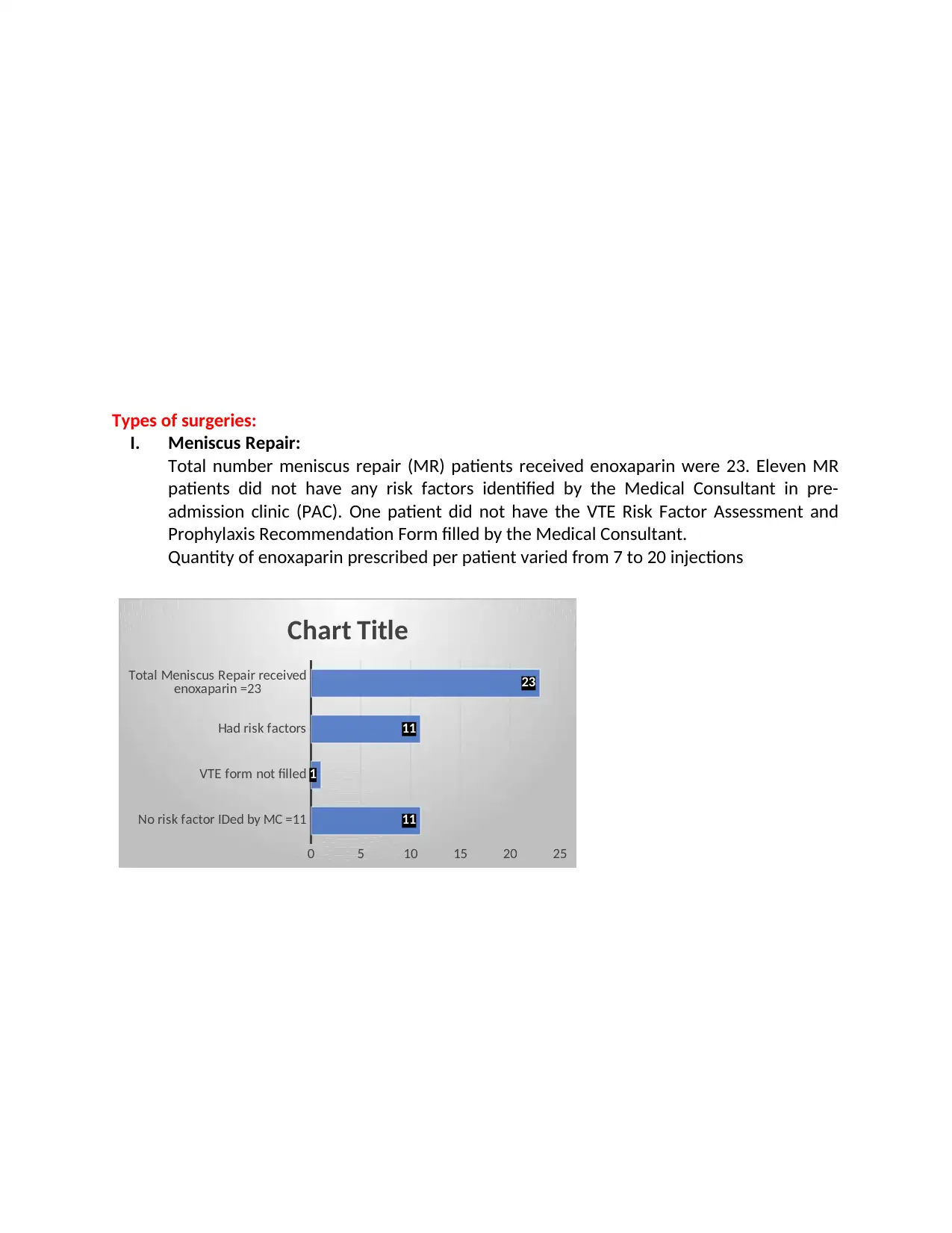
Types of surgeries:
I. Meniscus Repair:
Total number meniscus repair (MR) patients received enoxaparin were 23. Eleven MR
patients did not have any risk factors identified by the Medical Consultant in pre-
admission clinic (PAC). One patient did not have the VTE Risk Factor Assessment and
Prophylaxis Recommendation Form filled by the Medical Consultant.
