Practical Science Project: Comparing Antacid Effectiveness (Biology)
VerifiedAdded on 2020/07/23
|16
|4211
|220
Practical Assignment
AI Summary
This practical science project investigates the effectiveness of various antacids in neutralizing stomach acid, focusing on the treatment of heartburn and gastroesophageal reflux disease (GERD). The study compares the acid-neutralizing capabilities of Rennie, Milk of Magnesia, and Gaviscon through a titration experiment, measuring the amount of acid required to neutralize each antacid. The project includes background research on GERD and antacids, experimental protocols, data collection, and analysis, including graphical representation of results. The hypothesis suggests Milk of Magnesia will be the most effective. The project also addresses safety considerations, potential outcomes, and limitations, offering suggestions for improvement. The project aims to determine which antacid is most effective in neutralizing acid, providing insights into their use for relieving heartburn symptoms. The results are analyzed in reference to their reliability and validity, and compared to existing scientific knowledge.

Practical Science Project 1
Practical Science Project
Student’s Name:
Instructor’s Name:
Date:
Practical Science Project
Student’s Name:
Instructor’s Name:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Practical Science Project 2
Contents
Heartburn and gastrooesophageal reflux disease.......................................................................................2
1.0. Introduction:.....................................................................................................................................2
1.1. Proposal............................................................................................................................................2
1.2. Hypothesis........................................................................................................................................3
2.0. Background knowledge on area of research........................................................................................3
a) Gastrooesophageal reflux disease and heartburn...............................................................................3
b) Antacids...............................................................................................................................................4
2.1. Protocol and Experimental Plan.......................................................................................................5
a) Timescale and task priority..............................................................................................................6
b) Materials required and arranged.....................................................................................................6
2.2. Data collection and analytical method.............................................................................................7
2.3. Justification of hypothesis (controlled and uncontrolled variables, accuracy, and reliability)..........7
2.4. Probable outcomes and congruence with literature........................................................................7
2.5. Ethical and safety issues in practical execution along with proposed management........................8
2.6. Risk assessment................................................................................................................................8
3.1. Planned techniques for obtaining results: Methodology..................................................................8
a) Accuracy of results...........................................................................................................................9
b) Reliability.......................................................................................................................................10
3.2. Practical modifications to the proposed plan.................................................................................10
3.3. Record of the data..........................................................................................................................10
3.4. Record of errors..............................................................................................................................11
3.5. Health and safety measures...........................................................................................................11
4.1. Data processing – graphical and tabular, or diagrammatic representation:...................................11
Figure: Graph of pH range of Rennie, Milk of magnesia, and Gaviscon.................................................11
4.2. Data analysis with reference to reliability and validity...................................................................12
5.1. Relevant conclusions based on the results.....................................................................................12
5.2. Explanation of the conclusion with reference to scientific knowledge...........................................12
6.1. Evaluation of the strengths and limitations of the study design and protocol...............................12
6.2. Suggestion of justified improvements and modifications in the procedure...................................13
7.0. Conclusion......................................................................................................................................13
References:................................................................................................................................................14
Contents
Heartburn and gastrooesophageal reflux disease.......................................................................................2
1.0. Introduction:.....................................................................................................................................2
1.1. Proposal............................................................................................................................................2
1.2. Hypothesis........................................................................................................................................3
2.0. Background knowledge on area of research........................................................................................3
a) Gastrooesophageal reflux disease and heartburn...............................................................................3
b) Antacids...............................................................................................................................................4
2.1. Protocol and Experimental Plan.......................................................................................................5
a) Timescale and task priority..............................................................................................................6
b) Materials required and arranged.....................................................................................................6
2.2. Data collection and analytical method.............................................................................................7
2.3. Justification of hypothesis (controlled and uncontrolled variables, accuracy, and reliability)..........7
2.4. Probable outcomes and congruence with literature........................................................................7
2.5. Ethical and safety issues in practical execution along with proposed management........................8
2.6. Risk assessment................................................................................................................................8
3.1. Planned techniques for obtaining results: Methodology..................................................................8
a) Accuracy of results...........................................................................................................................9
b) Reliability.......................................................................................................................................10
3.2. Practical modifications to the proposed plan.................................................................................10
3.3. Record of the data..........................................................................................................................10
3.4. Record of errors..............................................................................................................................11
3.5. Health and safety measures...........................................................................................................11
4.1. Data processing – graphical and tabular, or diagrammatic representation:...................................11
Figure: Graph of pH range of Rennie, Milk of magnesia, and Gaviscon.................................................11
4.2. Data analysis with reference to reliability and validity...................................................................12
5.1. Relevant conclusions based on the results.....................................................................................12
5.2. Explanation of the conclusion with reference to scientific knowledge...........................................12
6.1. Evaluation of the strengths and limitations of the study design and protocol...............................12
6.2. Suggestion of justified improvements and modifications in the procedure...................................13
7.0. Conclusion......................................................................................................................................13
References:................................................................................................................................................14

Practical Science Project 3
Heartburn and gastrooesophageal reflux disease
1.0. Introduction:
The current topic of research is the analysis of the incessant rise in the incidence of heartburn
and consequent gastrooesophageal reflux disease [1]. There has been a notable increase in the
occurrence of number of cases of gastrooesophageal reflux and heartburn across the world [1].
