Thermodynamics Laboratory Report
VerifiedAdded on 2023/01/23
|14
|2017
|49
AI Summary
This work focuses on theoretical and experimental analysis on two different systems namely compressor with intercooler system and study of the refrigeration system. Both the systems were studied and all the relevant information about the working of the system are collected, various tests are conducted on the system, with the help of the collected information the appropriate calculations were made, graphs were plotted, and all the results were analysed effectively.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

School of Mechanical and Manufacturing Engineering Faculty of Engineering
The University of New South Wales, Sydney
Thermodynamics Laboratory Report
by
<Name>
MMAN2700 T1 2019
Submitted:
Course Convener: A/Prof. Person
Student ID: z1234567
Abstract
This work focuses on theoretical and experimental analysis on two different systems namely
compressor with intercooler system and study of the refrigeration system.both the systems were
studied and all the relevant information about the working of the system are collected, various tests
are conducted on the system, with the help of the collected information the appropriate calculations
were made, graphs were plotted, and all the results were analysed effectively.
The University of New South Wales, Sydney
Thermodynamics Laboratory Report
by
<Name>
MMAN2700 T1 2019
Submitted:
Course Convener: A/Prof. Person
Student ID: z1234567
Abstract
This work focuses on theoretical and experimental analysis on two different systems namely
compressor with intercooler system and study of the refrigeration system.both the systems were
studied and all the relevant information about the working of the system are collected, various tests
are conducted on the system, with the help of the collected information the appropriate calculations
were made, graphs were plotted, and all the results were analysed effectively.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Contents
Abstract.................................................................................................................................................1
1. Laboratory T2 Compressor Laboratory.........................................................................................3
1.1. Overview...............................................................................................................................3
1.2. Compression Ratio vs Polytropic Index.................................................................................3
1.1.1. Example Analysis..........................................................................................................3
1.1.2. Results...........................................................................................................................6
1.1.3. Discussion......................................................................................................................6
1.3. Intercooler Performance........................................................................................................7
1.4. Conclusion.............................................................................................................................7
2. Laboratory T4 Refrigeration Laboratory.......................................................................................8
3. Discussion and Conclusion..........................................................................................................11
References:..........................................................................................................................................12
Abstract.................................................................................................................................................1
1. Laboratory T2 Compressor Laboratory.........................................................................................3
1.1. Overview...............................................................................................................................3
1.2. Compression Ratio vs Polytropic Index.................................................................................3
1.1.1. Example Analysis..........................................................................................................3
1.1.2. Results...........................................................................................................................6
1.1.3. Discussion......................................................................................................................6
1.3. Intercooler Performance........................................................................................................7
1.4. Conclusion.............................................................................................................................7
2. Laboratory T4 Refrigeration Laboratory.......................................................................................8
3. Discussion and Conclusion..........................................................................................................11
References:..........................................................................................................................................12

1. Laboratory T2 Compressor Laboratory
1.1. Overview
In this Laboratory work, an experimental and theoretical investigation on a Compressor system with
intercooler will be carried out. The various calculation for finding the temperatures,
pressures,polytropic index, mass flow, etc. will be carried out. All the analysis will be answered
appropriately with proper graphs if required.
1.2. Compression Ratio vs Polytropic Index
1.1.1.Example Analysis
(a) Calculation of T3 from polytrophic relation:
P ( R
T )
n
=C
T
Pn−1/ n =C
T 1
T 3 =( P 1
P 3 )
n −1 /n
Now we have:
For adiabatic process, n = 3/2
WKt,
P3: Atmospheric pressure: 1.005 Bar
P1 = 3 Bar
T1 = 294.15 K
Now,
T 1
( P3
P 1 )
n−1/ n =T 3
T 3=423.54 K
(b)
Given:
T1: 294.15
T3: 438.15
P1: 3 bar
To find: n
1.1. Overview
In this Laboratory work, an experimental and theoretical investigation on a Compressor system with
intercooler will be carried out. The various calculation for finding the temperatures,
pressures,polytropic index, mass flow, etc. will be carried out. All the analysis will be answered
appropriately with proper graphs if required.