Quantity of enoxaparin prescribed per patient varied from 7 to 20 injections
No risk factor IDed by MC =11
VTE form not filled
Had risk factors
Total Meniscus Repair received
enoxaparin =23
0 5 10 15 20 25
11
1
11
23
Chart Title
I. Meniscus Repair:
Total number meniscus repair (MR) patients received enoxaparin were 23. Eleven MR
patients did not have any risk factors identified by the Medical Consultant in pre-
admission clinic (PAC). One patient did not have the VTE Risk Factor Assessment and
Prophylaxis Recommendation Form filled by the Medical Consultant.
Quantity of enoxaparin prescribed per patient varied from 7 to 20 injections
No risk factor IDed by MC =11
VTE form not filled
Had risk factors
Total Meniscus Repair received
enoxaparin =23
0 5 10 15 20 25
11
1
11
23
Chart Title
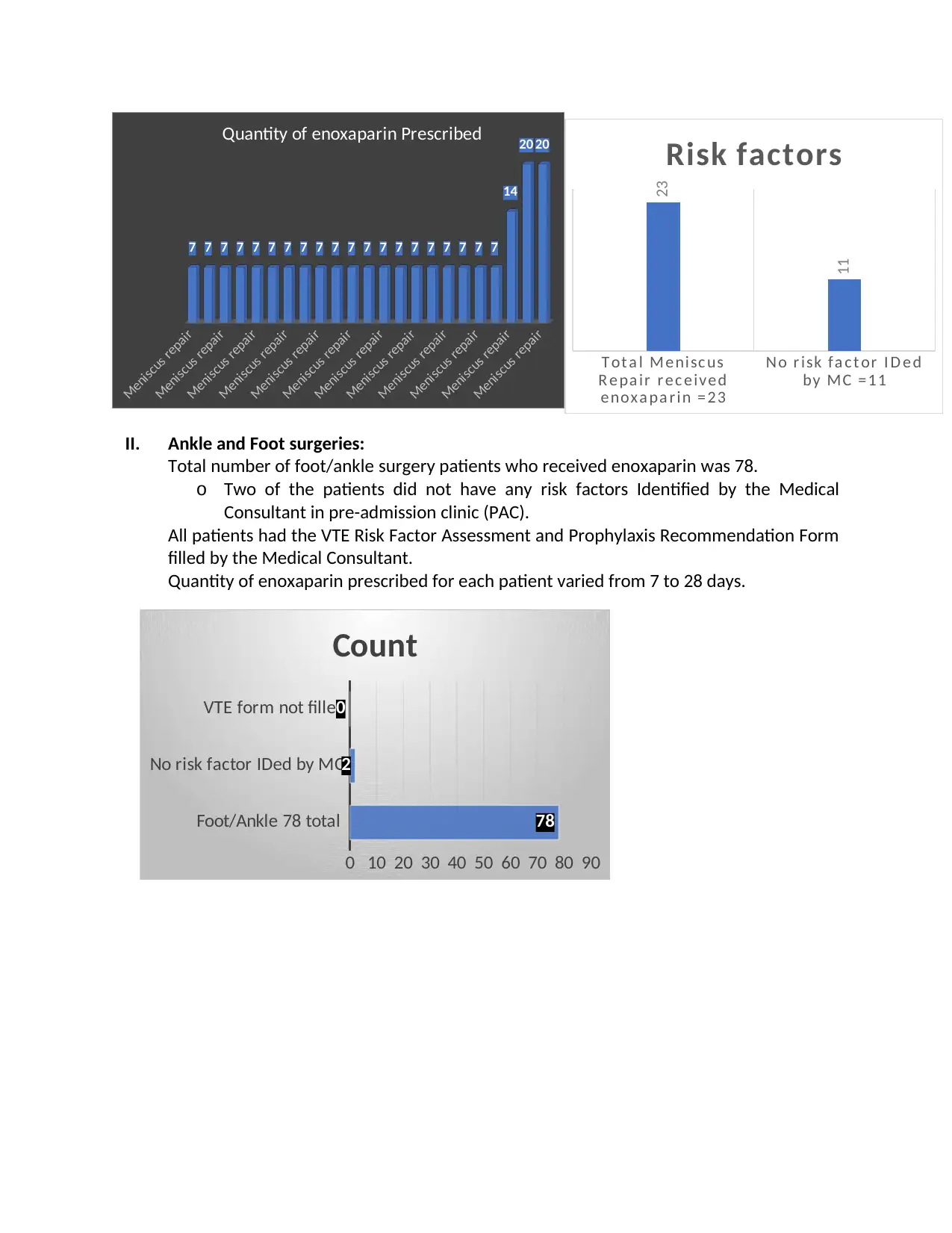
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7
14
20 20
Quantity of enoxaparin Prescribed
II. Ankle and Foot surgeries:
Total number of foot/ankle surgery patients who received enoxaparin was 78.