The clinical manifestation of gastrooesophageal reflux is the presentation of mild to severe
heartburn [1]. Most healthy individuals present with episodic presentations of heartburn [1, 2].
Self medication with antacids is a popular solution to the episodic instances of heartburn [1, 2].
Antacids are used extensively in the treatment of heartburn [2]. However, they are found to have
limited efficacy in the treatment of symptoms that are similar to heartburn that include diseases
related to peptic ulcer, advanced disease of the oesophagus etc [2]. The common diseases of the
oesophagus include erosive gastrooesophageal reflux disease (GERD) and columnar metaplasia
present in the oesophagus or Barrett’s oesophagus [1, 2]. Most individuals tend to neglect the
symptom of heartburn as an unimportant presentation [1, 2]. Thus, the symptoms of heartburn or
heartburnlike are commonly ignored and treated with antacids [1, 2]. There is a considerable lack
of data concerning the characterization of disorders of the upper region of the gastrointestinal
tract in individuals who use anatacids on a regular basis [1, 2].
1.1. Proposal: The current experiment focuses on the assessment of the action of antacids on
neutralizing acid reflux [3]. The study primarily focuses on understanding the implications of
gastrooesophageal reflux disease manifested in heartburn or heartburnlike symptoms in most
patients. The study assesses the different antacids available for the treatment of the symptoms
and the remedy for the disease. The experiment examines the efficacy of various antacids in the
Heartburn and gastrooesophageal reflux disease
1.0. Introduction:
The current topic of research is the analysis of the incessant rise in the incidence of heartburn
and consequent gastrooesophageal reflux disease [1]. There has been a notable increase in the
occurrence of number of cases of gastrooesophageal reflux and heartburn across the world [1].
The clinical manifestation of gastrooesophageal reflux is the presentation of mild to severe
heartburn [1]. Most healthy individuals present with episodic presentations of heartburn [1, 2].
Self medication with antacids is a popular solution to the episodic instances of heartburn [1, 2].
Antacids are used extensively in the treatment of heartburn [2]. However, they are found to have
limited efficacy in the treatment of symptoms that are similar to heartburn that include diseases
related to peptic ulcer, advanced disease of the oesophagus etc [2]. The common diseases of the
oesophagus include erosive gastrooesophageal reflux disease (GERD) and columnar metaplasia
present in the oesophagus or Barrett’s oesophagus [1, 2]. Most individuals tend to neglect the
symptom of heartburn as an unimportant presentation [1, 2]. Thus, the symptoms of heartburn or
heartburnlike are commonly ignored and treated with antacids [1, 2]. There is a considerable lack
of data concerning the characterization of disorders of the upper region of the gastrointestinal
tract in individuals who use anatacids on a regular basis [1, 2].
1.1. Proposal: The current experiment focuses on the assessment of the action of antacids on
neutralizing acid reflux [3]. The study primarily focuses on understanding the implications of
gastrooesophageal reflux disease manifested in heartburn or heartburnlike symptoms in most
patients. The study assesses the different antacids available for the treatment of the symptoms
and the remedy for the disease. The experiment examines the efficacy of various antacids in the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Practical Science Project 4
neutralization of the reflux of acids. The experimental protocol includes the analysis of three
types of antacids namely: Rennie, Milk of magnesium, and gaviscon. The current study involves
the measurement of the amount of acid required for the neutralization of the antacid. These
values indicate the efficiency of the antacid in the relief from the symptoms of acid reflux.
1.2. Hypothesis: The hypothetical basis of the current experiment is that all antacids display a
similar range of efficacy. However, milk of magnesia has a higher level of efficiency in the
neutralization of the antacids. Milk of magnesia is capable of neutralizing the highest amount of
acids.
