Research Report: Sample Application for HREC Approval (NURBN3022)
VerifiedAdded on 2022/10/02
|14
|4182
|351
Report
AI Summary
This report presents a sample application for Human Research Ethics Committee (HREC) approval for a research project investigating the effectiveness of Continuous Subcutaneous Insulin Infusion (CSII) versus Multiple Dose Injections (MDI) in treating Type 1 diabetes in children and young adults. The project aims to determine which treatment method is more effective, considering factors such as age of dose initiation and HbA1c levels. The research involves collecting data from participants, categorized into CSII and MDI groups, and analyzing it using statistical tools. The methodology includes a detailed outline of data collection techniques, participant recruitment strategies, and data analysis methods. The report also addresses the significance of the research, supported by relevant literature, and outlines ethical considerations, including participant recruitment, data privacy, and informed consent. The study intends to recruit 1000 participants aged 0-18 years and will collect data at three stages: prior to medication, after six months, and after one year. The report highlights inclusive and exclusive criteria for participant selection and describes the statistical tools to be used for data analysis, aiming to provide comprehensive insights into the comparative effectiveness of CSII and MDI treatments.
Sample Application for
HREC Approval
Total word limit for this assessment item is 2000 words
1. PROJECT DETAILS
Project title:
What type of project is this? (Tick as many as apply)
Masters project Clinical Trial Postgraduate Diploma
Undergraduate Student Research Project Honours
PhD Staff Research Project Other
Through which School/Section is the research to be conducted?
2. RESEARCHERS
Principal Researcher (STAFF MEMBER ONLY)
Title & Name: Dr ABCD
Position: Senior Lecturer
School/Section: School of Nursing and Healthcare Professions
Phone number: xxxxxx
Email address: abcd@mystudy.com.au
Please list academic qualifications: PhD RN
Describe what this researcher will do in
the context of this project:
The lead researcher will be an expert guide though all
aspects of the project.
Include a brief summary of relevant
experience for this project:
Supervision of higher degree students, multiple
publications to peer reviewed journals and successful
competitive grants applications.
Student/Other Researcher/s
Title & Name:
Position:
School/Section:
Phone number:
Email address:
Student ID number:
Please list academic qualifications:
Describe what this researcher will do in
the context of this project:
Include a brief summary of relevant
1
HREC Approval
Total word limit for this assessment item is 2000 words
1. PROJECT DETAILS
Project title:
What type of project is this? (Tick as many as apply)
Masters project Clinical Trial Postgraduate Diploma
Undergraduate Student Research Project Honours
PhD Staff Research Project Other
Through which School/Section is the research to be conducted?
2. RESEARCHERS
Principal Researcher (STAFF MEMBER ONLY)
Title & Name: Dr ABCD
Position: Senior Lecturer
School/Section: School of Nursing and Healthcare Professions
Phone number: xxxxxx
Email address: abcd@mystudy.com.au
Please list academic qualifications: PhD RN
Describe what this researcher will do in
the context of this project:
The lead researcher will be an expert guide though all
aspects of the project.
Include a brief summary of relevant
experience for this project:
Supervision of higher degree students, multiple
publications to peer reviewed journals and successful
competitive grants applications.
Student/Other Researcher/s
Title & Name:
Position:
School/Section:
Phone number:
Email address:
Student ID number:
Please list academic qualifications:
Describe what this researcher will do in
the context of this project:
Include a brief summary of relevant
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sample Application for
HREC Approval
experience for this project:
3. LAY DESCRIPTION
Provide a brief outline of the project describing in everyday, jargon-free language the key aspects
of the research (e.g., who will be participating, what information will be collected and by what
means, what participants will be required to do, etc.) and the key research aims. (300 words max.)
Type 1 diabetes is of the most harmful health condition among the children and young adults.
Approximately 26000 or more individual or more in UK is affected by the disease and excessive
carbohydrate in tale in the form of junk foods and soft drinks are considered as main reason behind
this tendency. This research aims to investigate better medical opinion between CSII and MDI for
treatment of Type 1 diabetes in children of age 0yr to 18 yr. The research associated with the factors
such as age of dose initiation and HbA1c percentage of body which are affecting glycemic
condition of the body . Data will be taken from the primary sources by observing participants of the
research. The data will be collected in the supervision of our professor. The participants will be
categorized into two groups such as patients completed 2 year of treatment of CSII and the patients
have completed the medication MDI. The participants were children that is why concerns will be
taken from their parents. As an investigator, it is always important to maintain dignity of the
participants and their opinions should be considered. Samples will be collected from the local
hospitals with appropriate concerns of authority. Dosages of CSII (Continuous subcutaneous insulin
infusion) and MDI (Multiple dose injection), initial age for starting the dose, activation of HbA1c
and its percentage in the body will be collected (Chlup et al.,2018). Some demographic parameters
will also take under the consideration, as the effect in body of certain dosage can be different for
different individuals and can varies in families. The demographic questions will be asked for
maintaining same base lines among the group of participants. These two different therapies are
highly acclaimed therapy for diabetic children or patients around the world. The research aims to
understand the efficiencies of the two therapies with comparison.
2
HREC Approval
experience for this project:
3. LAY DESCRIPTION
Provide a brief outline of the project describing in everyday, jargon-free language the key aspects
of the research (e.g., who will be participating, what information will be collected and by what
means, what participants will be required to do, etc.) and the key research aims. (300 words max.)
