Pathogenic E. coli Isolates and Diarrhea in Humans and Animals
VerifiedAdded on 2022/08/17
|8
|5519
|15
Report
AI Summary
This report, published in the American Journal of Animal and Veterinary Sciences, investigates Escherichia coli pathotypes associated with diarrhea in humans and domestic animals. The study focuses on characterizing pathogenic E. coli isolates from cattle and sheep, examining their pathotypes, serotypes, and genotypes. The research reveals that VTEC is the most prevalent pathotype, followed by EAEC, EPEC, ETEC, and EIEC. The study highlights the molecular relatedness between E. coli O157:H7 isolates from human patients, sheep, and cattle, supporting the hypothesis that ruminants act as reservoirs for human infection. The findings emphasize the importance of understanding the role of VTEC and other DEC strains in causing diarrheal diseases, and the potential for zoonotic transmission. The research utilized methods such as PCR and PFGE to identify virulence factors and determine the genetic relatedness between strains, providing valuable insights into the epidemiology and control of DEC epidemics.
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/280778600
Escherichia coli pathotypes associated with diarrhea in human and domestic
animals
Article in American Journal of Animal and Veterinary Sciences · September 2014
DOI: 10.3844/ajavssp.2014.155.161
CITATIONS
4
READS
147
1 author:
Some of the authors of this publication are also working on these related projects:
a fellowshipView project
Iman Shabana
Suez Canal University
19PUBLICATIONS154CITATIONS
SEE PROFILE
All content following this page was uploaded by Iman Shabana on 12 June 2017.
The user has requested enhancement of the downloaded file.
Escherichia coli pathotypes associated with diarrhea in human and domestic
animals
Article in American Journal of Animal and Veterinary Sciences · September 2014
DOI: 10.3844/ajavssp.2014.155.161
CITATIONS
4
READS
147
1 author:
Some of the authors of this publication are also working on these related projects:
a fellowshipView project
Iman Shabana
Suez Canal University
19PUBLICATIONS154CITATIONS
SEE PROFILE
All content following this page was uploaded by Iman Shabana on 12 June 2017.
The user has requested enhancement of the downloaded file.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

American Journal of Animal and Veterinary Sciences 9 (3): 155-161, 2014
ISSN: 1557-4555
© 2014 I.I. Shabana, This open access article is distributed under a Creative Commons Attribution
(CC-BY) 3.0 license
doi:10.3844/ajavssp.2014.155.161 Published Online 9 (3) 2014 (http://www.thescipub.com/ajavs.toc)
155Science Publications AJAVS
ESCHERICHIA COLI PATHOTYPES ASSOCIATED WITH
DIARRHEA IN HUMAN AND DOMESTIC ANIMALS
Shabana, I.I.
Department of Bacteriology, Faculty of Veterinary Medicine,
Immunology and Mycology, Suez Canal University, Egypt
Received 2014-06-14; Revised 2014-07-27; Accepted 2014-08-08
ABSTRACT
Ruminants are important reservoirs for zoonotic pathogenic E. coli. The objective of this study was to
characterize pathogenic E. coli isolates from cattle and sheep linked to human illness with respect to their path
types, serotypes and genotypes. E. coli O157:H7 isolated from cattle, sheep and human patients were
compared for their genomic similarity by Pulsed-Field Gel-Electrophoresis (PFGE). PCR detection of
virulence factors associated with different E. coli path types (VTEC, ETEC, EPEC, EAEC and EIEC) revealed
that VTEC was the most prevalent path type (22/45; 48.9%), followed by EAEC (3/45; 6.7%), EPEC (1/45;
2.2%), a EPEC (3/45; 6.7%), ETEC (1/45; 2.2%) and EIEC (1/45; 2.2%). E. coli O157:H7 represented the
most prevalent VTEC serotypes (11/22; 50%). Pulsed field gel electrophoresis typing revealed exact matches
between E. coli O157:H7 isolates from the human patients, sheep and cattle in the same municipality. VTEC
play an important cause of diarrhea in human, sheep and cattle. The molecular relatedness between PFGE
profiles of E. coli O157:H7 isolates from human, sheep and cattle supported the hypothesis that ruminants
especially cattle and sheep act as reservoirs of E. coli O157:H7 for human infection.
Keywords: DEC, Human, Ruminants, O157, PFGE
1. INTRODUCTION
Diarrheal diseases are a leading cause of morbidity and
mortality in developing countries. Escherichia coli is
recognized as an important cause of both sporadic cases and
outbreaks of diarrhea all over the world. Nguyen et al.
(2005). Diarrheagenic Escherichia Coli (DEC) strains can
be classified into five major categories on the basis of the
nature of their infection and pathogenic mechanisms:
They are Enter Pathogenic E. Coli (EPEC), Enter
Hemorrhagic E. Coli (EHEC), Enterotoxigenic E. Coli
(ETEC), enter invasive E. Coli (EIEC) and Enter
Aggregative E. Coli (EAEC) (Tamaki et al., 2005).
O157:H7 and related non-O157 VTEC strains are
pathogens of public health concern worldwide. They
may cause severe outbreaks of gastrointestinal illness
with clinical symptoms ranging from diarrhea and
hemorrhagic colitis to the life-threatening Hemolytic
Uraemic Syndrome (HUS) (Lynn et al., 2005). VTEC
strains have been isolated from a variety of animals and
cattle are considered to be the major reservoir for VTEC
strains (Ferens and Hovde, 2011). However, recent
evidence has indicated that small domestic ruminants,
including sheep and goats, are also key reservoirs of
VTEC (La Ragione et al., 2009). In particular, sheep and
their products have been documented as reservoirs for
VTECs that belong to a diverse set of non-O157
Serogroup that harbor genes encoding key virulence
factors that have been implicated in human disease
(Djordjevic et al., 2001). Transmission of VTEC to
humans occurs through direct or indirect contact with
animals or their environment, consumption of
contaminated food or water and through person-to-
person contact (Gillespie et al., 2005).
In order to prevent and control DEC epidemics, a
reliable procedure should be followed to identify and
categorize DEC isolates. Since several virulence factors
have been identified in DEC strains, modern molecular
detection methods, including PCR have been developed
(Teng et al., 2004). They are performed at the genetic
ISSN: 1557-4555
© 2014 I.I. Shabana, This open access article is distributed under a Creative Commons Attribution
(CC-BY) 3.0 license
doi:10.3844/ajavssp.2014.155.161 Published Online 9 (3) 2014 (http://www.thescipub.com/ajavs.toc)
155Science Publications AJAVS
ESCHERICHIA COLI PATHOTYPES ASSOCIATED WITH
DIARRHEA IN HUMAN AND DOMESTIC ANIMALS
Shabana, I.I.
Department of Bacteriology, Faculty of Veterinary Medicine,
Immunology and Mycology, Suez Canal University, Egypt
Received 2014-06-14; Revised 2014-07-27; Accepted 2014-08-08
ABSTRACT
Ruminants are important reservoirs for zoonotic pathogenic E. coli. The objective of this study was to
characterize pathogenic E. coli isolates from cattle and sheep linked to human illness with respect to their path
types, serotypes and genotypes. E. coli O157:H7 isolated from cattle, sheep and human patients were
compared for their genomic similarity by Pulsed-Field Gel-Electrophoresis (PFGE). PCR detection of
virulence factors associated with different E. coli path types (VTEC, ETEC, EPEC, EAEC and EIEC) revealed
that VTEC was the most prevalent path type (22/45; 48.9%), followed by EAEC (3/45; 6.7%), EPEC (1/45;
2.2%), a EPEC (3/45; 6.7%), ETEC (1/45; 2.2%) and EIEC (1/45; 2.2%). E. coli O157:H7 represented the
most prevalent VTEC serotypes (11/22; 50%). Pulsed field gel electrophoresis typing revealed exact matches
between E. coli O157:H7 isolates from the human patients, sheep and cattle in the same municipality. VTEC
play an important cause of diarrhea in human, sheep and cattle. The molecular relatedness between PFGE
profiles of E. coli O157:H7 isolates from human, sheep and cattle supported the hypothesis that ruminants
especially cattle and sheep act as reservoirs of E. coli O157:H7 for human infection.
Keywords: DEC, Human, Ruminants, O157, PFGE
1. INTRODUCTION
Diarrheal diseases are a leading cause of morbidity and
mortality in developing countries. Escherichia coli is
recognized as an important cause of both sporadic cases and
outbreaks of diarrhea all over the world. Nguyen et al.