2.0. Background knowledge on area of research:
The current experiment researches the efficiency of the antacids rennie, milk of magnesia, and
gaviscon in neutralizing acids in the human body in instances of heartburn and
gastrooesophageal reflux disease. In recent times, there has been a considerable increase in the
number of cases of gastrooesophageal reflux disease and heartburn [3, 4]. The incidences have
been increasing in children as well as adults [3, 4]. The current experiment measures the amount
of acid required to neutralize the antacids used for analysis [3, 4]. The efficacy of the antacid is
determined in terms of the amount of acid neutralized [3, 4].
a) Gastrooesophageal reflux disease and heartburn: The initial introduction of the
concept of gastrooesophageal reflux accompanied by symptoms of heartburn [4, 5]. The initial
observations suggest that there are several gastric secretions that lead to damage to the mucosal
layer the gastrooesophageal system [4, 5]. The basis pathway of the pathophysiological origin of
the disease indicates that there is the occurrence of a reflux mechanism caused by the acids in the
system [4, 5]. Literature observes that there is the presence of a reflux injury that may lead to
neutralization of the reflux of acids. The experimental protocol includes the analysis of three
types of antacids namely: Rennie, Milk of magnesium, and gaviscon. The current study involves
the measurement of the amount of acid required for the neutralization of the antacid. These
values indicate the efficiency of the antacid in the relief from the symptoms of acid reflux.
1.2. Hypothesis: The hypothetical basis of the current experiment is that all antacids display a
similar range of efficacy. However, milk of magnesia has a higher level of efficiency in the
neutralization of the antacids. Milk of magnesia is capable of neutralizing the highest amount of
acids.
2.0. Background knowledge on area of research:
The current experiment researches the efficiency of the antacids rennie, milk of magnesia, and
gaviscon in neutralizing acids in the human body in instances of heartburn and
gastrooesophageal reflux disease. In recent times, there has been a considerable increase in the
number of cases of gastrooesophageal reflux disease and heartburn [3, 4]. The incidences have
been increasing in children as well as adults [3, 4]. The current experiment measures the amount
of acid required to neutralize the antacids used for analysis [3, 4]. The efficacy of the antacid is
determined in terms of the amount of acid neutralized [3, 4].
a) Gastrooesophageal reflux disease and heartburn: The initial introduction of the
concept of gastrooesophageal reflux accompanied by symptoms of heartburn [4, 5]. The initial
observations suggest that there are several gastric secretions that lead to damage to the mucosal
layer the gastrooesophageal system [4, 5]. The basis pathway of the pathophysiological origin of
the disease indicates that there is the occurrence of a reflux mechanism caused by the acids in the
system [4, 5]. Literature observes that there is the presence of a reflux injury that may lead to
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Practical Science Project 5
heartburn [4, 5]. The heartburn or heartburnlike symptoms may occur without the presence of
oesophagitis and thus the term gastrooesophageal reflux disease (GERD) is being used to
describe the various symptomatic events that include heartburn [4, 5]. The condition of GERD
and heartburn is commonly accompanied by several complications of oesophageal origin
including strictures, oesophagitis, Barrett’s oesophagus or Barrett’s metaplasia [4, 5]. There are
several histopathological events that comprise the pathogenesis of the disease [4, 5]. The disease
process includes the occurrence of the disease in several organs even apart from the oesophagus
[4, 5]. The symptoms of reflux occur along with a consistent presentation of severe symptoms [4,
5]. Research has not been able to successfully establish a connection between heartburn and
gastrooesophageal reflux disease [4, 5]. There are several instances of reflux in the
extreoesophageal regions or the regions present outside of the oesophagus [4, 5].
b) Antacids: Antacids are some of the most commonly used medicines for the cure of
heartburn across the world [4, 5]. Antacids are available over the counter in most pharmacies [4,
5]. Gastritis and symptoms of heartburn can be cured with the use of antacids [4, 5]. Antacids
commonly act by neutralizing the acids in the gastrooesophageal system [4, 5]. The incidence of
heartburn is accompanied by the decrease in the physiological pH value of the human body [4,
5]. Due to the ingestion of food, there is an excessive production of acids in the stomach [4, 5].
The excessive production of acids in the stomach leads to the pH becoming acidic in nature [4,
5]. The decline in the level of physiological pH due to overconsumption of food having acidic
pH enhances the symptoms of heartburn [4, 5]. The physiological pH is reduced to a level below
2 which leads to heartburnlike symptoms and when uncontrolled, can result in gastrooesophageal
disorder [4, 5]. Antacids primarily function by increasing the pH in the stomach to pH 3-4 [4, 5].