1.2. Compression Ratio vs Polytropic Index
1.1.1.Example Analysis
(a) Calculation of T3 from polytrophic relation:
P ( R
T )
n
=C
T
Pn−1/ n =C
T 1
T 3 =( P 1
P 3 )
n −1 /n
Now we have:
For adiabatic process, n = 3/2
WKt,
P3: Atmospheric pressure: 1.005 Bar
P1 = 3 Bar
T1 = 294.15 K
Now,
T 1
( P3
P 1 )
n−1/ n =T 3
T 3=423.54 K
(b)
Given:
T1: 294.15
T3: 438.15
P1: 3 bar
To find: n
The process is assumed to be reversible adiabatic (Taylor, 2017):
T 1
T 3 =( P 1
P 3 )
n −1 /n
294.15
438.15 =(2.9850)n−1 /n
Log (n/ (n-1)) (0.6713) = 2.9850)
n=−17.24 n+17.2450
n=0.94
(c)
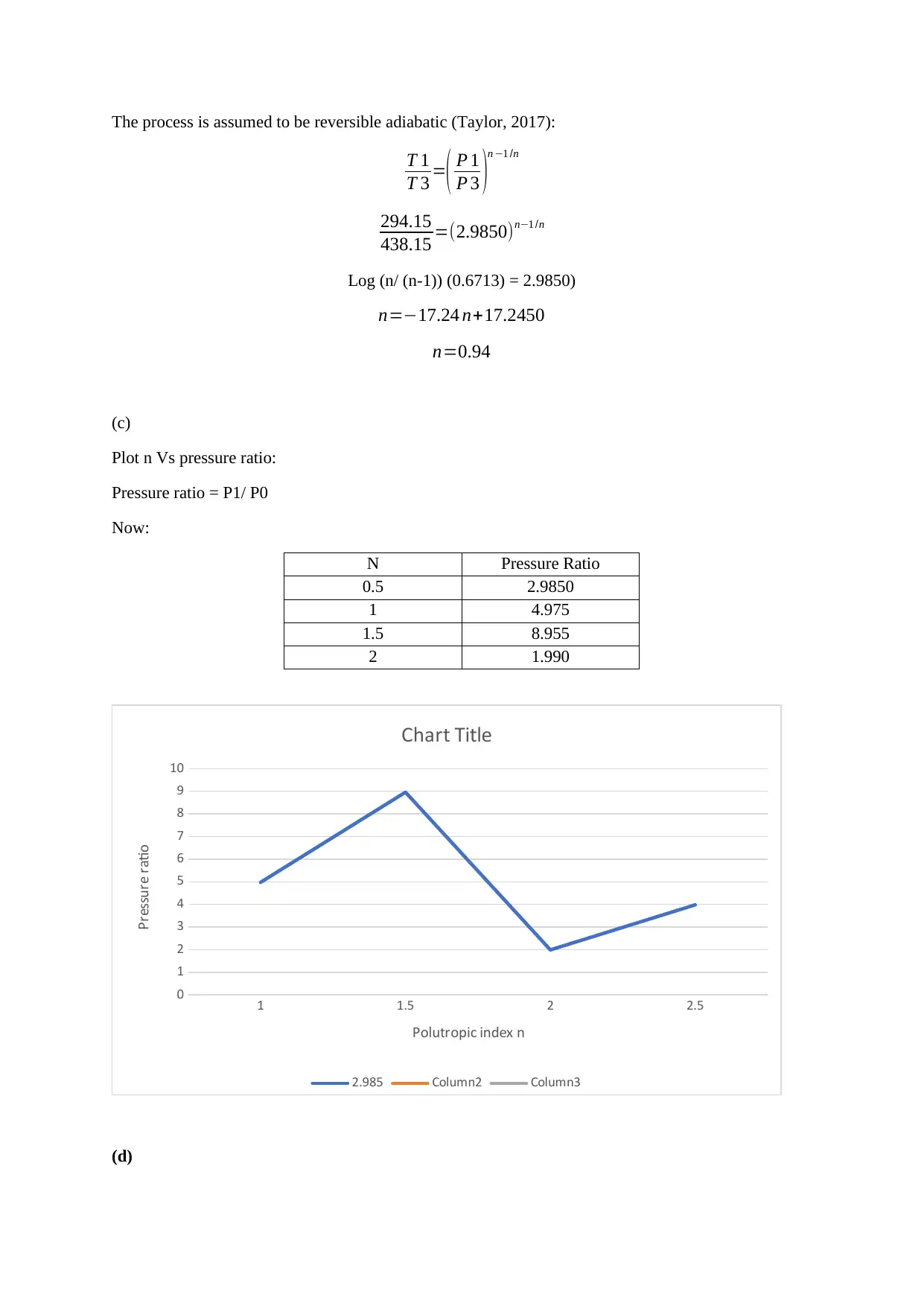
Plot n Vs pressure ratio:
Pressure ratio = P1/ P0
Now:
N Pressure Ratio
0.5 2.9850
1 4.975
1.5 8.955
2 1.990
1 1.5 2 2.5
0
1
2
3
4
5
6
7
8
9
10
Chart Title
2.985 Column2 Column3
Polutropic index n
Pressure ratio
(d)
T 1
T 3 =( P 1
P 3 )
n −1 /n
294.15
438.15 =(2.9850)n−1 /n
Log (n/ (n-1)) (0.6713) = 2.9850)
n=−17.24 n+17.2450
n=0.94
(c)
Plot n Vs pressure ratio:
Pressure ratio = P1/ P0
Now:
N Pressure Ratio
0.5 2.9850
1 4.975
1.5 8.955
2 1.990
1 1.5 2 2.5
0
1
2
3
4
5
6
7
8
9
10
Chart Title
2.985 Column2 Column3
Polutropic index n
Pressure ratio
(d)
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Wmech=nRT ln ( P 1