o Two of the patients did not have any risk factors Identified by the Medical
Consultant in pre-admission clinic (PAC).
All patients had the VTE Risk Factor Assessment and Prophylaxis Recommendation Form
filled by the Medical Consultant.
Quantity of enoxaparin prescribed for each patient varied from 7 to 28 days.
Foot/Ankle 78 total
No risk factor IDed by MC
VTE form not filled
0 10 20 30 40 50 60 70 80 90
78
2
0
Count
T ot a l M e nisc us
R e pa ir r e c e ive d
e nox a pa r in = 23
No r isk fa c t or I D e d
by MC = 11
23
11
Risk factors
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
Meniscus repair
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7
14
20 20
Quantity of enoxaparin Prescribed
II. Ankle and Foot surgeries:
Total number of foot/ankle surgery patients who received enoxaparin was 78.
o Two of the patients did not have any risk factors Identified by the Medical
Consultant in pre-admission clinic (PAC).
All patients had the VTE Risk Factor Assessment and Prophylaxis Recommendation Form
filled by the Medical Consultant.
Quantity of enoxaparin prescribed for each patient varied from 7 to 28 days.
Foot/Ankle 78 total
No risk factor IDed by MC
VTE form not filled
0 10 20 30 40 50 60 70 80 90
78
2
0
Count
T ot a l M e nisc us
R e pa ir r e c e ive d
e nox a pa r in = 23
No r isk fa c t or I D e d
by MC = 11
23
11
Risk factors
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
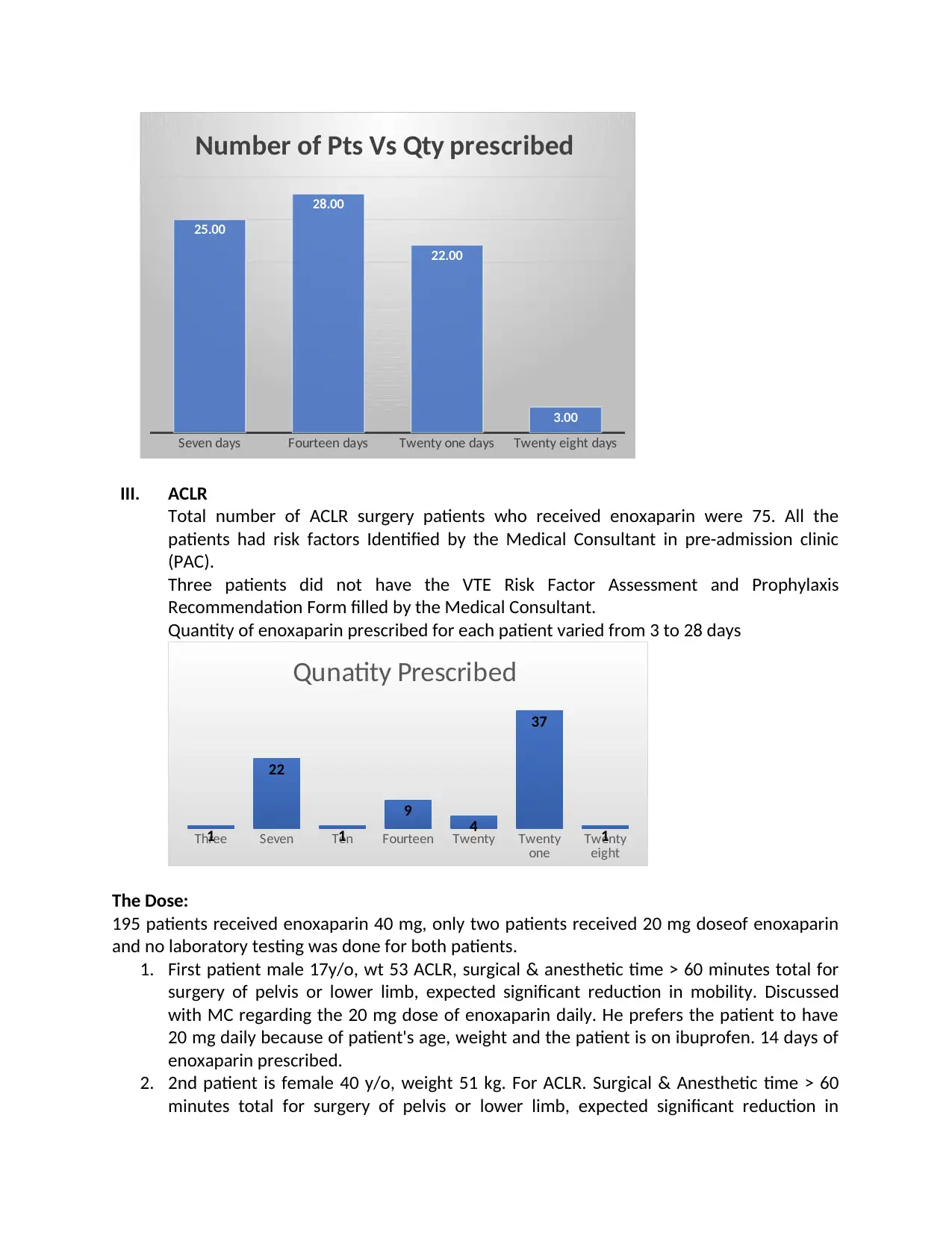
Seven days Fourteen days Twenty one days Twenty eight days
25.00
28.00
22.00
3.00
Number of Pts Vs Qty prescribed
III. ACLR
Total number of ACLR surgery patients who received enoxaparin were 75. All the
patients had risk factors Identified by the Medical Consultant in pre-admission clinic