The aforementioned increase in alkaline pH results in the neutralization of the acid in the
heartburn [4, 5]. The heartburn or heartburnlike symptoms may occur without the presence of
oesophagitis and thus the term gastrooesophageal reflux disease (GERD) is being used to
describe the various symptomatic events that include heartburn [4, 5]. The condition of GERD
and heartburn is commonly accompanied by several complications of oesophageal origin
including strictures, oesophagitis, Barrett’s oesophagus or Barrett’s metaplasia [4, 5]. There are
several histopathological events that comprise the pathogenesis of the disease [4, 5]. The disease
process includes the occurrence of the disease in several organs even apart from the oesophagus
[4, 5]. The symptoms of reflux occur along with a consistent presentation of severe symptoms [4,
5]. Research has not been able to successfully establish a connection between heartburn and
gastrooesophageal reflux disease [4, 5]. There are several instances of reflux in the
extreoesophageal regions or the regions present outside of the oesophagus [4, 5].
b) Antacids: Antacids are some of the most commonly used medicines for the cure of
heartburn across the world [4, 5]. Antacids are available over the counter in most pharmacies [4,
5]. Gastritis and symptoms of heartburn can be cured with the use of antacids [4, 5]. Antacids
commonly act by neutralizing the acids in the gastrooesophageal system [4, 5]. The incidence of
heartburn is accompanied by the decrease in the physiological pH value of the human body [4,
5]. Due to the ingestion of food, there is an excessive production of acids in the stomach [4, 5].
The excessive production of acids in the stomach leads to the pH becoming acidic in nature [4,
5]. The decline in the level of physiological pH due to overconsumption of food having acidic
pH enhances the symptoms of heartburn [4, 5]. The physiological pH is reduced to a level below
2 which leads to heartburnlike symptoms and when uncontrolled, can result in gastrooesophageal
disorder [4, 5]. Antacids primarily function by increasing the pH in the stomach to pH 3-4 [4, 5].
The aforementioned increase in alkaline pH results in the neutralization of the acid in the

Practical Science Project 6
stomach along with curing the symptoms of heartburn [4, 5]. Gastrooesophageal reflux disorders
such as peptic oesophagitis are treated by increasing the value of intragstric pH and the sphincter
strength of the lower oesophagus [4, 5]. These measures help in the enhancement of the
clearance of oesophageal acids thus resulting in improved clearance of the gastric system [4, 5].
Antacids have been found to have a considerable amount of effectiveness in their ability to
facilitate the neutralization of contents of the gastric system along with enhancing the tone of the
lower oesophageal sphincter [4, 5]. Antacids, however, may cause severe alkalinisation of the
stomach in most cases [4, 5]. There is a lack of considerable research in literature for the
assessment of the effects of antacid in the treatment of peptic oesophagitis and gastrooesphageal
reflux disease [4, 5]. The common methods of antacid action include the ingredients of H2
blockers or inhibitors of the proton flux pump in the cellular dynamics in conditions of heartburn
due to gastric disturbance [4, 5]. Antacids commonly lack the ability to neutralize the acids in
instances of heartburn independently [4, 5]. Additionally, they require the incorporation of
ingredients such as sodium, calcium, aluminium, and magnesium [4, 5]. These aforementioned
ingredients aid the process of increase of the physiological pH [4, 5].
2.1. Protocol and Experimental Plan: The current experiment focuses on the
determination of the most effective antacid in the treatment of heartburn and gastrooesophageal
reflux disorder. The current experiment focuses on the antacids rennie, milk of magnesium, and
gaviscon [4, 5]. Milk of magnesium is believed to have the highest efficacy in terms of the
neutralization of acids in the system [4, 5]. Rennie is a commonly available antacid which is
available over the counter in most pharmacies [4, 5]. It is primarily composed of calcium and
magnesium carbonate composition [4, 5]. They function in the improvement of the emptying of
stomach along with curing the symptoms of heartburn [4, 5]. Gastrooesophageal reflux disorders
such as peptic oesophagitis are treated by increasing the value of intragstric pH and the sphincter
strength of the lower oesophagus [4, 5]. These measures help in the enhancement of the
clearance of oesophageal acids thus resulting in improved clearance of the gastric system [4, 5].
Antacids have been found to have a considerable amount of effectiveness in their ability to
facilitate the neutralization of contents of the gastric system along with enhancing the tone of the
lower oesophageal sphincter [4, 5]. Antacids, however, may cause severe alkalinisation of the
stomach in most cases [4, 5]. There is a lack of considerable research in literature for the
assessment of the effects of antacid in the treatment of peptic oesophagitis and gastrooesphageal
reflux disease [4, 5]. The common methods of antacid action include the ingredients of H2
blockers or inhibitors of the proton flux pump in the cellular dynamics in conditions of heartburn
due to gastric disturbance [4, 5]. Antacids commonly lack the ability to neutralize the acids in
instances of heartburn independently [4, 5]. Additionally, they require the incorporation of
ingredients such as sodium, calcium, aluminium, and magnesium [4, 5]. These aforementioned
ingredients aid the process of increase of the physiological pH [4, 5].