P 2 )=1.09
Now,
𝑊𝑒𝑙𝑒𝑐 = 𝑉1𝐴1 = 89 (10) = 890 = 0.89
Now comparing the work done mechanical and electrical the mechanical work is done is more than
that of the electrical work done.
(e)
Heat loss in compressor:
From the first law of thermodynamics:
ΔU =Q+W
As we consider the Compressor system to operate in Reversible Adiabatic process the heat loss in the
total system is assumed to be 0.
In reversible adiabatic process: Q = 0
(f)
Heat gained by water = Heat lost by the air in intercooler
Qg = m.Cp .dt = QL = M.Cp.dt
Now the heat Gained by the water can be found as:
Ql = Mw (1) (dt)
We have:
Mass of air flow,
𝑚̇𝑎 = 𝐶𝑑𝐸𝐴 √2 ρ ∆ P
Change in pressure ΔP = 58 mmHgo=7.73 ¯¿
𝐸 = (1 − ( /𝐷 )4 ) −0.5
D = 25.4 mm = 0.0254 m
d- 12.7 mm 0.0127 m
E = 1.032
A = 0.000127 m2
Cd =0.62
𝜌 = 𝑃0 / 𝑅𝑎𝑖𝑟 (T1) = 1.190
Ma = 8.13e-5 ( √2 ρ ∆ P)
P 2 )=1.09
Now,
𝑊𝑒𝑙𝑒𝑐 = 𝑉1𝐴1 = 89 (10) = 890 = 0.89
Now comparing the work done mechanical and electrical the mechanical work is done is more than
that of the electrical work done.
(e)
Heat loss in compressor:
From the first law of thermodynamics:
ΔU =Q+W
As we consider the Compressor system to operate in Reversible Adiabatic process the heat loss in the
total system is assumed to be 0.