(2005). Diarrheagenic Escherichia Coli (DEC) strains can
be classified into five major categories on the basis of the
nature of their infection and pathogenic mechanisms:
They are Enter Pathogenic E. Coli (EPEC), Enter
Hemorrhagic E. Coli (EHEC), Enterotoxigenic E. Coli
(ETEC), enter invasive E. Coli (EIEC) and Enter
Aggregative E. Coli (EAEC) (Tamaki et al., 2005).
O157:H7 and related non-O157 VTEC strains are
pathogens of public health concern worldwide. They
may cause severe outbreaks of gastrointestinal illness
with clinical symptoms ranging from diarrhea and
hemorrhagic colitis to the life-threatening Hemolytic
Uraemic Syndrome (HUS) (Lynn et al., 2005). VTEC
strains have been isolated from a variety of animals and
cattle are considered to be the major reservoir for VTEC
strains (Ferens and Hovde, 2011). However, recent
evidence has indicated that small domestic ruminants,
including sheep and goats, are also key reservoirs of
VTEC (La Ragione et al., 2009). In particular, sheep and
their products have been documented as reservoirs for
VTECs that belong to a diverse set of non-O157
Serogroup that harbor genes encoding key virulence
factors that have been implicated in human disease
(Djordjevic et al., 2001). Transmission of VTEC to
humans occurs through direct or indirect contact with
animals or their environment, consumption of
contaminated food or water and through person-to-
person contact (Gillespie et al., 2005).
In order to prevent and control DEC epidemics, a
reliable procedure should be followed to identify and
categorize DEC isolates. Since several virulence factors
have been identified in DEC strains, modern molecular
detection methods, including PCR have been developed
(Teng et al., 2004). They are performed at the genetic

Shabana, I.I. / American Journal of Animal and Veterinary Sciences 9 (3): 155-161, 2014
156Science Publications AJAVS
level and directly detect genes for specific virulence
factors, which themselves determine the pathogenicity.
The presence of these genes is the evidence that renders
the virulence and that can be used to categorize DEC
strains (Yang et al., 2007).
This study aimed to document the association of
pathogenic Escherichia coli isolates with diarrhea in
human and animals, based on the PCR-based
identification of their intrinsic virulence. And try to find
the genetic relatedness among VTEC strains from human,
cattle and sheep to identify the zoonotic possibility.
2. MATERIALS AND METHODS
2.1. Bacterial Strains
Stool samples were collected from patients with
diarrhea and rectal swabs were collected from cow and
sheep with diarrhea. Typical E. coli isolates selected
were selected from Eosin Methylene Blue (EMB) agar,
which discourage the growth of gram positive bacteria and
give a distinctive metallic green sheen, these isolates
were then subjected to the IMViC test for the further
selection of E. coli strains.
2.2. Reference Strains
E. coli strains used as positive controls were: 241
(LT), 422 (aggR), 418 (bfp) 226 (ST), S103 (E-hly),
G200 (ipaH), 206 (EAST1), ED33 (V1, V2 and eae) and
E. coli A300 was used as a negative control. Reference
strains were kindly provided by the Niigata Prefectural
Institute of Public Health and Environmental Sciences,
Department of Bacteriology, Niigata, Japan.
2.3. Somatic and Flagella Serotyping
Isolates were grown on nutrient agar plates at 37 8C
overnight, then the cells were collected and suspended in
0.9% sterile normal saline (154 mEq/L sodium and 154
mEq/L chloride, pH adjusted to 7) and autoclaved at 121
8C for 15 min and then the cells were collected by
centrifugation and resuspended in an appropriate volume
of sterile normal saline. Detection of O-serogroup was
performed using a commercially available O-
serogrouping Kit (Denka Seiken, Tokyo, Japan)
(Takahashi et al., 2008). The Flagellar phase inversion
was carried out using the standard Craigie tube technique
by passage through semi-solid agar containing the
appropriate flagellar antis era (Davies and Wray, 1997).
• O-antis era used were,
• Polyvalent I: O1, O26, O86a, O111, O119, O127a,
O128
• Polyvalent 2: O44, O55, O125, O126, O146, O166
• Polyvalent 3: O18, O114, O142, O151, O157, O158
• Polyvalent 4: O6, O27, O78, O148, O159, O168
• Polyvalent 5: O20, O25, O63, O153, O167
• Polyvalent 6: O8, O15, O115, O169
• Polyvalent 7: O28ac, O112ac, O124, O136, O144
• Polyvalent 8: O29, O143, O152, O164
• Polyvalent 9: O74, O91, O103, O121, O145,
O161, O165
• Flagellar antis era (H-antis era) used were, H2, H4, H5
• H6, H7, H9, H10, H11, H12, H16, H18, H19, H20,
H21, H27,H28, H34, H40, H41, H42, H45, H51
2.4. Template DNA Preparation
Single colonies obtained from nutrient agar were
inoculated into Luria Bertani (LB) broth (Merck) and
were grown at 37°C for 12 h. Cells were harvested from
2 mL of the cell suspension by centrifuging at 16 000×g
for10 min and discarding the supernatant. Genomic DNA
was then isolated by boiling method (Usein et al., 2009).
2.5. Detection of Virulence Factors
Detection of virulence factors was performed by
PCR. Primer sequences and PCR conditions used for the
study listed in Table 1, (Moyo et al., 2007; Ochoa et al.,
2008; Vu-Khac et al., 2007; Mendez-Arancibia et al.,
2008: Zweifel et al., 2006; Osek, 2003; Shabana et al.,
2013). PCR performed in the Takara thermal cycler
(Takara, Tokyo, Japan). PCR products were separated and
visualized by gel electrophoresis in 1.5% agarose
(Wako, Japan) in Tris-Acetate-EDTA (TAE) buffer at
100 V. And a 100 or 500 bp DNA ladder (one-step
ladder, Wako) was included in each agarose run,
accordingly the amplified product.
2.6. Pulsed Field Gel Electrophoresis (PFGE)
PFGE was performed according to CDC (the Pulse
Net protocol of the (CDCP, 1998). The agarose-
embedded bacterial genomic DNA was digested with
restriction enzyme XbaI at 37 8C for 4 h.
Electrophoresis was performed on 1% agarose gel
with 0.5-Tris-borate-EDTA buffer. The
electrophoretic conditions were optimized for the
separation of the 24-to 600-kB XbaI-digested macro
restriction fragments. The following PFGE parameters
were applied: Voltage of 6 v/cm, initial switch time of
2.2 s, final switch time of 54.2 s and run time of 19 h.
Electrophoresis was conducted by using a CHEF-DRII
(Bio-Rad Laboratories, Tokyo, Japan). The gel was
stained with ethidium bromide and imaged with the
Gel Doc 2000 and Multi-Analyst program (Bio-Rad).
156Science Publications AJAVS
level and directly detect genes for specific virulence
factors, which themselves determine the pathogenicity.
The presence of these genes is the evidence that renders
the virulence and that can be used to categorize DEC
strains (Yang et al., 2007).
This study aimed to document the association of
pathogenic Escherichia coli isolates with diarrhea in
human and animals, based on the PCR-based
identification of their intrinsic virulence. And try to find
the genetic relatedness among VTEC strains from human,
cattle and sheep to identify the zoonotic possibility.
2. MATERIALS AND METHODS
2.1. Bacterial Strains
Stool samples were collected from patients with
diarrhea and rectal swabs were collected from cow and
sheep with diarrhea. Typical E. coli isolates selected
were selected from Eosin Methylene Blue (EMB) agar,
which discourage the growth of gram positive bacteria and
give a distinctive metallic green sheen, these isolates
were then subjected to the IMViC test for the further
selection of E. coli strains.
2.2. Reference Strains
E. coli strains used as positive controls were: 241
(LT), 422 (aggR), 418 (bfp) 226 (ST), S103 (E-hly),
G200 (ipaH), 206 (EAST1), ED33 (V1, V2 and eae) and
E. coli A300 was used as a negative control. Reference
strains were kindly provided by the Niigata Prefectural
Institute of Public Health and Environmental Sciences,
Department of Bacteriology, Niigata, Japan.
2.3. Somatic and Flagella Serotyping
Isolates were grown on nutrient agar plates at 37 8C
overnight, then the cells were collected and suspended in
0.9% sterile normal saline (154 mEq/L sodium and 154
mEq/L chloride, pH adjusted to 7) and autoclaved at 121
8C for 15 min and then the cells were collected by
centrifugation and resuspended in an appropriate volume
of sterile normal saline. Detection of O-serogroup was
performed using a commercially available O-
serogrouping Kit (Denka Seiken, Tokyo, Japan)
(Takahashi et al., 2008). The Flagellar phase inversion
was carried out using the standard Craigie tube technique
by passage through semi-solid agar containing the
appropriate flagellar antis era (Davies and Wray, 1997).