2.1. Protocol and Experimental Plan: The current experiment focuses on the
determination of the most effective antacid in the treatment of heartburn and gastrooesophageal
reflux disorder. The current experiment focuses on the antacids rennie, milk of magnesium, and
gaviscon [4, 5]. Milk of magnesium is believed to have the highest efficacy in terms of the
neutralization of acids in the system [4, 5]. Rennie is a commonly available antacid which is
available over the counter in most pharmacies [4, 5]. It is primarily composed of calcium and
magnesium carbonate composition [4, 5]. They function in the improvement of the emptying of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Practical Science Project 7
the gastric contents, neutralization of stomach acid and hydroxylation of the acid to form water
molecules [4, 5].
a) Timescale and task priority: The current experiment is based on a timescale of about one
hour for preparation of the required materials and the preparation of the standard operating
procedure. Preparation of the reagents required may require an additional thirty minutes.
b) Materials required and arranged: The materials required for the current experiment include
the following:
Glassware:
i) 4-5 beakers of assorted capacities (100 ml, 200/250 ml)
ii) 800 ml of clear water
iii) 80 ml of moderately concentrated hydrochloric acid
iv) Antacid (three different brands) – Milk of magnesia, rennie, and gaviscon
v) One burette apparatus
vi) One mortar and pestle apparatus
vii) One pH meter
viii) One spatula or spoon for measurement
ix) One glass marker
the gastric contents, neutralization of stomach acid and hydroxylation of the acid to form water
molecules [4, 5].
a) Timescale and task priority: The current experiment is based on a timescale of about one
hour for preparation of the required materials and the preparation of the standard operating
procedure. Preparation of the reagents required may require an additional thirty minutes.
b) Materials required and arranged: The materials required for the current experiment include
the following:
Glassware:
i) 4-5 beakers of assorted capacities (100 ml, 200/250 ml)
ii) 800 ml of clear water
iii) 80 ml of moderately concentrated hydrochloric acid
iv) Antacid (three different brands) – Milk of magnesia, rennie, and gaviscon
v) One burette apparatus
vi) One mortar and pestle apparatus
vii) One pH meter
viii) One spatula or spoon for measurement
ix) One glass marker
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Practical Science Project 8
In order to carry out the experiment, the access to the basic equipment and the reagents of the
laboratory is required. The materials required were obtained on request from the university lab
upon due submission of the standard operating procedure.
2.2. Data collection and analytical method: The collection of the required data was
carried out by means of the experimental protocol that consisted of recording the amount of acid
required for the neutralization of each antacid in a statistically significant number of repeats. The
observed values were recorded manually along with the time taken for the complete and effective
neutralization of the acid. The results were tabulated and graphically represented. The analysis of
the results indicated the antacid having the highest degree of effectiveness in the neutralization of
the acid.
2.3. Justification of hypothesis (controlled and uncontrolled variables,
accuracy, and reliability): The hypothesis of the current experiment states that milk of
magnesia has the highest efficacy in the neutralization of the acids. The controlled variable is the
amount of the different brands of antacids used in each run of the experiment. The uncontrolled
variable is the amount of acid that is required for the neutralization of the antacid. The accuracy
of the results is dependent on the variability of the dependent variable i.e. the amount of acid
required for neutralization. The results are made reliable by carrying out statistically significant
number of repeats.
2.4. Probable outcomes and congruence with literature: The probable outcomes of
this experiment will be that all the antacids have a similar level of acid neutralization [4, 5].
However, milk of magnesia is likely to display highest efficacy by neutralizing a large amount of
In order to carry out the experiment, the access to the basic equipment and the reagents of the
laboratory is required. The materials required were obtained on request from the university lab
upon due submission of the standard operating procedure.
2.2. Data collection and analytical method: The collection of the required data was
carried out by means of the experimental protocol that consisted of recording the amount of acid
required for the neutralization of each antacid in a statistically significant number of repeats. The
observed values were recorded manually along with the time taken for the complete and effective
neutralization of the acid. The results were tabulated and graphically represented. The analysis of
the results indicated the antacid having the highest degree of effectiveness in the neutralization of
the acid.
2.3. Justification of hypothesis (controlled and uncontrolled variables,
accuracy, and reliability): The hypothesis of the current experiment states that milk of
magnesia has the highest efficacy in the neutralization of the acids. The controlled variable is the
amount of the different brands of antacids used in each run of the experiment. The uncontrolled
variable is the amount of acid that is required for the neutralization of the antacid. The accuracy
of the results is dependent on the variability of the dependent variable i.e. the amount of acid
required for neutralization. The results are made reliable by carrying out statistically significant
number of repeats.