In reversible adiabatic process: Q = 0
(f)
Heat gained by water = Heat lost by the air in intercooler
Qg = m.Cp .dt = QL = M.Cp.dt
Now the heat Gained by the water can be found as:
Ql = Mw (1) (dt)
We have:
Mass of air flow,
𝑚̇𝑎 = 𝐶𝑑𝐸𝐴 √2 ρ ∆ P
Change in pressure ΔP = 58 mmHgo=7.73 ¯¿
𝐸 = (1 − ( /𝐷 )4 ) −0.5
D = 25.4 mm = 0.0254 m
d- 12.7 mm 0.0127 m
E = 1.032
A = 0.000127 m2
Cd =0.62
𝜌 = 𝑃0 / 𝑅𝑎𝑖𝑟 (T1) = 1.190
Ma = 8.13e-5 ( √2 ρ ∆ P)

Ma = 0.1102 Kg/s
Now, heat loss can be found as:
Q=0.1102 ( 1 ) ( T 3−T 5 )=15.5382 W
The heat lost by the air is the heat gained by the water, QL = Qg = 15.5382 W
1.1.2. Results
The experiment was carried out successfully and al the question in the Analysis section was answered
with the help of the observed data. The overall results are provided here:
a. The temperature T3 was found using the Polytropic relation as (Liu, etal., 2017):
T 1
( P3
P 1 )
n−1/ n =T 3
T 3=423.54 K
b. The polytrpoic index was found usiong the P1 and T3 as:
n=−17.24 n+17.2450
n=0.94
d. Work done by the system was found as:
𝑊𝑒𝑙𝑒𝑐 = 𝑉1𝐴1 = 89 (10) = 890 = 0.89
f. The total heat gained by the water was calculated as:
Q=0.1102 ( 1 ) ( T 3−T 5 )=15.5382 W
The heat lost by the air is the heat gained by the water, QL = Qg = 15.5382 W
1.1.3.Discussion
(g) In general, any thermodynamic system must be studied by applying basic thermodynamic process
that it is related to the compressor system was studied by implementing the reversible adiabatic
process. The C task was to plot a graph between the polytropic index and the pressure ratio the curve
was an uncertain curve with periodic downward and upward which shows that the pressure ratio is
affected by the polytropic index.
(h) The dynamometer to compressor drive system consists of various Mechanical and electrical
losses, in case of dynamometer various losses such as eddy current loss, hysteresis loss, heat loss, etc.
will occur so that some form of input will always be lost in form of various losses.
(i) The heat gained by the water and lost by the air will have some difference as the heat energy
initially is used to increase the kinetic energy of the working fluid in case of intercooler, the initial
Now, heat loss can be found as:
Q=0.1102 ( 1 ) ( T 3−T 5 )=15.5382 W
The heat lost by the air is the heat gained by the water, QL = Qg = 15.5382 W
1.1.2. Results
The experiment was carried out successfully and al the question in the Analysis section was answered
with the help of the observed data. The overall results are provided here:
a. The temperature T3 was found using the Polytropic relation as (Liu, etal., 2017):
T 1
( P3
P 1 )
n−1/ n =T 3
T 3=423.54 K
b. The polytrpoic index was found usiong the P1 and T3 as:
n=−17.24 n+17.2450
n=0.94
d. Work done by the system was found as:
𝑊𝑒𝑙𝑒𝑐 = 𝑉1𝐴1 = 89 (10) = 890 = 0.89
f. The total heat gained by the water was calculated as:
Q=0.1102 ( 1 ) ( T 3−T 5 )=15.5382 W
The heat lost by the air is the heat gained by the water, QL = Qg = 15.5382 W
1.1.3.Discussion
(g) In general, any thermodynamic system must be studied by applying basic thermodynamic process
that it is related to the compressor system was studied by implementing the reversible adiabatic
process. The C task was to plot a graph between the polytropic index and the pressure ratio the curve
was an uncertain curve with periodic downward and upward which shows that the pressure ratio is
affected by the polytropic index.
(h) The dynamometer to compressor drive system consists of various Mechanical and electrical
losses, in case of dynamometer various losses such as eddy current loss, hysteresis loss, heat loss, etc.
will occur so that some form of input will always be lost in form of various losses.
(i) The heat gained by the water and lost by the air will have some difference as the heat energy
initially is used to increase the kinetic energy of the working fluid in case of intercooler, the initial

heat of the air is used by the water to increase its kinetic energy and thus it experiences loss of heat
energy.
1.3. Intercooler Performance
The intercooler must be utilized in every compressor to decrease the temperature of the air after it is
being compressed. The intercooler acts as a fluid conditioning system. It is very much important to
reduce the temperature of the air in case of two-stage compression, if the temperature of the air is
higher then its density will be very low, to increase the density of air while compressing it is highly
recommended to reduce the temperature also cooling help to reduce the wear and thermal load applied
on all other mechanical parts in the compressor system.
1.4. Conclusion
Thus the intercooler performance in a compressor system was theoretically and experimentally
studied and all the related results are presented, discussions were made and the calculations are
performed as per the requirements.
energy.
1.3. Intercooler Performance
The intercooler must be utilized in every compressor to decrease the temperature of the air after it is
being compressed. The intercooler acts as a fluid conditioning system. It is very much important to
reduce the temperature of the air in case of two-stage compression, if the temperature of the air is
higher then its density will be very low, to increase the density of air while compressing it is highly
recommended to reduce the temperature also cooling help to reduce the wear and thermal load applied
on all other mechanical parts in the compressor system.