• O-antis era used were,
• Polyvalent I: O1, O26, O86a, O111, O119, O127a,
O128
• Polyvalent 2: O44, O55, O125, O126, O146, O166
• Polyvalent 3: O18, O114, O142, O151, O157, O158
• Polyvalent 4: O6, O27, O78, O148, O159, O168
• Polyvalent 5: O20, O25, O63, O153, O167
• Polyvalent 6: O8, O15, O115, O169
• Polyvalent 7: O28ac, O112ac, O124, O136, O144
• Polyvalent 8: O29, O143, O152, O164
• Polyvalent 9: O74, O91, O103, O121, O145,
O161, O165
• Flagellar antis era (H-antis era) used were, H2, H4, H5
• H6, H7, H9, H10, H11, H12, H16, H18, H19, H20,
H21, H27,H28, H34, H40, H41, H42, H45, H51
2.4. Template DNA Preparation
Single colonies obtained from nutrient agar were
inoculated into Luria Bertani (LB) broth (Merck) and
were grown at 37°C for 12 h. Cells were harvested from
2 mL of the cell suspension by centrifuging at 16 000×g
for10 min and discarding the supernatant. Genomic DNA
was then isolated by boiling method (Usein et al., 2009).
2.5. Detection of Virulence Factors
Detection of virulence factors was performed by
PCR. Primer sequences and PCR conditions used for the
study listed in Table 1, (Moyo et al., 2007; Ochoa et al.,
2008; Vu-Khac et al., 2007; Mendez-Arancibia et al.,
2008: Zweifel et al., 2006; Osek, 2003; Shabana et al.,
2013). PCR performed in the Takara thermal cycler
(Takara, Tokyo, Japan). PCR products were separated and
visualized by gel electrophoresis in 1.5% agarose
(Wako, Japan) in Tris-Acetate-EDTA (TAE) buffer at
100 V. And a 100 or 500 bp DNA ladder (one-step
ladder, Wako) was included in each agarose run,
accordingly the amplified product.
2.6. Pulsed Field Gel Electrophoresis (PFGE)
PFGE was performed according to CDC (the Pulse
Net protocol of the (CDCP, 1998). The agarose-
embedded bacterial genomic DNA was digested with
restriction enzyme XbaI at 37 8C for 4 h.
Electrophoresis was performed on 1% agarose gel
with 0.5-Tris-borate-EDTA buffer. The
electrophoretic conditions were optimized for the
separation of the 24-to 600-kB XbaI-digested macro
restriction fragments. The following PFGE parameters
were applied: Voltage of 6 v/cm, initial switch time of
2.2 s, final switch time of 54.2 s and run time of 19 h.
Electrophoresis was conducted by using a CHEF-DRII
(Bio-Rad Laboratories, Tokyo, Japan). The gel was
stained with ethidium bromide and imaged with the
Gel Doc 2000 and Multi-Analyst program (Bio-Rad).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Shabana, I.I. / American Journal of Animal and Veterinary Sciences 9 (3): 155-161, 2014
157Science Publications AJAVS
Table 1. PCR primers and conditions for amplification of virulence genes
Primer PCR PCR
Target gene designation Primer sequence(5'-3') conditions * product (bp) Reference
Vero toxin KS7 CCCGGATCCATGAAAAAAACATTATTAATAGC 95°C,30 s; 285 Zweifel et al.
1 (V1) KS8 CCCGAATTCAGCTATTCTGAGTCAACG 52°C,30 s; (2006)
72°C,30 s
Vero toxin VT2e AATACATTATGGGAAAGTAATA 95°C,30 s; 348 Zweifel et al.
2 (V2) VT2f TAAACTGCACTTCAGCAAAT 52°C,30 s; (2006)
72°C,30 s
eae (all intimin SK1 CCCGAATTCGGCACAAGCATAAGC 95°C,30 s; 863 Zweifel et al.
genes) SK2 CCCGGATCCGTCTCGCCAGTATTCG 61°C,30 s; (2006)
72°C,30 s
E-hly (EHEC- HlyA1 GGTGCAGCAGAAAAAGTTGTAG 95°C,30 s; 1550 Zweifel et al.
hemolysin) HlyA4 TCTCGCCTGATAGTGTTTGGTA 61°C,30 s; (2006)
72°C,30 s
(LT) Heat-labile elt1 ATTTACGGCGTTACTATCCTC 95°C,30 s; 281 Vu-Khac et al.
Enterotoxin elt2 TTTTGGTCTCGGTCAGATATG 58°C,30 (2007)
72°C,30 s
(St)Heat-stable St5 TTAATAGCACCCGGTACAAGCAGG 95°C,30 s; 147 Mendez-
Enterotoxin St3 CTTGACTCTTCAAAAGAGAAAATTAC 52°C,30 s; Arancibia et al.
72°C,30 s (2008)
(EAST1) Eagg AstA1-1 CCATCAACACAGTATATC 95°C,30 s; 111 Osek (2003)
EaggEC heat AstA1-2 GGTCGCGAGTGACGGCTTTGT 52°C,30 s;
-stable enterotoxin 72°C,30 s
(aggR) Fimbrial aggR1 CTAATTGTACAATCGATGTA 95°C,30 s; 457 Moyo et al.
antigen-specific aggR2 AGAGTCCATCTCTTTGATAAG 55°C,30 s (2007)
gene 72°C,30 s;
(bfp) bundle- EP1 AATGGTGCTTGCGCTTGCTGC 95°C,30 s; 326 Ochoa et al.
forming pilus EP2 GCCGCTTTATCCAACCTGGTA 52°C,30 s; (2008)
(ipaH)Invasion EI-1 GCTGGAAAAACTCAGTGCCT 95°C,30 s; 424 Shabana et al.
plasmid antigen EI-2 CCAGTCCGTAAATTCATTCT 52°C,30 s; (2013)
72°C,30 s
Dendrograms were created with a Molecular Analyst
(Bio-Rad) by using the Dice coefficient, Un weighted
Pair Group Method with Arithmetic means (UPGMA)
and a position tolerance of 1.3%.
3. RESULTS
3.1. Isolation and Confirmation of E. Coli
Isolates from Diarrheic Cases
A total of 45 presumptive E. coli isolates were
obtained from diarrheic human (n = 29), cow (n = 9) and
sheep (n = 7). The presumptive E. coli isolates were
confirmed as E. coli as they were UidA-positive by PCR,
UidA-F, CCAAAAGCCAGACAGAGT, UidA-R
GCACAGCACATCAA AGAG (Moyo et al., 2007).
3.2. Serotyping
O antigen and H antigen typing using conventional
method revealed a variety of sertypes, 22 E. coli isolates
correlated with VTEC (O157:H7, O157:H11, O26:H11,
O121: HNM, O165: HUT), there was only one ETEC
isolate (O8:H12), three EAEC belonging to (OUT: H16,
OUT: H28, OUT: HNM), EPEC represented by only one
serotype (O115:HUT), three serotypes were aEPEC
(OUT:H21, OUT:HNM, O128:H2), 1 EIEC belonging to
serotype O74:H9. Fourteen serotypes were negative for
the tested virulence genes belonging to serotypes (O166:
HUT, O18: HUT, OUT: HUT, OUT: H27, O1: HUT,
O153: HUT, O8: HUT, OUT: H21, OUT: H4 (Table 2).
3.3. Virulence Genes Detection and
Categorization of DEC:
From the total of 45 isolates, 31 isolates were positive
for at least one of the targeted virulence genes. The
minimal criteria for the determination of DEC were as
follows: The presence of V1 and/or V2 for VTEC, the
presence of LT and /or ST for ETEC, the presence of
aggR and or astA for EAEC, the presence of bfpA and
eae for typical EPEC and the presence of eae only for
atypical EPEC, the presence of ipaH for EIEC. Based on
these criteria, the 31 isolates were classified as VTEC
(22 isolates) expressed either V1 or V2 or both, ETEC (1
isolate) from calf, two isolates were EAEC positive for
aggR and astA and one isolate EAEC (astA) was
heterogenous among the putative DEC, typical EPEC
represented by only one strain, 3 a EPEC isolates and 1
isolate EIEC, respectively (Table 2).