2.4. Probable outcomes and congruence with literature: The probable outcomes of
this experiment will be that all the antacids have a similar level of acid neutralization [4, 5].
However, milk of magnesia is likely to display highest efficacy by neutralizing a large amount of

Practical Science Project 9
acid [4, 5]. The reliability of the results is ensured by carrying out a significant number of
experimental runs [4, 5].
The results of certain studies present in literature reflect that milk of magnesia is an efficacious
antacid as compared to several other antacids. Similar experiments in literature have displayed
the efficacy of milk of magnesia against antacids such as gaviscon, rennie, tagament, turns etc [4,
5]. Milk of magnesia has been found to have a higher capacity to neutralize acids in the system
in comparison to most antacids containing H2 blockers or proton pump inhibitors [4, 5].
2.5. Ethical and safety issues in practical execution along with proposed
management: The current experiment included basic safety practices. The use of safety wear
and experimental gear in the laboratory had to be used at all times. Additionally, the consistent
use of gloves for handling each step of the experiment was ensured. Extra precaution practiced
whilst handling acids and all other required reagents.
2.6. Risk assessment: The probable risk factor is inhalation of fumes and risk during the
andling of concentrated Hydrochloric acid. Basic risks whilst handling other reagents and risk of
ingestion;
3.1. Planned techniques for obtaining results: Methodology
The methodology firstly included the identification of controlled (independent) and uncontrolled
(dependent) variables during the measurement of the acid required for neutralization. The
controlled variable in the present experiment is the amount of brands of antacids used for each
repeat cycle of the experimental protocol. The uncontrolled or dependent variable is the amount
of acid required for neutralization. The procedure used is that of titration using the burette
apparatus. The uncontrolled variable has to be measured in each cycle of the experiment. This
acid [4, 5]. The reliability of the results is ensured by carrying out a significant number of
experimental runs [4, 5].
The results of certain studies present in literature reflect that milk of magnesia is an efficacious
antacid as compared to several other antacids. Similar experiments in literature have displayed
the efficacy of milk of magnesia against antacids such as gaviscon, rennie, tagament, turns etc [4,
5]. Milk of magnesia has been found to have a higher capacity to neutralize acids in the system
in comparison to most antacids containing H2 blockers or proton pump inhibitors [4, 5].
2.5. Ethical and safety issues in practical execution along with proposed
management: The current experiment included basic safety practices. The use of safety wear
and experimental gear in the laboratory had to be used at all times. Additionally, the consistent
use of gloves for handling each step of the experiment was ensured. Extra precaution practiced
whilst handling acids and all other required reagents.
2.6. Risk assessment: The probable risk factor is inhalation of fumes and risk during the
andling of concentrated Hydrochloric acid. Basic risks whilst handling other reagents and risk of
ingestion;
3.1. Planned techniques for obtaining results: Methodology
The methodology firstly included the identification of controlled (independent) and uncontrolled
(dependent) variables during the measurement of the acid required for neutralization. The
controlled variable in the present experiment is the amount of brands of antacids used for each
repeat cycle of the experimental protocol. The uncontrolled or dependent variable is the amount
of acid required for neutralization. The procedure used is that of titration using the burette
apparatus. The uncontrolled variable has to be measured in each cycle of the experiment. This
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Practical Science Project 10
measurement of the amount of acid is carried out by dropping the acid from the burette and
measuring the amount of the acid required to neutralize each different brand of antacid in the
beaker placed under the dropper of the burette. The neutralization event is ascertained by
measuring the pH of the solution in the beaker using a pH meter. The constant variables or the
controlled variables are now the amount of water present in the beaker (product of the reaction
between acid and antacid) and the concentration of the solution of acids in the beaker. Three
beakers are selected and labelled with the names milk of magnesia, rennie, and milk of magnesia
with the help of the glass marker. The beakers are filled with 200 ml of water each. The
prescribed dosages of the three types of antacids are blended with the water present in the beaker
labelled according to the brands of the antacid. The antacid tablets are crushed initially with the
help of mortar and pestle. This crushed antacid is then mixed with water. The antacid powder is
dissolved in water and dispensed in the experiment by the use of measuring spatula or spoon.