Type 1 diabetes is of the most harmful health condition among the children and young adults.
Approximately 26000 or more individual or more in UK is affected by the disease and excessive
carbohydrate in tale in the form of junk foods and soft drinks are considered as main reason behind
this tendency. This research aims to investigate better medical opinion between CSII and MDI for
treatment of Type 1 diabetes in children of age 0yr to 18 yr. The research associated with the factors
such as age of dose initiation and HbA1c percentage of body which are affecting glycemic
condition of the body . Data will be taken from the primary sources by observing participants of the
research. The data will be collected in the supervision of our professor. The participants will be
categorized into two groups such as patients completed 2 year of treatment of CSII and the patients
have completed the medication MDI. The participants were children that is why concerns will be
taken from their parents. As an investigator, it is always important to maintain dignity of the
participants and their opinions should be considered. Samples will be collected from the local
hospitals with appropriate concerns of authority. Dosages of CSII (Continuous subcutaneous insulin
infusion) and MDI (Multiple dose injection), initial age for starting the dose, activation of HbA1c
and its percentage in the body will be collected (Chlup et al.,2018). Some demographic parameters
will also take under the consideration, as the effect in body of certain dosage can be different for
different individuals and can varies in families. The demographic questions will be asked for
maintaining same base lines among the group of participants. These two different therapies are
highly acclaimed therapy for diabetic children or patients around the world. The research aims to
understand the efficiencies of the two therapies with comparison.
2

Sample Application for
HREC Approval
4. RESEARCH AIMS & SIGNIFICANCE
State the aims, key research questions, and significance of the project. Also provide a brief
description of the relevance of your proposed project to current research, supported by the
literature. (500 words max.)
The research question is whether Continuous subcutaneous insulin infusion is more
effective than Multiple Dose Insulin for treating Type 1 Diabetes in children or young adults with the
reference of some physiological conditions. There are some factor, which make this research
significant and scientifically relevant. The factors are the research refers several scholarly article
and peer- reviewed journals by maintaining the ethical responsibilities towards the participants.
The project will include quantitative data under supervision of health professionals and qualified
professors and this methodology will make it accurate with proper information. The standard errors
and deviation, standard error and other statistically significant data will be collected by interpreting
probability values and t values. The data interpretation will make the research more evident and
reliable.
According to Batajoo, Messina & Wilson ,(2012) the T1DM(Type 1 Diabetes Mellitus ) can be
controlled with prompt outcome when CSII is applied rather than application of MDI. This research
was based on ten-year long observation from 1999 to 2009 on patients of a certain endocrinologist.
Data were collected in several phases such as 6 months prior to the dosage of CSII, after
continuing the dose for 6 months and after one year of that dosage. Glycated Haemoglobin
(HbA1c) was considered as controlling factor for health of the participants(Bergenstal et al.,2016).
The experiment showed only children over the age of 11 year were affected by then dosages but
no persistent result were found for children who are below 1 years. CSII and MDI therapy represent
injecting insulin under the cutaneous membrane according to the application method; these
therapies are called insulin pumps (Pickup, Reznik & Sutton,2017). Injections are contained with
different dose of insulin. Comparison of these4 two method has no proper RCT evidences but
there are some literature which supports efficiency of CSII. For example, a therapeutic experiment
was done to calculate the basal insulin rate in controlling euglycemia without any her medicinal
treatments during the early morning scans (Krause, Barrington & Cranston, 2019). There are some
studies, which shows 75% reduction of hypoglycemia during treatment in comparison of MDI.
Various Studies regarding MDI and CSII by mentioning appropriate analyses have improved quality
of life, freedom to choose the treatment by understanding the outcomes. The studies also help
doctors and medical professionals in their decisions (Danne et. al, 2018). Hypoglycaemia and
diabetic ketoacidosis are two health conditions arise due to Type 1 Diabetes these conditions are
lowered by the application of insulin pumps. A 6-year long population based study was conducted
3
HREC Approval
4. RESEARCH AIMS & SIGNIFICANCE
State the aims, key research questions, and significance of the project. Also provide a brief
description of the relevance of your proposed project to current research, supported by the
literature. (500 words max.)
The research question is whether Continuous subcutaneous insulin infusion is more
effective than Multiple Dose Insulin for treating Type 1 Diabetes in children or young adults with the
reference of some physiological conditions. There are some factor, which make this research
significant and scientifically relevant. The factors are the research refers several scholarly article
and peer- reviewed journals by maintaining the ethical responsibilities towards the participants.
The project will include quantitative data under supervision of health professionals and qualified
professors and this methodology will make it accurate with proper information. The standard errors
and deviation, standard error and other statistically significant data will be collected by interpreting
probability values and t values. The data interpretation will make the research more evident and
reliable.
According to Batajoo, Messina & Wilson ,(2012) the T1DM(Type 1 Diabetes Mellitus ) can be
controlled with prompt outcome when CSII is applied rather than application of MDI. This research
was based on ten-year long observation from 1999 to 2009 on patients of a certain endocrinologist.
Data were collected in several phases such as 6 months prior to the dosage of CSII, after
continuing the dose for 6 months and after one year of that dosage. Glycated Haemoglobin
(HbA1c) was considered as controlling factor for health of the participants(Bergenstal et al.,2016).