1.4. Conclusion
Thus the intercooler performance in a compressor system was theoretically and experimentally
studied and all the related results are presented, discussions were made and the calculations are
performed as per the requirements.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

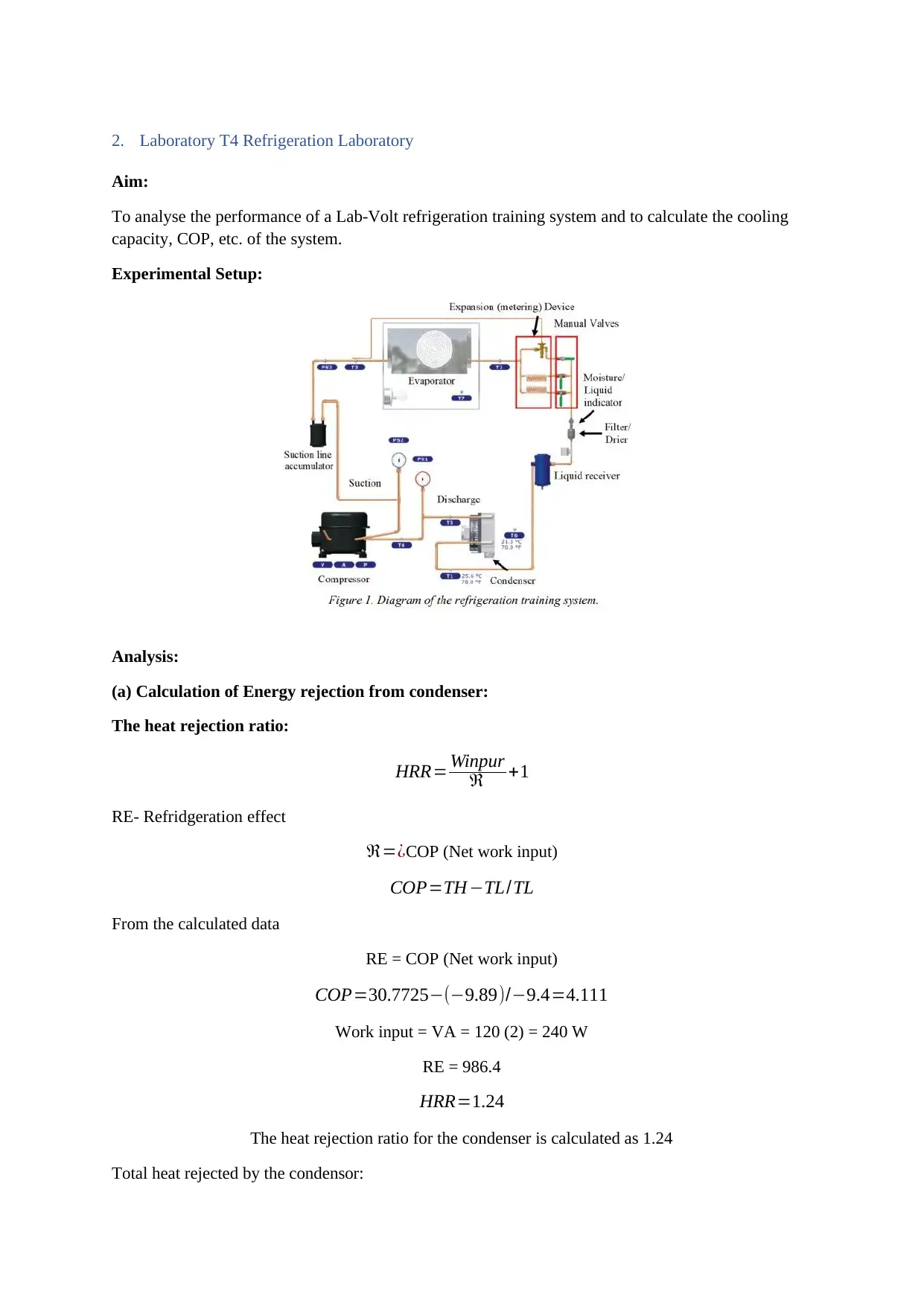
2. Laboratory T4 Refrigeration Laboratory
Aim:
To analyse the performance of a Lab-Volt refrigeration training system and to calculate the cooling
capacity, COP, etc. of the system.