The concentrated hydrochloric acid is measured into 40ml portion. The measured hydrochloric
acid solution is slowly poured into the apparatus of the burette. For each cycle of the experiment,
different beakers containing different antacid solutions are placed under the burette dropper. The
acid is dispensed into burette and is released into the beaker containing specifically measured
amount of antacid with water. Following the release of each drop, the solution is thoroughly
mixed in with the help of the stirrer and the reading of the pH is taken with the help of the pH
meter. At the point where the value of pH reaches an alkaline range i.e. 7.0, no more acid is
released from the burette. pH value of 7.0 is the normal physiological pH of a healthy
gastrooesophageal system. Thus, the amount of acid that is required for the neutralization of the
solution present in the beaker is tabulated and recorded for each repeat.
measurement of the amount of acid is carried out by dropping the acid from the burette and
measuring the amount of the acid required to neutralize each different brand of antacid in the
beaker placed under the dropper of the burette. The neutralization event is ascertained by
measuring the pH of the solution in the beaker using a pH meter. The constant variables or the
controlled variables are now the amount of water present in the beaker (product of the reaction
between acid and antacid) and the concentration of the solution of acids in the beaker. Three
beakers are selected and labelled with the names milk of magnesia, rennie, and milk of magnesia
with the help of the glass marker. The beakers are filled with 200 ml of water each. The
prescribed dosages of the three types of antacids are blended with the water present in the beaker
labelled according to the brands of the antacid. The antacid tablets are crushed initially with the
help of mortar and pestle. This crushed antacid is then mixed with water. The antacid powder is
dissolved in water and dispensed in the experiment by the use of measuring spatula or spoon.
The concentrated hydrochloric acid is measured into 40ml portion. The measured hydrochloric
acid solution is slowly poured into the apparatus of the burette. For each cycle of the experiment,
different beakers containing different antacid solutions are placed under the burette dropper. The
acid is dispensed into burette and is released into the beaker containing specifically measured
amount of antacid with water. Following the release of each drop, the solution is thoroughly
mixed in with the help of the stirrer and the reading of the pH is taken with the help of the pH
meter. At the point where the value of pH reaches an alkaline range i.e. 7.0, no more acid is
released from the burette. pH value of 7.0 is the normal physiological pH of a healthy
gastrooesophageal system. Thus, the amount of acid that is required for the neutralization of the
solution present in the beaker is tabulated and recorded for each repeat.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Practical Science Project 11
a) Accuracy of results: The accuracy of results is ensured by the measurement of pH with each
new drop of acid into the beaker. The mixture in the beaker is mixed thoroughly with the help of
a glass stirrer until the mixture is fully blended. The accuracy of the measurement of pH is
ensured with the use of pH meter.
b) Reliability: The reliability of the results is based on the number of repeats and the consistency
of results over the several repeats. 40 ml of concentrated hydrochloric acid is refilled in the
burette for the various repeats.
3.2. Practical modifications to the proposed plan: There are no significant
modifications required to the practical protocol of the experiment. The experiment is carried out
in the prescribed protocol. The amount of reagents and the proportions of the materials used is
consistent with the suggested procedure.
3.3. Record of the data: The data is recorded and tabulated as follows:
1. Amount of acid to neutralize the acid:
Brand of antacid Amount of acid required for neutralization of antacid
Milk of magnesia Rennie Gaviscon
Amount of acid
required 28ml 32.5 ml 6.9 ml
2. Graph depicting the time factor:
Brand of antacid Final pH Time taken for the attainment
a) Accuracy of results: The accuracy of results is ensured by the measurement of pH with each
new drop of acid into the beaker. The mixture in the beaker is mixed thoroughly with the help of
a glass stirrer until the mixture is fully blended. The accuracy of the measurement of pH is
ensured with the use of pH meter.
b) Reliability: The reliability of the results is based on the number of repeats and the consistency
of results over the several repeats. 40 ml of concentrated hydrochloric acid is refilled in the
burette for the various repeats.
3.2. Practical modifications to the proposed plan: There are no significant
modifications required to the practical protocol of the experiment. The experiment is carried out
in the prescribed protocol. The amount of reagents and the proportions of the materials used is
consistent with the suggested procedure.