157Science Publications AJAVS
Table 1. PCR primers and conditions for amplification of virulence genes
Primer PCR PCR
Target gene designation Primer sequence(5'-3') conditions * product (bp) Reference
Vero toxin KS7 CCCGGATCCATGAAAAAAACATTATTAATAGC 95°C,30 s; 285 Zweifel et al.
1 (V1) KS8 CCCGAATTCAGCTATTCTGAGTCAACG 52°C,30 s; (2006)
72°C,30 s
Vero toxin VT2e AATACATTATGGGAAAGTAATA 95°C,30 s; 348 Zweifel et al.
2 (V2) VT2f TAAACTGCACTTCAGCAAAT 52°C,30 s; (2006)
72°C,30 s
eae (all intimin SK1 CCCGAATTCGGCACAAGCATAAGC 95°C,30 s; 863 Zweifel et al.
genes) SK2 CCCGGATCCGTCTCGCCAGTATTCG 61°C,30 s; (2006)
72°C,30 s
E-hly (EHEC- HlyA1 GGTGCAGCAGAAAAAGTTGTAG 95°C,30 s; 1550 Zweifel et al.
hemolysin) HlyA4 TCTCGCCTGATAGTGTTTGGTA 61°C,30 s; (2006)
72°C,30 s
(LT) Heat-labile elt1 ATTTACGGCGTTACTATCCTC 95°C,30 s; 281 Vu-Khac et al.
Enterotoxin elt2 TTTTGGTCTCGGTCAGATATG 58°C,30 (2007)
72°C,30 s
(St)Heat-stable St5 TTAATAGCACCCGGTACAAGCAGG 95°C,30 s; 147 Mendez-
Enterotoxin St3 CTTGACTCTTCAAAAGAGAAAATTAC 52°C,30 s; Arancibia et al.
72°C,30 s (2008)
(EAST1) Eagg AstA1-1 CCATCAACACAGTATATC 95°C,30 s; 111 Osek (2003)
EaggEC heat AstA1-2 GGTCGCGAGTGACGGCTTTGT 52°C,30 s;
-stable enterotoxin 72°C,30 s
(aggR) Fimbrial aggR1 CTAATTGTACAATCGATGTA 95°C,30 s; 457 Moyo et al.
antigen-specific aggR2 AGAGTCCATCTCTTTGATAAG 55°C,30 s (2007)
gene 72°C,30 s;
(bfp) bundle- EP1 AATGGTGCTTGCGCTTGCTGC 95°C,30 s; 326 Ochoa et al.
forming pilus EP2 GCCGCTTTATCCAACCTGGTA 52°C,30 s; (2008)
(ipaH)Invasion EI-1 GCTGGAAAAACTCAGTGCCT 95°C,30 s; 424 Shabana et al.
plasmid antigen EI-2 CCAGTCCGTAAATTCATTCT 52°C,30 s; (2013)
72°C,30 s
Dendrograms were created with a Molecular Analyst
(Bio-Rad) by using the Dice coefficient, Un weighted
Pair Group Method with Arithmetic means (UPGMA)
and a position tolerance of 1.3%.
3. RESULTS
3.1. Isolation and Confirmation of E. Coli
Isolates from Diarrheic Cases
A total of 45 presumptive E. coli isolates were
obtained from diarrheic human (n = 29), cow (n = 9) and
sheep (n = 7). The presumptive E. coli isolates were
confirmed as E. coli as they were UidA-positive by PCR,
UidA-F, CCAAAAGCCAGACAGAGT, UidA-R
GCACAGCACATCAA AGAG (Moyo et al., 2007).
3.2. Serotyping
O antigen and H antigen typing using conventional
method revealed a variety of sertypes, 22 E. coli isolates
correlated with VTEC (O157:H7, O157:H11, O26:H11,
O121: HNM, O165: HUT), there was only one ETEC
isolate (O8:H12), three EAEC belonging to (OUT: H16,
OUT: H28, OUT: HNM), EPEC represented by only one
serotype (O115:HUT), three serotypes were aEPEC
(OUT:H21, OUT:HNM, O128:H2), 1 EIEC belonging to
serotype O74:H9. Fourteen serotypes were negative for
the tested virulence genes belonging to serotypes (O166:
HUT, O18: HUT, OUT: HUT, OUT: H27, O1: HUT,
O153: HUT, O8: HUT, OUT: H21, OUT: H4 (Table 2).
3.3. Virulence Genes Detection and
Categorization of DEC:
From the total of 45 isolates, 31 isolates were positive
for at least one of the targeted virulence genes. The
minimal criteria for the determination of DEC were as
follows: The presence of V1 and/or V2 for VTEC, the
presence of LT and /or ST for ETEC, the presence of
aggR and or astA for EAEC, the presence of bfpA and
eae for typical EPEC and the presence of eae only for
atypical EPEC, the presence of ipaH for EIEC. Based on
these criteria, the 31 isolates were classified as VTEC
(22 isolates) expressed either V1 or V2 or both, ETEC (1
isolate) from calf, two isolates were EAEC positive for
aggR and astA and one isolate EAEC (astA) was
heterogenous among the putative DEC, typical EPEC
represented by only one strain, 3 a EPEC isolates and 1
isolate EIEC, respectively (Table 2).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Shabana, I.I. / American Journal of Animal and Veterinary Sciences 9 (3): 155-161, 2014
158Science Publications AJAVS
Table 2. Genotypic profile of diarrhegenic Escherichia coli
DEC Genotype Serogroup (no. of isolates) Host
VTEC VT1, eaeA O26:H11(5) Human
O157:H7(1) sheep
VT2, eaeA O157:H7(1), O121:HNM (1), O165:HUT (1) Human
O157:H7(1) sheep
VT1,VT2, Ehly, eaeA O157:H7(4) Human
O157:H7(2), O8:H4(2) sheep
O157:H11(2)), O157:H7( 2) cow
ETEC LT, ST, astA O8:H12(1) cow
EAEC aggR, astA OUT:H16(1) Human
astA OUT:H28(1), OUT:HNM(1) Human
EPEC eaeA, bfpA O115:HUT(1) Human
aEPEC eaeA OUT:H21(1), OUT:HNM(1), O128:H2(1) Human
EIEC ipaH, astA O74:H9(1) cow
Untypable O166:HUT(2), O18:HUT(3), OUT:HUT(2), Human
O8:HUT(1), OUT:H21(2) cow
OUT:H4(1) Sheep
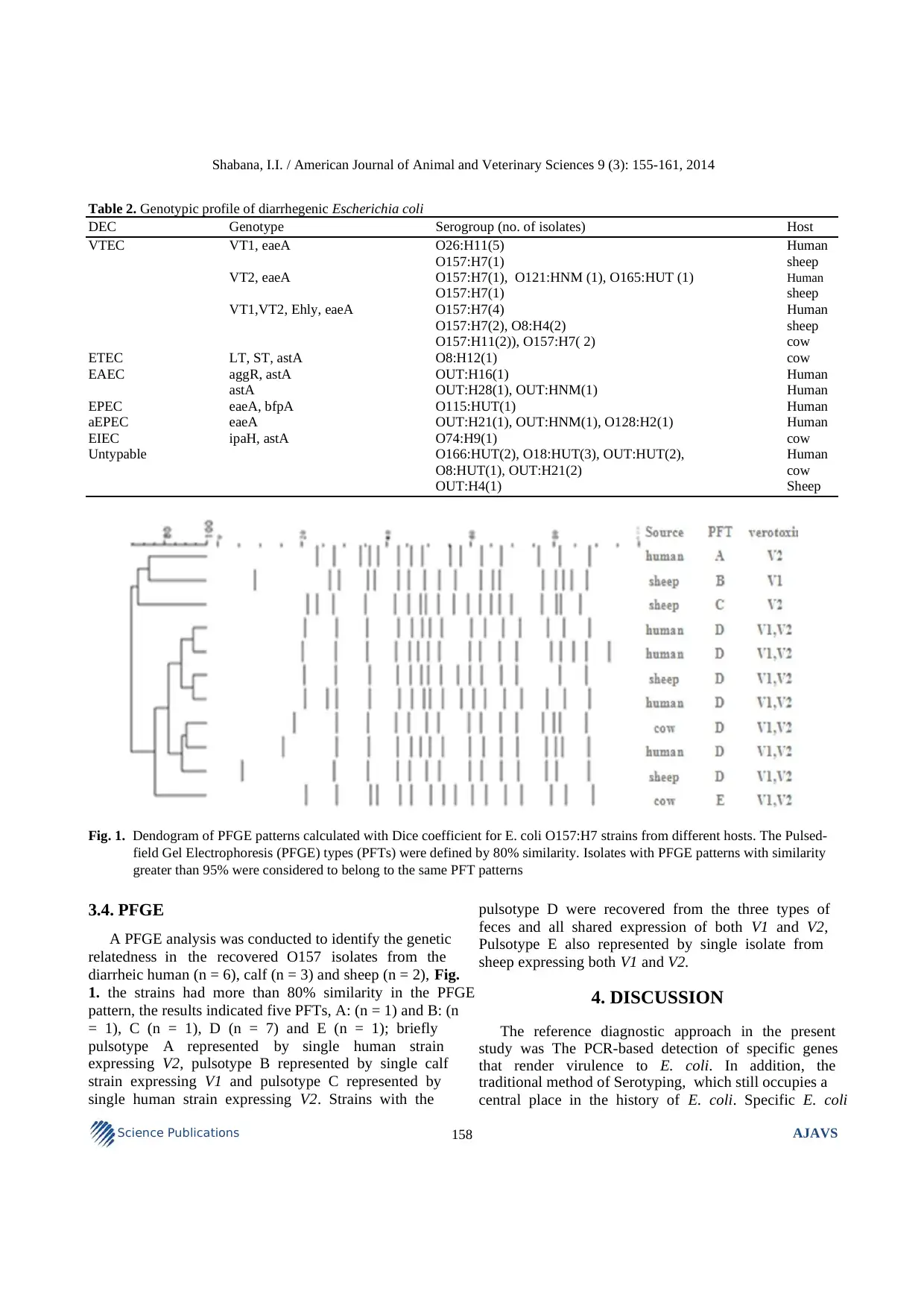
Fig. 1. Dendogram of PFGE patterns calculated with Dice coefficient for E. coli O157:H7 strains from different hosts. The Pulsed-
field Gel Electrophoresis (PFGE) types (PFTs) were defined by 80% similarity. Isolates with PFGE patterns with similarity
greater than 95% were considered to belong to the same PFT patterns
3.4. PFGE
A PFGE analysis was conducted to identify the genetic
relatedness in the recovered O157 isolates from the
diarrheic human (n = 6), calf (n = 3) and sheep (n = 2), Fig.