(PAC).
Three patients did not have the VTE Risk Factor Assessment and Prophylaxis
Recommendation Form filled by the Medical Consultant.
Quantity of enoxaparin prescribed for each patient varied from 3 to 28 days
Three Seven Ten Fourteen Twenty Twenty
one Twenty
eight
1
22
1
9
4
37
1
Qunatity Prescribed
The Dose:
195 patients received enoxaparin 40 mg, only two patients received 20 mg doseof enoxaparin
and no laboratory testing was done for both patients.
1. First patient male 17y/o, wt 53 ACLR, surgical & anesthetic time > 60 minutes total for
surgery of pelvis or lower limb, expected significant reduction in mobility. Discussed
with MC regarding the 20 mg dose of enoxaparin daily. He prefers the patient to have
20 mg daily because of patient's age, weight and the patient is on ibuprofen. 14 days of
enoxaparin prescribed.
2. 2nd patient is female 40 y/o, weight 51 kg. For ACLR. Surgical & Anesthetic time > 60
minutes total for surgery of pelvis or lower limb, expected significant reduction in
25.00
28.00
22.00
3.00
Number of Pts Vs Qty prescribed
III. ACLR
Total number of ACLR surgery patients who received enoxaparin were 75. All the
patients had risk factors Identified by the Medical Consultant in pre-admission clinic
(PAC).
Three patients did not have the VTE Risk Factor Assessment and Prophylaxis
Recommendation Form filled by the Medical Consultant.
Quantity of enoxaparin prescribed for each patient varied from 3 to 28 days
Three Seven Ten Fourteen Twenty Twenty
one Twenty
eight
1
22
1
9
4
37
1
Qunatity Prescribed
The Dose:
195 patients received enoxaparin 40 mg, only two patients received 20 mg doseof enoxaparin
and no laboratory testing was done for both patients.