The experiment showed only children over the age of 11 year were affected by then dosages but
no persistent result were found for children who are below 1 years. CSII and MDI therapy represent
injecting insulin under the cutaneous membrane according to the application method; these
therapies are called insulin pumps (Pickup, Reznik & Sutton,2017). Injections are contained with
different dose of insulin. Comparison of these4 two method has no proper RCT evidences but
there are some literature which supports efficiency of CSII. For example, a therapeutic experiment
was done to calculate the basal insulin rate in controlling euglycemia without any her medicinal
treatments during the early morning scans (Krause, Barrington & Cranston, 2019). There are some
studies, which shows 75% reduction of hypoglycemia during treatment in comparison of MDI.
Various Studies regarding MDI and CSII by mentioning appropriate analyses have improved quality
of life, freedom to choose the treatment by understanding the outcomes. The studies also help
doctors and medical professionals in their decisions (Danne et. al, 2018). Hypoglycaemia and
diabetic ketoacidosis are two health conditions arise due to Type 1 Diabetes these conditions are
lowered by the application of insulin pumps. A 6-year long population based study was conducted
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Sample Application for
HREC Approval
with 446 diabetic patients in Australia to assess metabolic control by applying insulin pumps. This
research covers certain unplanned conditions during the research and exposures of insulin pump
and multiple daily insulin injections were assessed. Insulin pumps were more effective in better
glycaemic control as risks related to hypoglycaemia and diabetic ketoacidosis lowered among
children, young adult and others (Karges et. al , 2017). Another analysis revealed that an important
rate of reduction (mean difference 0.37) is observed in glycosylated hemoglobin in patients while
they were treated with continuous subcutaneous insulin infusion. On the contrary, the patients
treated with multiple daily injections did not show higher reduction rate than CSII. This result was
established in both children and adult and no significance in hypoglycaemia was found (Karges et.
al , 2017).
5. RESEARCH METHODOLOGY
Provide an outline of the proposed method, including details of data collection techniques, tasks
participants will be asked to do, the estimated time commitment involved, and how data will be
analysed. (500 words max).
The data for the research were collected by random selection with 1:1 randomisation.
There are inclusive and exclusive criteria for selection of the participants.
The inclusive criteria are:
Participants should be patient of T1DM
They should be aged from 0-18 year
4
HREC Approval
with 446 diabetic patients in Australia to assess metabolic control by applying insulin pumps. This
research covers certain unplanned conditions during the research and exposures of insulin pump
and multiple daily insulin injections were assessed. Insulin pumps were more effective in better
glycaemic control as risks related to hypoglycaemia and diabetic ketoacidosis lowered among
children, young adult and others (Karges et. al , 2017). Another analysis revealed that an important
rate of reduction (mean difference 0.37) is observed in glycosylated hemoglobin in patients while
they were treated with continuous subcutaneous insulin infusion. On the contrary, the patients
treated with multiple daily injections did not show higher reduction rate than CSII. This result was
established in both children and adult and no significance in hypoglycaemia was found (Karges et.
al , 2017).
5. RESEARCH METHODOLOGY
Provide an outline of the proposed method, including details of data collection techniques, tasks
participants will be asked to do, the estimated time commitment involved, and how data will be
analysed. (500 words max).
The data for the research were collected by random selection with 1:1 randomisation.
There are inclusive and exclusive criteria for selection of the participants.
The inclusive criteria are:
Participants should be patient of T1DM
They should be aged from 0-18 year
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sample Application for
HREC Approval
Parents or legal guardians should give consent for the observation
Doctor of the patient should give consent
The participants or their parents are agreed to use insulin pumps as medium of treatment
The exclusive criteria are:
Patients with other severe medical conditions rather than T1DM
Patients with conterminous diseases
Patients with thyroid disease
Patients with eating disorder or incapable of leaving gluten contain foods
Patients over 18 years
The patients will be chosen in randomised manner and their details will be collected from
the computer based data of hospitals. An informing text message or mail will be sent to everye
chosen candidates. Mails will forward to the parents and legal guardians for those candidates
who are below 16. Those mails will invite the candidates for the documentation and
authentication for concern giving.
The candidates or the parents will be provided some documents which will explain every
detail regarding the research and they will be requested to sign the documents if they would
agree with the terms.
Data will be collected from the computer-based records and flow sheets provided by the
hospitals. The collected data then revised by statistical tools for example: SAS. SAS is used as
cost effective analytical tool. Every detail is compared and evaluated by the tools(Benkhadra et
al.,2017). The statistical analysis will be done by finding the variances to find out mean, standard
deviation , proportion and frequencies. There are multiple variances for the experiments so t-test
will be executed by ANOVA analysis tool (Kucuk et al.,2016). Demographic questionnaires will
be recorded or analysed by SPSS tool. Samples t-test will be executed for comparing clinical
values over the conditions of applying CSII of Application of MDI. SPSS tool is ideal to interpret
numerous demographical questions (Fragkioudaki, Mavragani & Moutsopoulos, 2016). Agreed
participants will be asked some demographical questions regarding their age, sex, birthplace,
parents, body mass, BMI.