1. the strains had more than 80% similarity in the PFGE
pattern, the results indicated five PFTs, A: (n = 1) and B: (n
= 1), C (n = 1), D (n = 7) and E (n = 1); briefly
pulsotype A represented by single human strain
expressing V2, pulsotype B represented by single calf
strain expressing V1 and pulsotype C represented by
single human strain expressing V2. Strains with the
pulsotype D were recovered from the three types of
feces and all shared expression of both V1 and V2,
Pulsotype E also represented by single isolate from
sheep expressing both V1 and V2.
4. DISCUSSION
The reference diagnostic approach in the present
study was The PCR-based detection of specific genes
that render virulence to E. coli. In addition, the
traditional method of Serotyping, which still occupies a
central place in the history of E. coli. Specific E. coli
158Science Publications AJAVS
Table 2. Genotypic profile of diarrhegenic Escherichia coli
DEC Genotype Serogroup (no. of isolates) Host
VTEC VT1, eaeA O26:H11(5) Human
O157:H7(1) sheep
VT2, eaeA O157:H7(1), O121:HNM (1), O165:HUT (1) Human
O157:H7(1) sheep
VT1,VT2, Ehly, eaeA O157:H7(4) Human
O157:H7(2), O8:H4(2) sheep
O157:H11(2)), O157:H7( 2) cow
ETEC LT, ST, astA O8:H12(1) cow
EAEC aggR, astA OUT:H16(1) Human
astA OUT:H28(1), OUT:HNM(1) Human
EPEC eaeA, bfpA O115:HUT(1) Human
aEPEC eaeA OUT:H21(1), OUT:HNM(1), O128:H2(1) Human
EIEC ipaH, astA O74:H9(1) cow
Untypable O166:HUT(2), O18:HUT(3), OUT:HUT(2), Human
O8:HUT(1), OUT:H21(2) cow
OUT:H4(1) Sheep
Fig. 1. Dendogram of PFGE patterns calculated with Dice coefficient for E. coli O157:H7 strains from different hosts. The Pulsed-
field Gel Electrophoresis (PFGE) types (PFTs) were defined by 80% similarity. Isolates with PFGE patterns with similarity
greater than 95% were considered to belong to the same PFT patterns
3.4. PFGE
A PFGE analysis was conducted to identify the genetic
relatedness in the recovered O157 isolates from the
diarrheic human (n = 6), calf (n = 3) and sheep (n = 2), Fig.
1. the strains had more than 80% similarity in the PFGE
pattern, the results indicated five PFTs, A: (n = 1) and B: (n
= 1), C (n = 1), D (n = 7) and E (n = 1); briefly
pulsotype A represented by single human strain
expressing V2, pulsotype B represented by single calf
strain expressing V1 and pulsotype C represented by
single human strain expressing V2. Strains with the
pulsotype D were recovered from the three types of
feces and all shared expression of both V1 and V2,
Pulsotype E also represented by single isolate from
sheep expressing both V1 and V2.
4. DISCUSSION
The reference diagnostic approach in the present
study was The PCR-based detection of specific genes
that render virulence to E. coli. In addition, the
traditional method of Serotyping, which still occupies a
central place in the history of E. coli. Specific E. coli

Shabana, I.I. / American Journal of Animal and Veterinary Sciences 9 (3): 155-161, 2014
159Science Publications AJAVS
Serogroup can be associated reproducibly with certain
clinical syndromes, the serotypes and Serogroup serve as
readily identifiable chromosomal markers that correlate
with specific virulent clones (Whittam et al., 1993).
Almost half of the isolates carried virulence genes
common to Verotoxin-producing Escherichia Coli
(VTEC), which appeared to be the most prevalent DEC
category in this study (48.9%), VTEC distributed among
human, calf and sheep isolates and assigned to serotypes
O157:H7, O26:H11, O121: HNM, O165: HUT and
O8:H4 harbored either V1 or V2 or both in addition to
Ehly and eaeA. This is in concordance with other
report highlighting the involvement of VTEC in
human, calf and sheep illness (Blanco et al., 2003).
EPEC represented by four strains restricted to human
isolates; three were atypical EPEC possessed only the
virulence marker eae, indicative for the presence of the
Locus of Enterocyte Effacement (LEE) pathogenicity
island and single typical EPEC strain possessed an
inducible bundle-forming pilus (bfpA) associated with
the presence of the EPEC Adherence Factor (EAF)
plasmid and Localized Adherence (LA) on HEp-2 cells
in addition to LEE-encoded virulence factors. These
findings agreed with previous report about the prevalence
of EPEC and how atypical EPEC had more implication
than typical EPEC (Afset et al., 2003). The majority of
EPEC isolates belong to classic serotypes derived from 12
classical O serogroups (O26, O55, O86, O111, O114,
O119, O125, O126, O127, O128, O142 andO158)
(Scaletsky et al., 2010). This study reported O115: HUT
as EPEC which agreed with (Saito et al., 2005).
ETEC was detected in only one strain from cow
assigned for serotype O8:H12, the classical serotype
of ETEC (Huasai et al., 2012). It possessed heat-
labile, heat-stable enterotoxins and plasmid-borne
astA gene encoding the EAST1. Although the lack of
references, which monitors the presence of
Enteroinvasive Escherichia coli (EIEC) in cow, the
current study recorded one strain assigned for O74:H9 and
possessed Invasion plasmid antigens (Ipa) proteins
which encoded in the ipa operon.
Theoretical digestion of sequenced genomes of E.
coli O157 strains should generate 41 fragments with
XbaI. Some smaller bands run off the gel, while others
are too similar in size and co migrate. As a
consequence only half to three quarters of these bands
can usually be distinguished by PFGE analysis.
(Kudva et al., 2002) demonstrated that PFGE diversity
in E. coli O157 is primarily attributed to insertions and
deletions, not to point mutations. PFGE was used to
genetically discriminate VTEC O157:H7 isolates from
different hosts, similar patterns were found among
calf, sheep and human strains in Lineage D, which
accounted for 63.3% of the tested strains. The strains
had expressed the same virulence genes, both V1 and
V2, in addition to Ehly and eaeA, while other lineages
were distinctly different due to the variation in
expression of virulence genes.
Enterohaemorrhagic Escherichia Coli (EHEC)
O157:H7 is a zoonotic enteric pathogen of worldwide
importance; ruminants cited as its primary reservoirs.