Experimental Setup:
Analysis:
(a) Calculation of Energy rejection from condenser:
The heat rejection ratio:
HRR= Winpur
ℜ +1
RE- Refridgeration effect
ℜ=¿COP (Net work input)
COP=TH −TL/TL
From the calculated data
RE = COP (Net work input)
COP=30.7725−(−9.89)/−9.4=4.111
Work input = VA = 120 (2) = 240 W
RE = 986.4
HRR=1.24
The heat rejection ratio for the condenser is calculated as 1.24
Total heat rejected by the condensor:
Aim:
To analyse the performance of a Lab-Volt refrigeration training system and to calculate the cooling
capacity, COP, etc. of the system.
Experimental Setup:
Analysis:
(a) Calculation of Energy rejection from condenser:
The heat rejection ratio:
HRR= Winpur
ℜ +1
RE- Refridgeration effect
ℜ=¿COP (Net work input)
COP=TH −TL/TL
From the calculated data
RE = COP (Net work input)
COP=30.7725−(−9.89)/−9.4=4.111
Work input = VA = 120 (2) = 240 W
RE = 986.4
HRR=1.24
The heat rejection ratio for the condenser is calculated as 1.24
Total heat rejected by the condensor:

THR = RE + Work done = 240+ 986.4 = 1226.4 W
(b) Calculate the rate at which the energy is added to the system through the evaporator:
Energy added:
E=WinXQrejected
The heat rejected can be calculated as:
Qr=MCp(dt )
dt = T2-T3 = 5.465
Cp = 1
M = Mass flow of refridgerator = 0.00525 Kg/s
Qr=0.028418 W
E= ( 240 ) 0.028418W /s
E=6.82032W /s
The energy is added to the system at the rate of 6.82032 W/s.
(c) Calculation of the rate at which energy is added to the system by compressor:
Power input to compressor = mXWin
Where,
M is the mas flow rate of the refrigerant
M = 0.00525 Kg/s
Win = VA = 240 w
P=1.26 W /s
The energy is added in the rate of 1.26 W/s to the system by the refrigerator.
(d)
To determine, If the total heat added/rejected = Total work done on the system.
∑ Q=∑ W
Total Work is done:
W = VI = 240.60
Total heat rejected by the system:
Q = 180.60
(b) Calculate the rate at which the energy is added to the system through the evaporator:
Energy added:
E=WinXQrejected
The heat rejected can be calculated as:
Qr=MCp(dt )
dt = T2-T3 = 5.465
Cp = 1
M = Mass flow of refridgerator = 0.00525 Kg/s
Qr=0.028418 W
E= ( 240 ) 0.028418W /s
E=6.82032W /s
The energy is added to the system at the rate of 6.82032 W/s.
(c) Calculation of the rate at which energy is added to the system by compressor:
Power input to compressor = mXWin
Where,
M is the mas flow rate of the refrigerant
M = 0.00525 Kg/s
Win = VA = 240 w
P=1.26 W /s
The energy is added in the rate of 1.26 W/s to the system by the refrigerator.
(d)
To determine, If the total heat added/rejected = Total work done on the system.
∑ Q=∑ W
Total Work is done:
W = VI = 240.60
Total heat rejected by the system:
Q = 180.60
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

∑ Q ≠∑ W
Due to various heat losses, Q will not be equal to work input. There is always a form of heat loss as
per the kelvin planks statement no heat engine works at 100 per cent efficiency (street, Voorhis,
&shapiro, 2017).
(e)
COP of the system:
COP=TH−TL/TL
TH = 30.7725
TL = -9.34
Now,
COP=(30.7725− ( −9.89 ) )/−9.4=−4.111
COP of the system is calculated to be -4.11
(f)
Data available:
P1 = 7.965 bar
P2 = 1.0025
T1 = 30.77250 C
T4 = ?
Assuming Isotropic process:
( T 2
T 1 ) =( P 2
P 1 ) k−1
k
Take, K = Cp/Cv = 1.13 (For refrigerant R134a)
T2: T4 =?
( T 4
30.775 )= ( 0.1258 ) 0.115
T 4=24.3042
The T4 is found to be 24.30420 C.