1. First patient male 17y/o, wt 53 ACLR, surgical & anesthetic time > 60 minutes total for
surgery of pelvis or lower limb, expected significant reduction in mobility. Discussed
with MC regarding the 20 mg dose of enoxaparin daily. He prefers the patient to have
20 mg daily because of patient's age, weight and the patient is on ibuprofen. 14 days of
enoxaparin prescribed.
2. 2nd patient is female 40 y/o, weight 51 kg. For ACLR. Surgical & Anesthetic time > 60
minutes total for surgery of pelvis or lower limb, expected significant reduction in
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
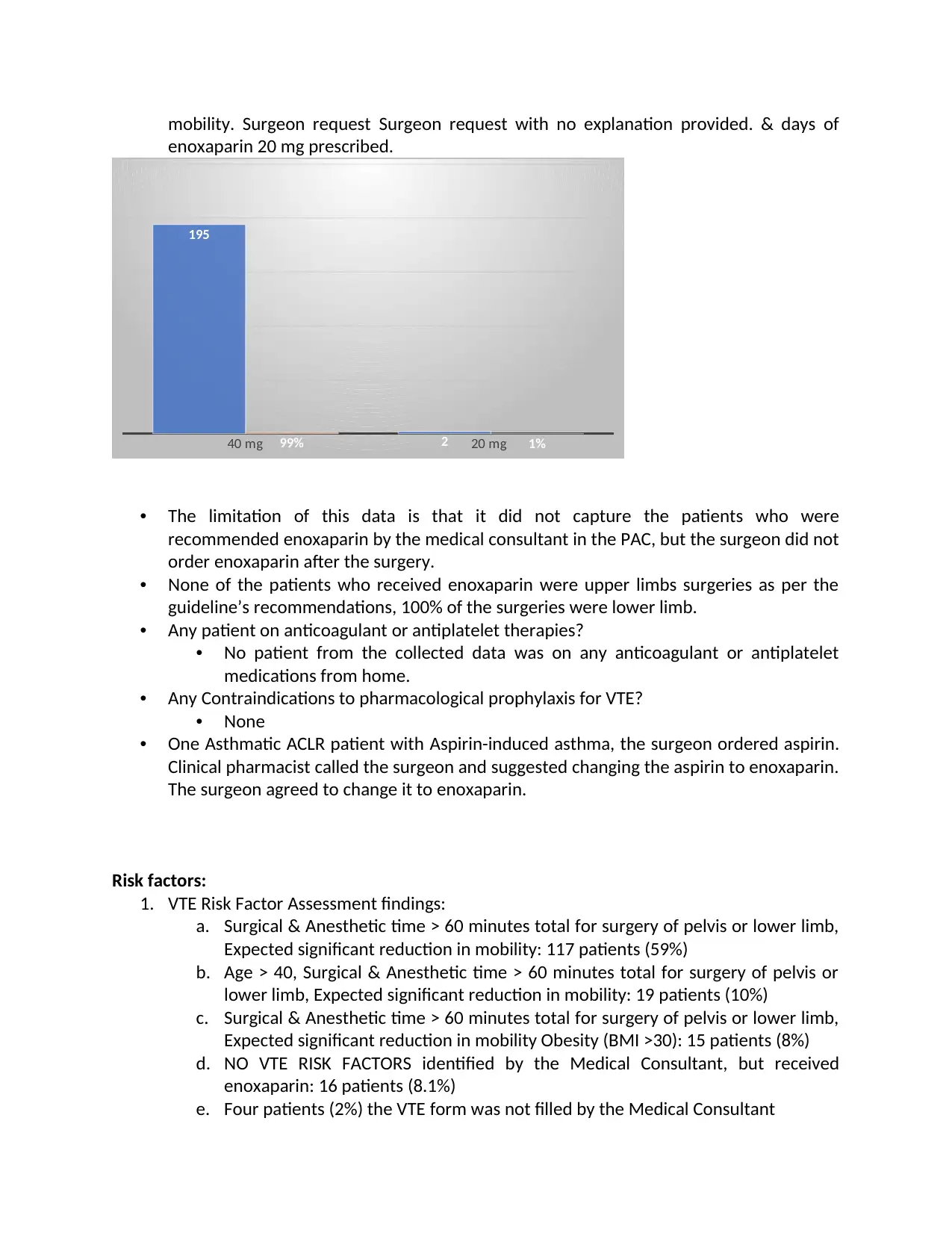
mobility. Surgeon request Surgeon request with no explanation provided. & days of
enoxaparin 20 mg prescribed.