Cattle considered to be the sole source of E. coli
O157:H7 outbreaks in humans; the pathobiology of E.
coli O157:H7 in small domestic ruminants does
appear to differ significantly from that described in
cattle and with cattle considered to be the primary
reservoir (La Ragione et al., 2009).
Transmission of E. coli O157:H7 to humans is
principally via contamination of food by animal feces
(Jaros et al., 2013). Food products of animal origin
have been confirmed as vehicles of disease
transmission in case-control studies of VTEC
outbreaks and sporadic VTEC infections; these
included raw milk (Guh et al., 2010) and undercooked
meat products (Chapman et al., 2001).
World Health Organization (WHO) listed
Escherichia coli spp. as one of the most important
Pathogenic bacteria which transmitted by food especially
Enterotoxigenic E. coli (ETEC), enteropathogenic E.
Coli (EPEC), Enterohaemorrhagic E. Coli (EHEC),
enteroinvasive E. coli (EIEC), however the restricted
number of the strains in the present study; EHEC, ETEC
and EIEC strains were reported in cattle,. The findings
refer to the importance of cattle and sheep as sources for
zoonotic Escherichia coli strains.
5. CONCLUSION
High prevalence of VTEC among diarrheic human,
sheep and cattle and highly similar genotypes of VTEC
O157:H7 are found in both cattle and sheep and cause
human illness suggest that pathogenic strains can circulate
freely between them. This information will be of
importance for future efforts to trace sources of infection
and reduce the burden of disease caused by O157:H7.
6. ACKNOWLEDGEMENT
I’d like to thank Dr. Hiroshi Sato, Niigata Prefectural
Institute of Public Health and Environmental Sciences,
Department Of Bacteriology, Niigata, Japan; for his
valuable help and contributions.
159Science Publications AJAVS
Serogroup can be associated reproducibly with certain
clinical syndromes, the serotypes and Serogroup serve as
readily identifiable chromosomal markers that correlate
with specific virulent clones (Whittam et al., 1993).
Almost half of the isolates carried virulence genes
common to Verotoxin-producing Escherichia Coli
(VTEC), which appeared to be the most prevalent DEC
category in this study (48.9%), VTEC distributed among
human, calf and sheep isolates and assigned to serotypes
O157:H7, O26:H11, O121: HNM, O165: HUT and
O8:H4 harbored either V1 or V2 or both in addition to
Ehly and eaeA. This is in concordance with other
report highlighting the involvement of VTEC in
human, calf and sheep illness (Blanco et al., 2003).
EPEC represented by four strains restricted to human
isolates; three were atypical EPEC possessed only the
virulence marker eae, indicative for the presence of the
Locus of Enterocyte Effacement (LEE) pathogenicity
island and single typical EPEC strain possessed an
inducible bundle-forming pilus (bfpA) associated with
the presence of the EPEC Adherence Factor (EAF)
plasmid and Localized Adherence (LA) on HEp-2 cells
in addition to LEE-encoded virulence factors. These
findings agreed with previous report about the prevalence
of EPEC and how atypical EPEC had more implication
than typical EPEC (Afset et al., 2003). The majority of
EPEC isolates belong to classic serotypes derived from 12
classical O serogroups (O26, O55, O86, O111, O114,
O119, O125, O126, O127, O128, O142 andO158)
(Scaletsky et al., 2010). This study reported O115: HUT
as EPEC which agreed with (Saito et al., 2005).
ETEC was detected in only one strain from cow
assigned for serotype O8:H12, the classical serotype
of ETEC (Huasai et al., 2012). It possessed heat-
labile, heat-stable enterotoxins and plasmid-borne
astA gene encoding the EAST1. Although the lack of
references, which monitors the presence of
Enteroinvasive Escherichia coli (EIEC) in cow, the
current study recorded one strain assigned for O74:H9 and
possessed Invasion plasmid antigens (Ipa) proteins
which encoded in the ipa operon.
Theoretical digestion of sequenced genomes of E.
coli O157 strains should generate 41 fragments with
XbaI. Some smaller bands run off the gel, while others
are too similar in size and co migrate. As a
consequence only half to three quarters of these bands
can usually be distinguished by PFGE analysis.
(Kudva et al., 2002) demonstrated that PFGE diversity
in E. coli O157 is primarily attributed to insertions and
deletions, not to point mutations. PFGE was used to
genetically discriminate VTEC O157:H7 isolates from
different hosts, similar patterns were found among
calf, sheep and human strains in Lineage D, which
accounted for 63.3% of the tested strains. The strains
had expressed the same virulence genes, both V1 and
V2, in addition to Ehly and eaeA, while other lineages
were distinctly different due to the variation in
expression of virulence genes.
Enterohaemorrhagic Escherichia Coli (EHEC)
O157:H7 is a zoonotic enteric pathogen of worldwide
importance; ruminants cited as its primary reservoirs.
Cattle considered to be the sole source of E. coli
O157:H7 outbreaks in humans; the pathobiology of E.
coli O157:H7 in small domestic ruminants does
appear to differ significantly from that described in
cattle and with cattle considered to be the primary
reservoir (La Ragione et al., 2009).
Transmission of E. coli O157:H7 to humans is
principally via contamination of food by animal feces
(Jaros et al., 2013). Food products of animal origin
have been confirmed as vehicles of disease
transmission in case-control studies of VTEC
outbreaks and sporadic VTEC infections; these
included raw milk (Guh et al., 2010) and undercooked
meat products (Chapman et al., 2001).
World Health Organization (WHO) listed
Escherichia coli spp. as one of the most important
Pathogenic bacteria which transmitted by food especially
Enterotoxigenic E. coli (ETEC), enteropathogenic E.
Coli (EPEC), Enterohaemorrhagic E. Coli (EHEC),
enteroinvasive E. coli (EIEC), however the restricted
number of the strains in the present study; EHEC, ETEC
and EIEC strains were reported in cattle,. The findings
refer to the importance of cattle and sheep as sources for
zoonotic Escherichia coli strains.
5. CONCLUSION
High prevalence of VTEC among diarrheic human,
sheep and cattle and highly similar genotypes of VTEC
O157:H7 are found in both cattle and sheep and cause
human illness suggest that pathogenic strains can circulate
freely between them. This information will be of
importance for future efforts to trace sources of infection
and reduce the burden of disease caused by O157:H7.
6. ACKNOWLEDGEMENT
I’d like to thank Dr. Hiroshi Sato, Niigata Prefectural
Institute of Public Health and Environmental Sciences,
Department Of Bacteriology, Niigata, Japan; for his
valuable help and contributions.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Shabana, I.I. / American Journal of Animal and Veterinary Sciences 9 (3): 155-161, 2014
160Science Publications AJAVS
7. REFERENCE
Afset, J.E., K. Bergh and L. Bevanger, 2003. High
prevalence of atypical Enteropathogenic Escherichia
Coli (EPEC) in Norwegian children with diarrhoea.
J. Med. Microbiol. 52: 1015-1019. PMID: 14532347
Blanco, J., M. Blanco, J.E. Blanco, A. Mora and E.A.
González et al., 2003. Verotoxin-producing
Escherichia coli in Spain: Prevalence, serotypes and
virulence genes of O157:H7 and non-O157 VTEC in
ruminants, raw beef products and humans. Exp.
Biol. Med., 228: 345-51. PMID: 12671177
CDCP, 1998. Standardized molecular subtyping of food
borne bacterial pathogens by pulsed field gel
electrophoresis. In: CDC Training Manual, Food
borne and Diarrheal Diseases Branch, Centers for
Disease Control and Prevention, Atlanta, GA.
Chapman, P.A., A.T. Cerdan Malo, M. Ellin and R.A.
Harkin, 2001. Escherichia coli O157 in cattle and
sheep at slaughter, on beef and lamb carcasses and
in raw beef and lamb products in South Yorkshire,
UK. Int. J. Food Microbiol., 64: 139-150. DOI:
10.1016/S0168-1605(00)00453-0
Davies, R.H. and C. Wray, 1997. Immunomagnetic
separation for enhanced flagellar antigen phase
inversion in salmonella. Lett. Appl. Microbiol., 24:
217-220. PMID: 9080704
Djordjevic, S.P., M.A. Hornitzky, G. Bailey, P. Gill and
B. Vanselow, 2001. Virulence properties and
serotypes of Shiga toxin-producing Escherichia coli
from healthy Australian slaughter-age sheep. J. Clin.