There are some commitments from the researcher’s end;
Details of every participants will be safe and secure
No information will passed by the researcher
Maintenance of privacy will be superior
The patients and their family will be informed for every minute details regarding
medications and application of any dosage
5
HREC Approval
Parents or legal guardians should give consent for the observation
Doctor of the patient should give consent
The participants or their parents are agreed to use insulin pumps as medium of treatment
The exclusive criteria are:
Patients with other severe medical conditions rather than T1DM
Patients with conterminous diseases
Patients with thyroid disease
Patients with eating disorder or incapable of leaving gluten contain foods
Patients over 18 years
The patients will be chosen in randomised manner and their details will be collected from
the computer based data of hospitals. An informing text message or mail will be sent to everye
chosen candidates. Mails will forward to the parents and legal guardians for those candidates
who are below 16. Those mails will invite the candidates for the documentation and
authentication for concern giving.
The candidates or the parents will be provided some documents which will explain every
detail regarding the research and they will be requested to sign the documents if they would
agree with the terms.
Data will be collected from the computer-based records and flow sheets provided by the
hospitals. The collected data then revised by statistical tools for example: SAS. SAS is used as
cost effective analytical tool. Every detail is compared and evaluated by the tools(Benkhadra et
al.,2017). The statistical analysis will be done by finding the variances to find out mean, standard
deviation , proportion and frequencies. There are multiple variances for the experiments so t-test
will be executed by ANOVA analysis tool (Kucuk et al.,2016). Demographic questionnaires will
be recorded or analysed by SPSS tool. Samples t-test will be executed for comparing clinical
values over the conditions of applying CSII of Application of MDI. SPSS tool is ideal to interpret
numerous demographical questions (Fragkioudaki, Mavragani & Moutsopoulos, 2016). Agreed
participants will be asked some demographical questions regarding their age, sex, birthplace,
parents, body mass, BMI.
There are some commitments from the researcher’s end;
Details of every participants will be safe and secure
No information will passed by the researcher
Maintenance of privacy will be superior
The patients and their family will be informed for every minute details regarding
medications and application of any dosage
5
Sample Application for
HREC Approval
Only the concerned medical authority will take decision for the patients
Patients can withdraw themselves from the research whenever they want
No ethical or physical harm will be conducted
The research will take at least a year to complete as three stages will be involved
Stage1 : prior to the medication
Stage 2 : after 6 months of medication
Stage 3 : after one year of the medication .
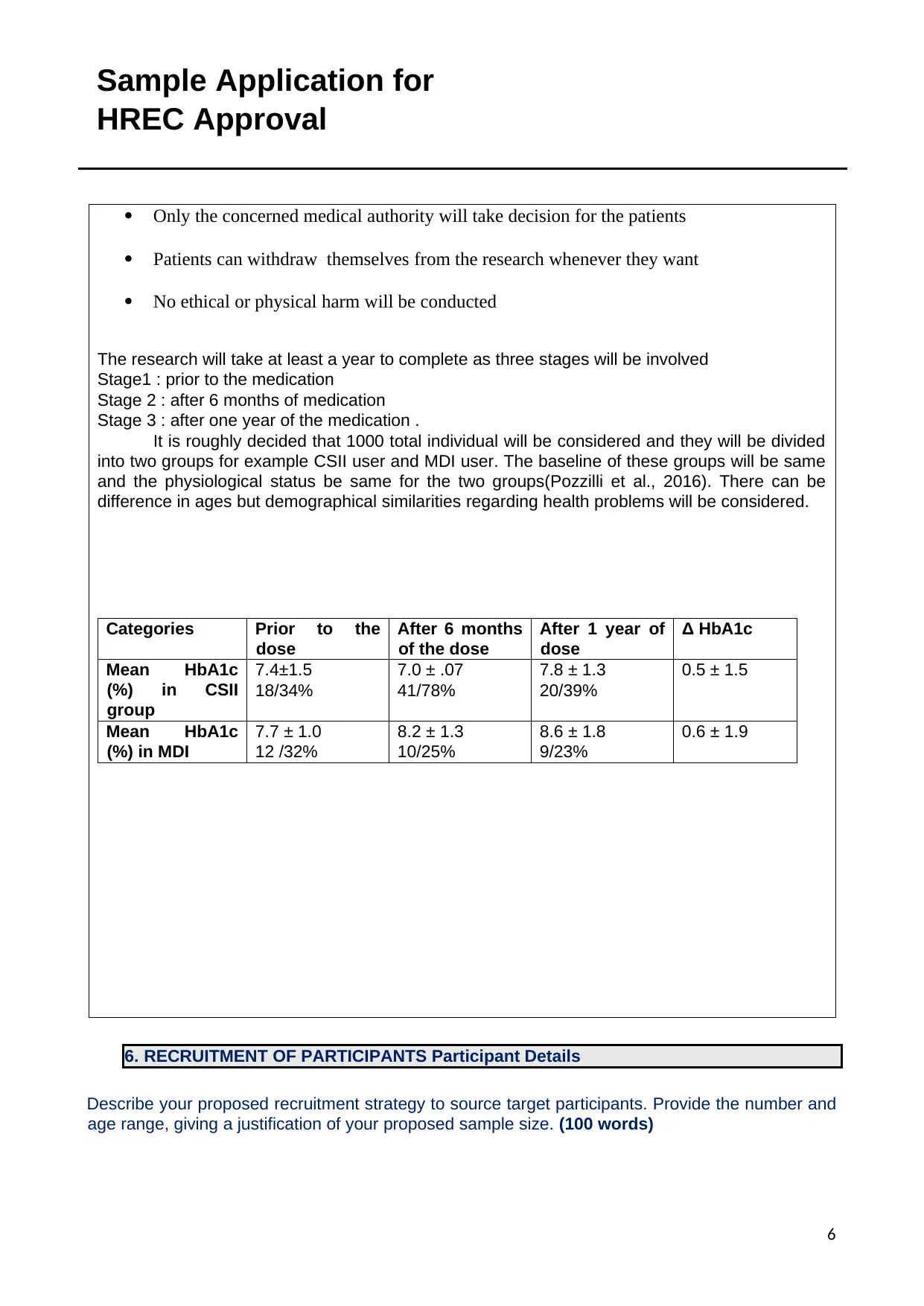
It is roughly decided that 1000 total individual will be considered and they will be divided
into two groups for example CSII user and MDI user. The baseline of these groups will be same
and the physiological status be same for the two groups(Pozzilli et al., 2016). There can be
difference in ages but demographical similarities regarding health problems will be considered.