40 mg 20 mg
195
299% 1%
• The limitation of this data is that it did not capture the patients who were
recommended enoxaparin by the medical consultant in the PAC, but the surgeon did not
order enoxaparin after the surgery.
• None of the patients who received enoxaparin were upper limbs surgeries as per the
guideline’s recommendations, 100% of the surgeries were lower limb.
• Any patient on anticoagulant or antiplatelet therapies?
• No patient from the collected data was on any anticoagulant or antiplatelet
medications from home.
• Any Contraindications to pharmacological prophylaxis for VTE?
• None
• One Asthmatic ACLR patient with Aspirin-induced asthma, the surgeon ordered aspirin.
Clinical pharmacist called the surgeon and suggested changing the aspirin to enoxaparin.
The surgeon agreed to change it to enoxaparin.
Risk factors:
1. VTE Risk Factor Assessment findings:
a. Surgical & Anesthetic time > 60 minutes total for surgery of pelvis or lower limb,
Expected significant reduction in mobility: 117 patients (59%)
b. Age > 40, Surgical & Anesthetic time > 60 minutes total for surgery of pelvis or
lower limb, Expected significant reduction in mobility: 19 patients (10%)
c. Surgical & Anesthetic time > 60 minutes total for surgery of pelvis or lower limb,
Expected significant reduction in mobility Obesity (BMI >30): 15 patients (8%)
d. NO VTE RISK FACTORS identified by the Medical Consultant, but received
enoxaparin: 16 patients (8.1%)
e. Four patients (2%) the VTE form was not filled by the Medical Consultant
enoxaparin 20 mg prescribed.
40 mg 20 mg
195
299% 1%
• The limitation of this data is that it did not capture the patients who were
recommended enoxaparin by the medical consultant in the PAC, but the surgeon did not
order enoxaparin after the surgery.
• None of the patients who received enoxaparin were upper limbs surgeries as per the
guideline’s recommendations, 100% of the surgeries were lower limb.
• Any patient on anticoagulant or antiplatelet therapies?
• No patient from the collected data was on any anticoagulant or antiplatelet
medications from home.
• Any Contraindications to pharmacological prophylaxis for VTE?
• None
• One Asthmatic ACLR patient with Aspirin-induced asthma, the surgeon ordered aspirin.
Clinical pharmacist called the surgeon and suggested changing the aspirin to enoxaparin.
The surgeon agreed to change it to enoxaparin.