Microbiol., 39: 2017-2021. DOI:
10.1128/JCM.39.5.2017-2021.2001
Ferens, W.A. and C.J. Hovde, 2011. Escherichia coli
O157:H7: Animal reservoir and sources of human
infection. Foodborne Pathog. Dis., 8: 465-48. DOI:
10.1089/fpd.2010.0673
Gillespie, I.A., S.J. O’Brien, G.K. Adak, T. Cheasty and
G. Willshaw, 2005. Foodborne general outbreaks of
Shiga toxin-producing Escherichia coli O157 in
England and Wales 1992-2002: where are the risks?
Epidemiol Infect. 133: 803-808. DOI:
10.1017/S0950268805004486
Guh, A., Q. Phan, R. Nelson, K. Purviance and E. Milardo
et al., 2010. Outbreak of Escherichia coli O157
associated with raw milk, Connecticut. Clin. Infect.
Dis., 51, 411-1417.8. PMID: 21058911
Huasai, S., A. Chen, C.J. Wang, Y. Li and B.
Tongrige, 2012. Occurrence and characteristics of
virulence genes of Escherichia coli strains
isolated from healthy dairy cows in Inner
Mongolia, China. Braz. J. Microbiol., 43: 528-34.
DOI: 10.1590/S1517-83822012000200013
Jaros, P., A.L. Cookson, D.M. Campbell, T.E. Besser and S.
Shringi et al., 2013. A prospective case-control and
molecular epidemiological study of human cases of
Shiga toxin-producing Escherichia coli in New
Zealand. BMC Infect. Dis., 30: 13-450. DOI:
10.1186/1471-2334-13-450
Kudva, I.T., P.S. Evans, N.T. Perna, T.J. Barrett and
F.M. Ausubel et al., 2002. Strains of Escherichia
coli O157:H7 differ primarily by insertions or
deletions, not single-nucleotide polymorphisms. J.
Bacteriol., 184: 1873-9. DOI:
10.1128/JB.184.7.1873-1879.2002
La Ragione, R.M., A. Best, M.J. Woodward and A.D.
Wales, 2009. Escherichia coli O157:H7 colonization
in small domestic ruminants. FEMS Microbial.
Rev., 33: 394-410. DOI: 10.1111/j.1574-
6976.2008.00138.x
Lynn, R.M., S.J. O’B0rien, C.M. Taylor, G.K. Adak and
H. Chart et al., 2005. Childhood hemolytic uremic
syndrome, United Kingdom and Ireland. Emerg.
Infect. Dis., 11: 590-6. DOI:
10.3201/eid1104.040833
Mendez-Arancibia, E., M. Vargas, S. Soto, J. Ruiz and
E. Kahigwa et al., 2008. Prevalence of different
virulence factors and biofilm production in
enteroaggregative Escherichia coli isolates causing
diarrhea in children in Ifakara (Tanzania). Am. J.
Trop. Med. Hyg., 78: 985-9. PMID: 18541781
Moyo, S.J., S.Y. Maselle, M.I. Matee, N. Langeland and
H. Mylvaganam, 2007. Identification of diarrheagenic
Escherichia coli isolated from infants and children in
Dar es Salaam, Tanzania, Tanzania. BMC Infect. Dis.
9: 7-92. DOI: 10.1186/1471-2334-7-92
Nguyen, T.V., P. Le Van, C. Le Huy, K.N. Gia and A.
Weintraub, 2005. Detection and characterization of
diarrheagenic Escherichia coli from young children
in Hanoi, Vietnam. J. Clin. Microbiol., 43: 755-760.
DOI: 10.1128/JCM.43.2.755-760.2005
Ochoa, T.J., F. Barletta, C. Contreras and E. Mercado,
2008. New insights into the epidemiology of
enteropathogenic Escherichia coli infection. Trans.
R. Soc. Trop. Med. Hyg., 102: 852-856. DOI:
10.1016/j.trstmh.2008.03.017
Osek, J., 2003. Detection of the enteroaggregative
Escherichia coli heat-Stable enterotoxin 1 (EAST1)
gene and its relationship with fimbrial and
enterotoxin markers in E. coli isolates from pigs
with diarrhea.Vet. Microbiol., 91: 65-72. DOI:
10.1016/S0378-1135(02)00262-6
160Science Publications AJAVS
7. REFERENCE
Afset, J.E., K. Bergh and L. Bevanger, 2003. High
prevalence of atypical Enteropathogenic Escherichia
Coli (EPEC) in Norwegian children with diarrhoea.
J. Med. Microbiol. 52: 1015-1019. PMID: 14532347
Blanco, J., M. Blanco, J.E. Blanco, A. Mora and E.A.
González et al., 2003. Verotoxin-producing
Escherichia coli in Spain: Prevalence, serotypes and
virulence genes of O157:H7 and non-O157 VTEC in
ruminants, raw beef products and humans. Exp.
Biol. Med., 228: 345-51. PMID: 12671177
CDCP, 1998. Standardized molecular subtyping of food
borne bacterial pathogens by pulsed field gel
electrophoresis. In: CDC Training Manual, Food
borne and Diarrheal Diseases Branch, Centers for
Disease Control and Prevention, Atlanta, GA.
Chapman, P.A., A.T. Cerdan Malo, M. Ellin and R.A.
Harkin, 2001. Escherichia coli O157 in cattle and
sheep at slaughter, on beef and lamb carcasses and
in raw beef and lamb products in South Yorkshire,
UK. Int. J. Food Microbiol., 64: 139-150. DOI:
10.1016/S0168-1605(00)00453-0
Davies, R.H. and C. Wray, 1997. Immunomagnetic
separation for enhanced flagellar antigen phase
inversion in salmonella. Lett. Appl. Microbiol., 24:
217-220. PMID: 9080704
Djordjevic, S.P., M.A. Hornitzky, G. Bailey, P. Gill and
B. Vanselow, 2001. Virulence properties and
serotypes of Shiga toxin-producing Escherichia coli
from healthy Australian slaughter-age sheep. J. Clin.
Microbiol., 39: 2017-2021. DOI:
10.1128/JCM.39.5.2017-2021.2001
Ferens, W.A. and C.J. Hovde, 2011. Escherichia coli
O157:H7: Animal reservoir and sources of human
infection. Foodborne Pathog. Dis., 8: 465-48. DOI:
10.1089/fpd.2010.0673
Gillespie, I.A., S.J. O’Brien, G.K. Adak, T. Cheasty and
G. Willshaw, 2005. Foodborne general outbreaks of
Shiga toxin-producing Escherichia coli O157 in
England and Wales 1992-2002: where are the risks?
Epidemiol Infect. 133: 803-808. DOI:
10.1017/S0950268805004486
Guh, A., Q. Phan, R. Nelson, K. Purviance and E. Milardo
et al., 2010. Outbreak of Escherichia coli O157
associated with raw milk, Connecticut. Clin. Infect.
Dis., 51, 411-1417.8. PMID: 21058911
Huasai, S., A. Chen, C.J. Wang, Y. Li and B.
Tongrige, 2012. Occurrence and characteristics of
virulence genes of Escherichia coli strains
isolated from healthy dairy cows in Inner
Mongolia, China. Braz. J. Microbiol., 43: 528-34.
DOI: 10.1590/S1517-83822012000200013
Jaros, P., A.L. Cookson, D.M. Campbell, T.E. Besser and S.
Shringi et al., 2013. A prospective case-control and
molecular epidemiological study of human cases of
Shiga toxin-producing Escherichia coli in New
Zealand. BMC Infect. Dis., 30: 13-450. DOI:
10.1186/1471-2334-13-450
Kudva, I.T., P.S. Evans, N.T. Perna, T.J. Barrett and
F.M. Ausubel et al., 2002. Strains of Escherichia
coli O157:H7 differ primarily by insertions or
deletions, not single-nucleotide polymorphisms. J.
Bacteriol., 184: 1873-9. DOI:
10.1128/JB.184.7.1873-1879.2002
La Ragione, R.M., A. Best, M.J. Woodward and A.D.
Wales, 2009. Escherichia coli O157:H7 colonization
in small domestic ruminants. FEMS Microbial.
Rev., 33: 394-410. DOI: 10.1111/j.1574-
6976.2008.00138.x
Lynn, R.M., S.J. O’B0rien, C.M. Taylor, G.K. Adak and
H. Chart et al., 2005. Childhood hemolytic uremic
syndrome, United Kingdom and Ireland. Emerg.