Categories Prior to the
dose
After 6 months
of the dose
After 1 year of
dose
Δ HbA1c
Mean HbA1c
(%) in CSII
group
7.4±1.5
18/34%
7.0 ± .07
41/78%
7.8 ± 1.3
20/39%
0.5 ± 1.5
Mean HbA1c
(%) in MDI
7.7 ± 1.0
12 /32%
8.2 ± 1.3
10/25%
8.6 ± 1.8
9/23%
0.6 ± 1.9
6. RECRUITMENT OF PARTICIPANTS Participant Details
Describe your proposed recruitment strategy to source target participants. Provide the number and
age range, giving a justification of your proposed sample size. (100 words)
6
HREC Approval
Only the concerned medical authority will take decision for the patients
Patients can withdraw themselves from the research whenever they want
No ethical or physical harm will be conducted
The research will take at least a year to complete as three stages will be involved
Stage1 : prior to the medication
Stage 2 : after 6 months of medication
Stage 3 : after one year of the medication .
It is roughly decided that 1000 total individual will be considered and they will be divided
into two groups for example CSII user and MDI user. The baseline of these groups will be same
and the physiological status be same for the two groups(Pozzilli et al., 2016). There can be
difference in ages but demographical similarities regarding health problems will be considered.
Categories Prior to the
dose
After 6 months
of the dose
After 1 year of
dose
Δ HbA1c
Mean HbA1c
(%) in CSII
group
7.4±1.5
18/34%
7.0 ± .07
41/78%
7.8 ± 1.3
20/39%
0.5 ± 1.5
Mean HbA1c
(%) in MDI
7.7 ± 1.0
12 /32%
8.2 ± 1.3
10/25%
8.6 ± 1.8
9/23%
0.6 ± 1.9
6. RECRUITMENT OF PARTICIPANTS Participant Details
Describe your proposed recruitment strategy to source target participants. Provide the number and
age range, giving a justification of your proposed sample size. (100 words)
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Sample Application for
HREC Approval
In this experiment,number of patrcipants will be 1000. 500 of them will be user of MDI and
500 of them will be treated with CSII. The age range is defined from 0-18years: this is a long
range of age . the reson behind choosing such wide age range among the sample is to interpret
various amount of data which will lead the research towards its relevance. The sample size large
as the more sample increase the probability of better result (Gignac & Szodorai, 2016). On the
contrary, the age range s wide so small sample cannot give the appropriate result.
Target participants
Who are the target participants? (Tick as many as applicable)
Students or staff of this University
Adults (over the age of 18 years and competent to give consent)
Children/legal minors (under the age of 18 years, with parental consent)*
Elderly individuals
Individuals from non–English-speaking backgrounds
Pensioners or welfare recipients
Intellectually or mentally impaired individuals unable/with compromised capacity to provide onsent
c
Physically disabled individuals
Patients or clients
Prisoners, parolees, or wards of the state
Individuals highly dependent on medical care with a compromised capacity to give consent
7
HREC Approval
In this experiment,number of patrcipants will be 1000. 500 of them will be user of MDI and
500 of them will be treated with CSII. The age range is defined from 0-18years: this is a long
range of age . the reson behind choosing such wide age range among the sample is to interpret
various amount of data which will lead the research towards its relevance. The sample size large
as the more sample increase the probability of better result (Gignac & Szodorai, 2016). On the
contrary, the age range s wide so small sample cannot give the appropriate result.