3.3. Record of the data: The data is recorded and tabulated as follows:
1. Amount of acid to neutralize the acid:
Brand of antacid Amount of acid required for neutralization of antacid
Milk of magnesia Rennie Gaviscon
Amount of acid
required 28ml 32.5 ml 6.9 ml
2. Graph depicting the time factor:
Brand of antacid Final pH Time taken for the attainment
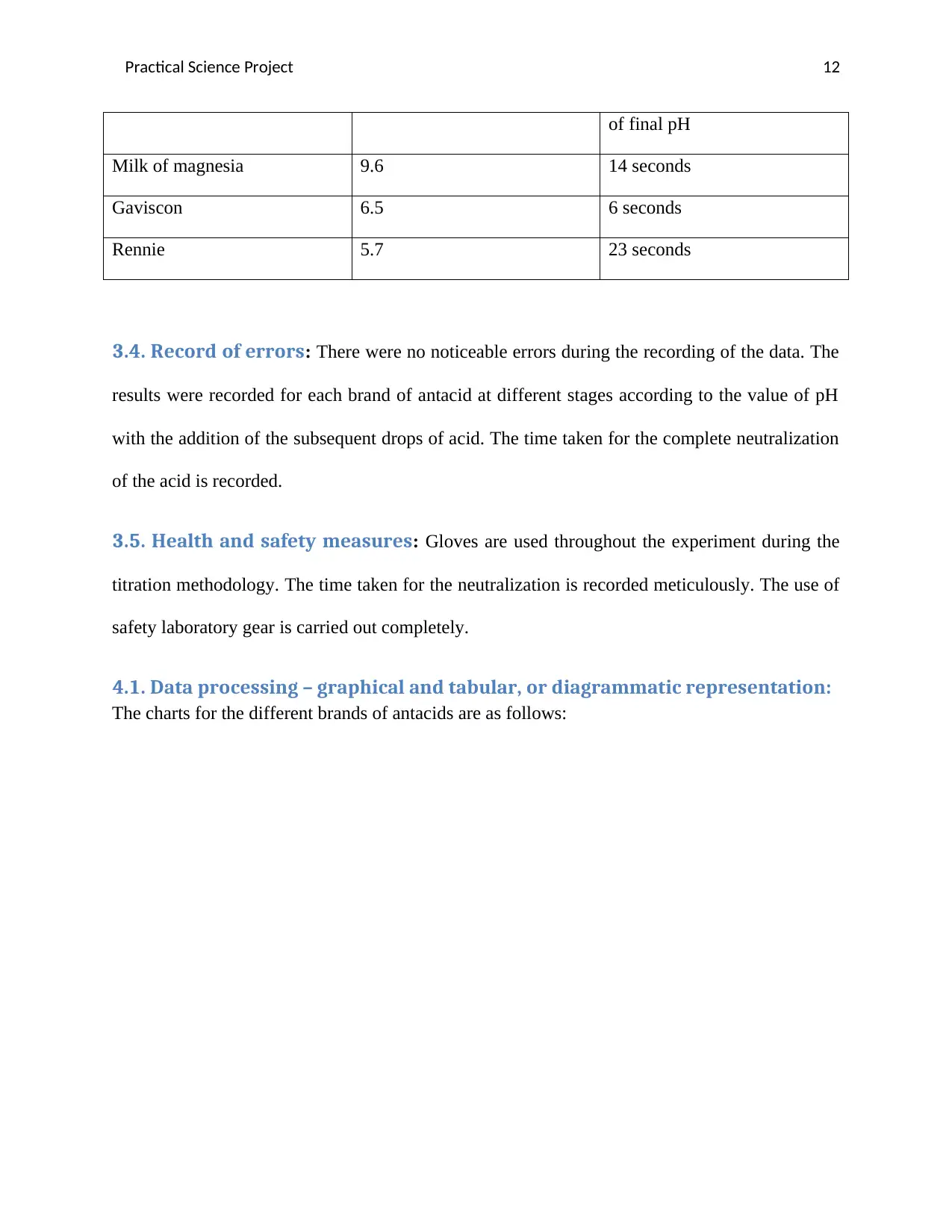
Practical Science Project 12
of final pH
Milk of magnesia 9.6 14 seconds
Gaviscon 6.5 6 seconds
Rennie 5.7 23 seconds
3.4. Record of errors: There were no noticeable errors during the recording of the data. The
results were recorded for each brand of antacid at different stages according to the value of pH
with the addition of the subsequent drops of acid. The time taken for the complete neutralization
of the acid is recorded.
3.5. Health and safety measures: Gloves are used throughout the experiment during the
titration methodology. The time taken for the neutralization is recorded meticulously. The use of
safety laboratory gear is carried out completely.
4.1. Data processing – graphical and tabular, or diagrammatic representation:
The charts for the different brands of antacids are as follows:
of final pH
Milk of magnesia 9.6 14 seconds
Gaviscon 6.5 6 seconds
Rennie 5.7 23 seconds
3.4. Record of errors: There were no noticeable errors during the recording of the data. The
results were recorded for each brand of antacid at different stages according to the value of pH
with the addition of the subsequent drops of acid. The time taken for the complete neutralization
of the acid is recorded.
3.5. Health and safety measures: Gloves are used throughout the experiment during the
titration methodology. The time taken for the neutralization is recorded meticulously. The use of
safety laboratory gear is carried out completely.
4.1. Data processing – graphical and tabular, or diagrammatic representation:
The charts for the different brands of antacids are as follows:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