Infect. Dis., 11: 590-6. DOI:
10.3201/eid1104.040833
Mendez-Arancibia, E., M. Vargas, S. Soto, J. Ruiz and
E. Kahigwa et al., 2008. Prevalence of different
virulence factors and biofilm production in
enteroaggregative Escherichia coli isolates causing
diarrhea in children in Ifakara (Tanzania). Am. J.
Trop. Med. Hyg., 78: 985-9. PMID: 18541781
Moyo, S.J., S.Y. Maselle, M.I. Matee, N. Langeland and
H. Mylvaganam, 2007. Identification of diarrheagenic
Escherichia coli isolated from infants and children in
Dar es Salaam, Tanzania, Tanzania. BMC Infect. Dis.
9: 7-92. DOI: 10.1186/1471-2334-7-92
Nguyen, T.V., P. Le Van, C. Le Huy, K.N. Gia and A.
Weintraub, 2005. Detection and characterization of
diarrheagenic Escherichia coli from young children
in Hanoi, Vietnam. J. Clin. Microbiol., 43: 755-760.
DOI: 10.1128/JCM.43.2.755-760.2005
Ochoa, T.J., F. Barletta, C. Contreras and E. Mercado,
2008. New insights into the epidemiology of
enteropathogenic Escherichia coli infection. Trans.
R. Soc. Trop. Med. Hyg., 102: 852-856. DOI:
10.1016/j.trstmh.2008.03.017
Osek, J., 2003. Detection of the enteroaggregative
Escherichia coli heat-Stable enterotoxin 1 (EAST1)
gene and its relationship with fimbrial and
enterotoxin markers in E. coli isolates from pigs
with diarrhea.Vet. Microbiol., 91: 65-72. DOI:
10.1016/S0378-1135(02)00262-6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Shabana, I.I. / American Journal of Animal and Veterinary Sciences 9 (3): 155-161, 2014
161Science Publications AJAVS
Saito, N., M. Kawano, T. Kobayashi, S. Watanabe and
W. Yamada et al., 2005. An outbreak of food
poisoning caused by an Enteropathogenic
Escherichia coli O115:H19 in Miyagi Prefecture.
Jpn. J. Infect. Dis., 58, 189-90.
Scaletsky, I.C., T.B. Souza, K.R. Aranda and I.N. Okeke,
2010. Genetic elements associated with antimicrobial
resistance in Enteropathogenic Escherichia Coli
(EPEC) from Brazil. BMC Microbiol., 27: 10-25.
DOI: 10.1186/1471-2180-10-25
Shabana, I.I., H. Zaraket and H. Suzuki, 2013. Molecular
studies on diarrhea-associated Escherichia coli
isolated from humans and animals in Egypt. Vet.
Microbiol., 167: 532-9. DOI:
10.1016/j.vetmic.2013.08.014
Takahashi, E., Z. Sultan, S. Shimada, W.W. Aung and
M.M. Nyein et al., 2008. Escherichia coli isolated
from children with diarrhea in Myanmar. Microbiol.
Immunol., 52: 2-8. DOI: 10.1111/j.1348-
0421.2008.00001.x
Tamaki, Y., H. Narimatsu, T. Miyazato, N. Nakasone and
N. Higa et al., 2005. The relationship between O-
antigens and pathogenic genes of diarrhea-
associated Escherichia coli. Jpn. J. Infect. Dis., 58:
65-9. PMID: 15858281
Teng, L.J., P.R. Hsueh, S.J. Liaw, S.W. Ho and J.C.
Tsai, 2004. Genetic detection of diarrheagenic
Escherichia coli isolated from children with
sporadic diarrhea. J. Microbiol. Immunol. Infect.,
37: 327-34. PMID: 15599464
Usein, C.R., D. Tatu-Chitoiu, S. Ciontea, M. Condei
and M. Damian, 2009. Escherichia coli
pathotypes associated with diarrhea in Romanian
children younger than 5 years of age. Jpn. J.
Infect. Dis., 62: 289- 93. PMID: 19628907
Vu-Khac, H., E. Holoda, E. Pilipcinec, M. Blanco and
J.E. Blanco et al., 2007. Serotypes, virulence
genes, intimin types and PFGE profiles of
Escherichia coli isolated from piglets with
diarrhea in Slovakia. Vet. J., 174: 176-87. DOI:
10.1016/j.tvjl.2006.05.019
Whittam, T.S., M.L. Wolfe, I.K. Wachsmuth, F.
Orskov and I. Orskov et al., 1993. Clonal
relationships among Escherichia coli strains that
cause hemorrhagic colitis and infantile diarrhea.
Infect. Immun., 61: 1619-29. PMID: 7682992
Yang J.H., S.T. Lee, J.A. Kim, S.H. Kim and S.Y.
Jang et al., 2007. Genetic analysis of three
Korean patients with clinical features of Ehlers-
Danlos syndrome type IV. J. Korean Med. Sci.,
PMID: 17728513
Zweifel, C., S. Schumacher, L. Beutin, J. Blanco and
R. Stephan, 2006. Virulence profiles of shiga
toxin 2e-producing Escherichia coli isolated from
healthy pig at slaughter. Vet. Microbiol., 117: 328-
32. DOI: 10.1016/j.vetmic.2006.06.017
View publication statsView publication stats
161Science Publications AJAVS
Saito, N., M. Kawano, T. Kobayashi, S. Watanabe and
W. Yamada et al., 2005. An outbreak of food
poisoning caused by an Enteropathogenic
Escherichia coli O115:H19 in Miyagi Prefecture.
Jpn. J. Infect. Dis., 58, 189-90.
Scaletsky, I.C., T.B. Souza, K.R. Aranda and I.N. Okeke,
2010. Genetic elements associated with antimicrobial
resistance in Enteropathogenic Escherichia Coli
(EPEC) from Brazil. BMC Microbiol., 27: 10-25.
DOI: 10.1186/1471-2180-10-25
Shabana, I.I., H. Zaraket and H. Suzuki, 2013. Molecular
studies on diarrhea-associated Escherichia coli
isolated from humans and animals in Egypt. Vet.
Microbiol., 167: 532-9. DOI:
10.1016/j.vetmic.2013.08.014
Takahashi, E., Z. Sultan, S. Shimada, W.W. Aung and
M.M. Nyein et al., 2008. Escherichia coli isolated
from children with diarrhea in Myanmar. Microbiol.
Immunol., 52: 2-8. DOI: 10.1111/j.1348-
0421.2008.00001.x
Tamaki, Y., H. Narimatsu, T. Miyazato, N. Nakasone and
N. Higa et al., 2005. The relationship between O-
antigens and pathogenic genes of diarrhea-
associated Escherichia coli. Jpn. J. Infect. Dis., 58:
65-9. PMID: 15858281
Teng, L.J., P.R. Hsueh, S.J. Liaw, S.W. Ho and J.C.
Tsai, 2004. Genetic detection of diarrheagenic
Escherichia coli isolated from children with
sporadic diarrhea. J. Microbiol. Immunol. Infect.,
37: 327-34. PMID: 15599464
Usein, C.R., D. Tatu-Chitoiu, S. Ciontea, M. Condei
and M. Damian, 2009. Escherichia coli
pathotypes associated with diarrhea in Romanian
children younger than 5 years of age. Jpn. J.
Infect. Dis., 62: 289- 93. PMID: 19628907
Vu-Khac, H., E. Holoda, E. Pilipcinec, M. Blanco and
J.E. Blanco et al., 2007. Serotypes, virulence
genes, intimin types and PFGE profiles of
Escherichia coli isolated from piglets with
diarrhea in Slovakia. Vet. J., 174: 176-87. DOI:
10.1016/j.tvjl.2006.05.019
Whittam, T.S., M.L. Wolfe, I.K. Wachsmuth, F.
Orskov and I. Orskov et al., 1993. Clonal
relationships among Escherichia coli strains that
cause hemorrhagic colitis and infantile diarrhea.
Infect. Immun., 61: 1619-29. PMID: 7682992
Yang J.H., S.T. Lee, J.A. Kim, S.H. Kim and S.Y.
Jang et al., 2007. Genetic analysis of three
Korean patients with clinical features of Ehlers-
Danlos syndrome type IV. J. Korean Med. Sci.,
PMID: 17728513
Zweifel, C., S. Schumacher, L. Beutin, J. Blanco and
R. Stephan, 2006. Virulence profiles of shiga
toxin 2e-producing Escherichia coli isolated from
healthy pig at slaughter. Vet. Microbiol., 117: 328-
32. DOI: 10.1016/j.vetmic.2006.06.017
View publication statsView publication stats
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