Target participants
Who are the target participants? (Tick as many as applicable)
Students or staff of this University
Adults (over the age of 18 years and competent to give consent)
Children/legal minors (under the age of 18 years, with parental consent)*
Elderly individuals
Individuals from non–English-speaking backgrounds
Pensioners or welfare recipients
Intellectually or mentally impaired individuals unable/with compromised capacity to provide onsent
c
Physically disabled individuals
Patients or clients
Prisoners, parolees, or wards of the state
Individuals highly dependent on medical care with a compromised capacity to give consent
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sample Application for
HREC Approval
Aboriginal and/or Torres Strait Island communities
Women who are pregnant and the human foetus
People who may be involved in illegal activities
*Parental consent may not be required in some instances - refer National Statement, 4.2.8 & 4.2.9
7. RISK MANAGEMENT
This section raises the issue of your duty of care toward research participants. To what risks are
participants subjected? What will you do should an emergency occur, or should a participant
become upset or distressed? What is your risk management strategy? Refer National Statement:
Section 2.1 Risk and Benefit
Research Activities
Which of the following activities will the research involve? (Tick as many as apply)
Use of a questionnaire (attach copy)
Interviews (attach interview questions)
Observation of participants without their knowledge
Participant observation
Audio- or video-taping of interviewees or events
Access to personal and/or confidential data (including student, patient or client data) without
participants’ specific consent
Administration of any stimuli, tasks, investigations or procedures which may be experienced
by participants as physically or mentally painful, stressful or unpleasant during or after the
research process
Performance of any acts which may diminish the self-esteem of participants or cause them to
experience embarrassment, regret or depression
Use of non-treatment of placebo control conditions
Collection of body tissues or fluid samples
8
HREC Approval
Aboriginal and/or Torres Strait Island communities
Women who are pregnant and the human foetus
People who may be involved in illegal activities
*Parental consent may not be required in some instances - refer National Statement, 4.2.8 & 4.2.9
7. RISK MANAGEMENT
This section raises the issue of your duty of care toward research participants. To what risks are
participants subjected? What will you do should an emergency occur, or should a participant
become upset or distressed? What is your risk management strategy? Refer National Statement:
Section 2.1 Risk and Benefit
Research Activities
Which of the following activities will the research involve? (Tick as many as apply)
Use of a questionnaire (attach copy)
Interviews (attach interview questions)
Observation of participants without their knowledge
Participant observation
Audio- or video-taping of interviewees or events
Access to personal and/or confidential data (including student, patient or client data) without
participants’ specific consent
Administration of any stimuli, tasks, investigations or procedures which may be experienced
by participants as physically or mentally painful, stressful or unpleasant during or after the
research process
Performance of any acts which may diminish the self-esteem of participants or cause them to
experience embarrassment, regret or depression
Use of non-treatment of placebo control conditions
Collection of body tissues or fluid samples
8
Sample Application for
HREC Approval
Identify as far as possible all potential risks to participants (e.g., physical, psychological, social,
legal, economic) associated with the proposed research. Explain what risk management
procedures will be put in place, along with contact details of an appropriately qualified organisation
for participant reference in case of distress, eg: Lifeline (200 words)
The participants will be assured with their dignity and the privacy of information. The information
will be secured and only accessed by the researcher. The data in computer are secured by definite
and private passwords. No aboriginal participants will hurt of face undesirable conditions.
Values principle and ethnicities will be uninterrupted as per national statement in ethical conduct
and human research (2007) and the research elements will be highly secured fro preventing any
miscommunication between participants and researcher. Section 2 of National Statement is highly
significant for this research as per term and conditions of the proposed research the participants
names will be ambiguous. Risk related physiological harm should be avoided and it is assured that
no medical decisions will be taken without any health professional (Roberts,2015). The participants
will be checked and diagnosed by the concerned professionals. The process of governance and
ethical review will not be violated as the data will be taken from the hospital and no decision for
clinical manifestation will be taken by the researcher (www.nhmrc.gov.au, 2019). Participants from
aboriginal or Torres island will not feel any dis comfort regarding their beliefs and ethnicity. If any
inconvenience will take place participants can proceed legally and no ambiguity in the concern
form will be there. Participants can contact the given number for any inconvenience.
8. CONSENT Obtaining and Documenting Consent
How will informed consent be obtained/recorded?
Signed consent form
Recorded verbal consent
Implied by return of survey
Other (Please specify):
9. INFORMATION PROTECTION (DATA STORAGE & SECURITY) Confidentiality
Tick which method will be used to guarantee confidentiality/anonymity?
Non-identifiable (anonymous) data, which have never been labelled with individual
9
HREC Approval
Identify as far as possible all potential risks to participants (e.g., physical, psychological, social,
legal, economic) associated with the proposed research. Explain what risk management
procedures will be put in place, along with contact details of an appropriately qualified organisation
for participant reference in case of distress, eg: Lifeline (200 words)
The participants will be assured with their dignity and the privacy of information. The information
will be secured and only accessed by the researcher. The data in computer are secured by definite
and private passwords. No aboriginal participants will hurt of face undesirable conditions.
Values principle and ethnicities will be uninterrupted as per national statement in ethical conduct
and human research (2007) and the research elements will be highly secured fro preventing any
miscommunication between participants and researcher. Section 2 of National Statement is highly
significant for this research as per term and conditions of the proposed research the participants
names will be ambiguous. Risk related physiological harm should be avoided and it is assured that
no medical decisions will be taken without any health professional (Roberts,2015). The participants
will be checked and diagnosed by the concerned professionals. The process of governance and
ethical review will not be violated as the data will be taken from the hospital and no decision for
clinical manifestation will be taken by the researcher (www.nhmrc.gov.au, 2019). Participants from
aboriginal or Torres island will not feel any dis comfort regarding their beliefs and ethnicity. If any
inconvenience will take place participants can proceed legally and no ambiguity in the concern
form will be there. Participants can contact the given number for any inconvenience.