The isentropic efficiency:
ηT = h 2 a−h 1
Wa = Ws
Wa
Due to various heat losses, Q will not be equal to work input. There is always a form of heat loss as
per the kelvin planks statement no heat engine works at 100 per cent efficiency (street, Voorhis,
&shapiro, 2017).
(e)
COP of the system:
COP=TH−TL/TL
TH = 30.7725
TL = -9.34
Now,
COP=(30.7725− ( −9.89 ) )/−9.4=−4.111
COP of the system is calculated to be -4.11
(f)
Data available:
P1 = 7.965 bar
P2 = 1.0025
T1 = 30.77250 C
T4 = ?
Assuming Isotropic process:
( T 2
T 1 ) =( P 2
P 1 ) k−1
k
Take, K = Cp/Cv = 1.13 (For refrigerant R134a)
T2: T4 =?
( T 4
30.775 )= ( 0.1258 ) 0.115
T 4=24.3042
The T4 is found to be 24.30420 C.
The isentropic efficiency:
ηT = h 2 a−h 1
Wa = Ws
Wa

Where,
h1 = Inlet enthalpy
h2a- Exit enthalpy of the isentropic process.
Now based on the Temperature values the enthalpy values are taken for the particular inlet and exit
temperatures of the refrigerant from refrigeration data table (Keith, Wang,& Norton, 2018).
h1 = 228 K/Kg
h2s = 412 KJ/Kg
ηT = h 2 a−h 1
Wa = 184
240 =0.766
The isentropic efficiency of the process is found to be 76%
3. Discussion and Conclusion
Thus in this project a detailed theoretical and experimental study was conducted on two systems
namely refrigeration system and the compressor with intercooling system, both the systems were
studied and all the relevant information about the working of the system are collected, various tests
are conducted on the system, with the help of the collected information the appropriate calculations
h1 = Inlet enthalpy
h2a- Exit enthalpy of the isentropic process.
Now based on the Temperature values the enthalpy values are taken for the particular inlet and exit
temperatures of the refrigerant from refrigeration data table (Keith, Wang,& Norton, 2018).
h1 = 228 K/Kg
h2s = 412 KJ/Kg
ηT = h 2 a−h 1
Wa = 184
240 =0.766
The isentropic efficiency of the process is found to be 76%
3. Discussion and Conclusion
Thus in this project a detailed theoretical and experimental study was conducted on two systems
namely refrigeration system and the compressor with intercooling system, both the systems were
studied and all the relevant information about the working of the system are collected, various tests
are conducted on the system, with the help of the collected information the appropriate calculations

were made, graphs were plotted, and all the results were analysed effectively. Thus both the
experiments were carried out appropriately and the required results are drawn.
experiments were carried out appropriately and the required results are drawn.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References:
Taylor, J. R. (2017). U.S. Patent No. 9,611,744. Washington, DC: U.S. Patent and Trademark Office.
Street, N. E., Voorhis, R. J., & Shapiro, D. (2017). U.S. Patent No. 9,696,059. Washington, DC: U.S.
Patent and Trademark Office.
Keith, F., Wang, S. K., & Norton, P. (2018). Air conditioning and refrigeration engineering. CRC
Press.
Liu, L. G., Li, X. S., Ren, X. D., &Gu, C. W. (2017, June). Effect of Cooling on the Aerodynamic
Performance in the Intercooled Compressor Vanes. In ASME Turbo Expo 2017: Turbomachinery
Technical Conference and Exposition (pp. V05BT22A008-V05BT22A008). American Society of
Mechanical Engineers.
Taylor, J. R. (2017). U.S. Patent No. 9,611,744. Washington, DC: U.S. Patent and Trademark Office.
Street, N. E., Voorhis, R. J., & Shapiro, D. (2017). U.S. Patent No. 9,696,059. Washington, DC: U.S.
Patent and Trademark Office.
Keith, F., Wang, S. K., & Norton, P. (2018). Air conditioning and refrigeration engineering. CRC
Press.
Liu, L. G., Li, X. S., Ren, X. D., &Gu, C. W. (2017, June). Effect of Cooling on the Aerodynamic
Performance in the Intercooled Compressor Vanes. In ASME Turbo Expo 2017: Turbomachinery
Technical Conference and Exposition (pp. V05BT22A008-V05BT22A008). American Society of
Mechanical Engineers.
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.