Risk factors:
1. VTE Risk Factor Assessment findings:
a. Surgical & Anesthetic time > 60 minutes total for surgery of pelvis or lower limb,
Expected significant reduction in mobility: 117 patients (59%)
b. Age > 40, Surgical & Anesthetic time > 60 minutes total for surgery of pelvis or
lower limb, Expected significant reduction in mobility: 19 patients (10%)
c. Surgical & Anesthetic time > 60 minutes total for surgery of pelvis or lower limb,
Expected significant reduction in mobility Obesity (BMI >30): 15 patients (8%)
d. NO VTE RISK FACTORS identified by the Medical Consultant, but received
enoxaparin: 16 patients (8.1%)
e. Four patients (2%) the VTE form was not filled by the Medical Consultant
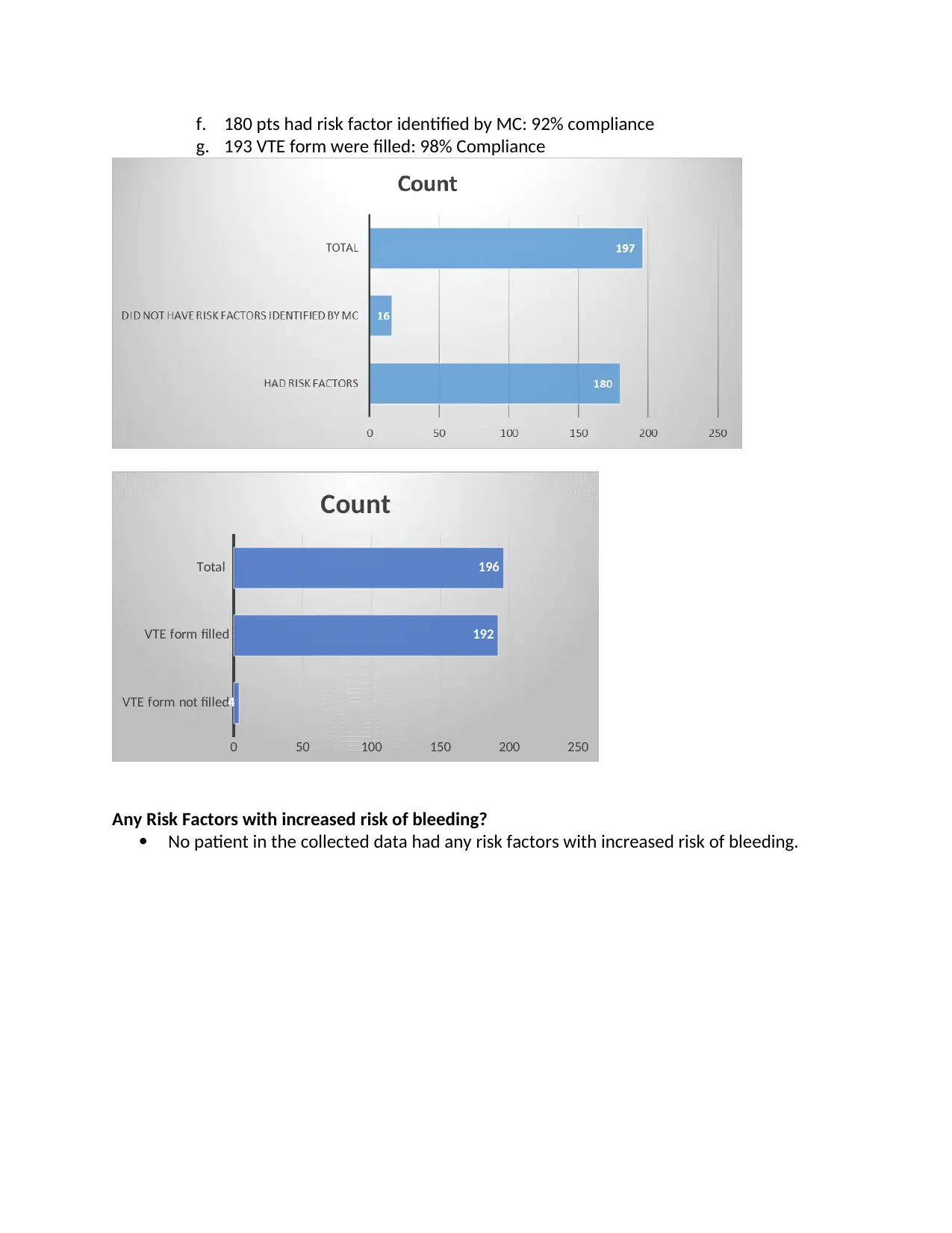
f. 180 pts had risk factor identified by MC: 92% compliance
g. 193 VTE form were filled: 98% Compliance
VTE form not filled
VTE form filled
Total
0 50 100 150 200 250
4
192
196
Count
Any Risk Factors with increased risk of bleeding?
No patient in the collected data had any risk factors with increased risk of bleeding.
g. 193 VTE form were filled: 98% Compliance
VTE form not filled
VTE form filled
Total
0 50 100 150 200 250
4
192
196
Count
Any Risk Factors with increased risk of bleeding?
No patient in the collected data had any risk factors with increased risk of bleeding.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 20
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.