8. CONSENT Obtaining and Documenting Consent
How will informed consent be obtained/recorded?
Signed consent form
Recorded verbal consent
Implied by return of survey
Other (Please specify):
9. INFORMATION PROTECTION (DATA STORAGE & SECURITY) Confidentiality
Tick which method will be used to guarantee confidentiality/anonymity?
Non-identifiable (anonymous) data, which have never been labelled with individual
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Sample Application for
HREC Approval
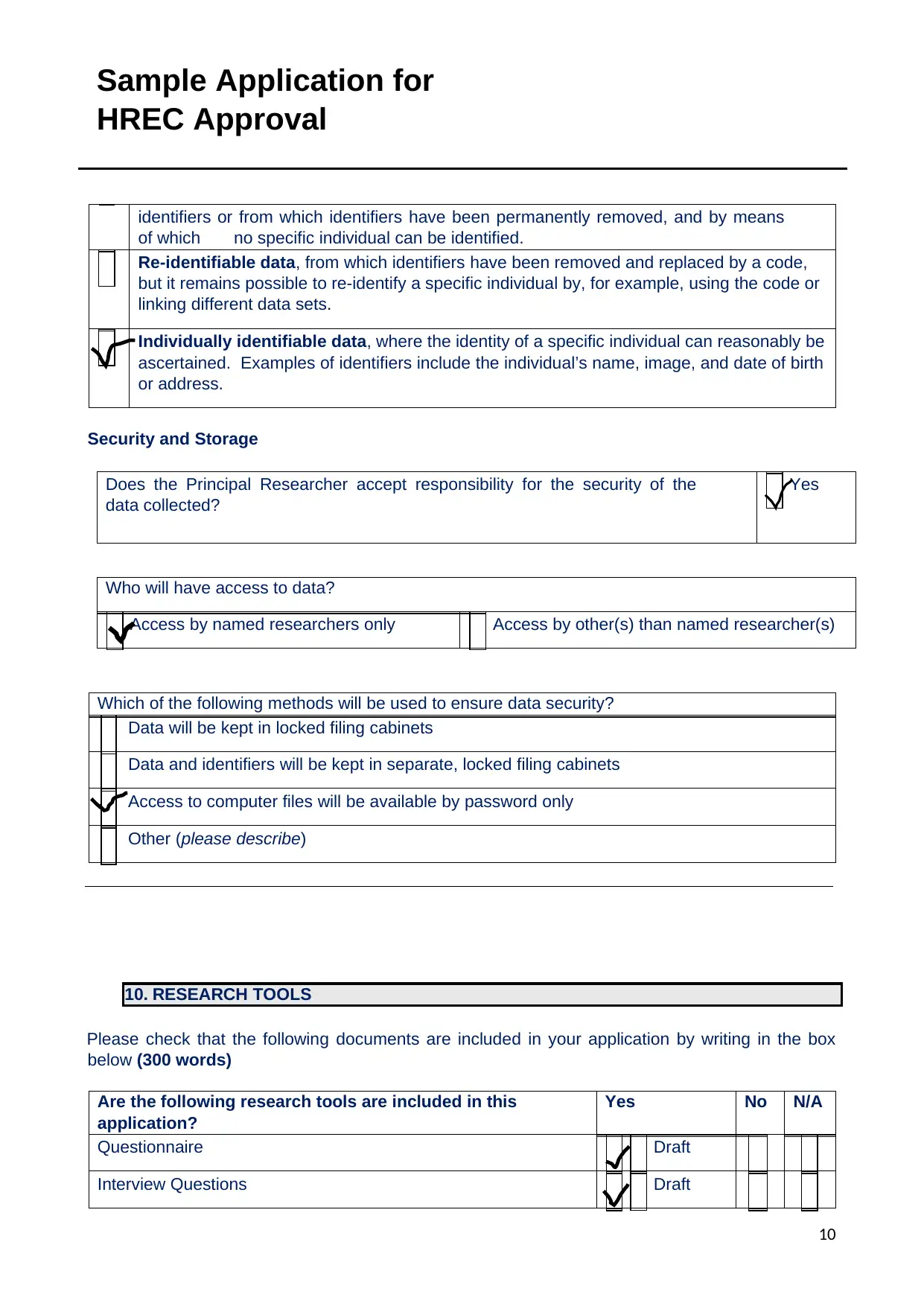
identifiers or from which identifiers have been permanently removed, and by means
of which no specific individual can be identified.
Re-identifiable data, from which identifiers have been removed and replaced by a code,
but it remains possible to re-identify a specific individual by, for example, using the code or
linking different data sets.
Individually identifiable data, where the identity of a specific individual can reasonably be
ascertained. Examples of identifiers include the individual’s name, image, and date of birth
or address.
Security and Storage
Does the Principal Researcher accept responsibility for the security of the
data collected?
Yes
Who will have access to data?
Access by named researchers only Access by other(s) than named researcher(s)
Which of the following methods will be used to ensure data security?
Data will be kept in locked filing cabinets
Data and identifiers will be kept in separate, locked filing cabinets
Access to computer files will be available by password only
Other (please describe)
10. RESEARCH TOOLS
Please check that the following documents are included in your application by writing in the box
below (300 words)
Are the following research tools are included in this
application?
Yes No N/A
Questionnaire Draft
Interview Questions Draft
10
HREC Approval
identifiers or from which identifiers have been permanently removed, and by means
of which no specific individual can be identified.
Re-identifiable data, from which identifiers have been removed and replaced by a code,
but it remains possible to re-identify a specific individual by, for example, using the code or
linking different data sets.
Individually identifiable data, where the identity of a specific individual can reasonably be
ascertained. Examples of identifiers include the individual’s name, image, and date of birth
or address.
Security and Storage
Does the Principal Researcher accept responsibility for the security of the
data collected?
Yes
Who will have access to data?
Access by named researchers only Access by other(s) than named researcher(s)
Which of the following methods will be used to ensure data security?
Data will be kept in locked filing cabinets
Data and identifiers will be kept in separate, locked filing cabinets
Access to computer files will be available by password only
Other (please describe)
10. RESEARCH TOOLS
Please check that the following documents are included in your application by writing in the box
below (300 words)
Are the following research tools are included in this
application?
Yes No N/A
Questionnaire Draft
Interview Questions Draft
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sample Application for
HREC Approval
Other
The demographic questions are
What is your / your child’s age?
What is your/ your child’s sex?
What is your / your child’s date of birth?
Where do you live?
Do you live with your parents? / Is he or she your own child?
Do you relate to any aboriginal place genetically?
Is there anybody in your family who is suffering from diabetes mellitus?
Survey questions:
How long you/ your child suffering from diabetes?
When do you come to know that you/ your child have T1DM?
What do you know about Type 1 Diabetes Mellitus?
Do you have any other severe disease?
Did you suffer from diabetes in your pregnancy?(for mothers)
How long you are having treatment?
Do you know what is the name of the treatment for serving your / your child’s diabetes?
Which type of insulin pump is used during treatment?
For each type of treatment
Is it effective for your child?
Or
Is it effective for the patient?
What is the difference between prior treatment and present condition?
Is the HbA1c showing some effect for the patient?(for doctors)
How can you assess that the CSII or the MDI is effective for the patient? (for doctors)
What is the dosage ?( for doctors)
Will there any change in dosage in future?( for doctors)
What will be changed in the dosage?( for doctors)
If no effect will measure what should you do? (for doctors)
Before asking any questions the client should be informed clarifying the objectives of the survey
with the client, investigating the question and methodology of the collecting data and guiding
research to get an insight with the features of various groups of the definite
population(www.abs.gov.au, 2019).
During the questions, no ambiguity should be there and query of the participants should be cleared
by researcher. The researcher will take care of dignity and self-respect of the participants. If
participant does not want to answer any question , he or she cannot be forced(Patten, 2016).
11
HREC Approval
Other
The demographic questions are
What is your / your child’s age?
What is your/ your child’s sex?
What is your / your child’s date of birth?
Where do you live?
Do you live with your parents? / Is he or she your own child?
Do you relate to any aboriginal place genetically?
Is there anybody in your family who is suffering from diabetes mellitus?
Survey questions:
How long you/ your child suffering from diabetes?
When do you come to know that you/ your child have T1DM?
What do you know about Type 1 Diabetes Mellitus?
Do you have any other severe disease?
Did you suffer from diabetes in your pregnancy?(for mothers)
How long you are having treatment?
Do you know what is the name of the treatment for serving your / your child’s diabetes?
Which type of insulin pump is used during treatment?
For each type of treatment
Is it effective for your child?
Or
Is it effective for the patient?
What is the difference between prior treatment and present condition?
Is the HbA1c showing some effect for the patient?(for doctors)
How can you assess that the CSII or the MDI is effective for the patient? (for doctors)
What is the dosage ?( for doctors)
Will there any change in dosage in future?( for doctors)
What will be changed in the dosage?( for doctors)
If no effect will measure what should you do? (for doctors)
Before asking any questions the client should be informed clarifying the objectives of the survey
with the client, investigating the question and methodology of the collecting data and guiding
research to get an insight with the features of various groups of the definite
population(www.abs.gov.au, 2019).
During the questions, no ambiguity should be there and query of the participants should be cleared
by researcher. The researcher will take care of dignity and self-respect of the participants. If
participant does not want to answer any question , he or she cannot be forced(Patten, 2016).
11

Sample Application for
HREC Approval
11. DECLARATIONS
Researcher Declarations:
The information contained herein is, to the best of my knowledge and belief, accurate. I have read
the University’s current human ethics guidelines, and accept responsibility for the conduct of the
procedures set out in the attached application in accordance with the guidelines. I and my
coresearchers have the appropriate qualifications, experience and facilities to conduct the research
set out in the attached application and to deal with any emergencies and contingencies related to
the research that may arise.
Principal Researcher
DR ABCD
(Print name in block letters)
Date: …..../…...../….....
12
HREC Approval
11. DECLARATIONS
Researcher Declarations:
The information contained herein is, to the best of my knowledge and belief, accurate. I have read
the University’s current human ethics guidelines, and accept responsibility for the conduct of the
procedures set out in the attached application in accordance with the guidelines. I and my
coresearchers have the appropriate qualifications, experience and facilities to conduct the research
set out in the attached application and to deal with any emergencies and contingencies related to
the research that may arise.
Principal Researcher
DR ABCD
(Print name in block letters)
Date: …..../…...../….....
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





