FZ2026: Organic and Inorganic Chemistry Assignment - Semester 1, UCLAN
VerifiedAdded on 2020/04/21
|17
|2405
|488
Homework Assignment
AI Summary
This document is a comprehensive solution to a chemistry assignment, covering various aspects of organic and inorganic chemistry. It begins with an analysis of the Jahn-Teller effect in coordination complexes, including the distortion of octahedral symmetry and the impact on bond lengths and crystal field stabilization energy (CFSE). The assignment then explores the structures and CFSE of spinel compounds like FeCr2O4 and Fe3O4, determining whether they exhibit normal or inverse spinel structures. Further, it delves into electrophilic aromatic substitution, predicting the regioselectivity of bromination reactions on substituted benzene rings, considering the effects of activating and deactivating groups and steric hindrance. The document also examines reaction mechanisms, including the generation of electrophiles and Friedel-Crafts alkylation, and outlines synthetic routes for the preparation of specific substituted benzene derivatives, employing strategies like protecting groups and the use of directing effects. Finally, the assignment analyzes the reduction of various functional groups (esters, amides, carboxylic acids, and ketones) by different reducing agents like NaBH4 and LiAlH4, predicting the products of these reactions.

FZ2026: ELEMENTS OF ORGANIC AND INORGANIC CHEMISTRY
Semester 1: Assignment
Shakir Alam Uddin
Suddin4@uclan.ac.uk
Semester 1: Assignment
Shakir Alam Uddin
Suddin4@uclan.ac.uk
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
1)
a)
The distortion in given chemical formula is due to Jahn-Teller effect. The Jahn-Teller
effect describes that if the ground electronic configuration of a nonlinear complex is
orbitally degenerate, and asymmetrically filled, then the complex distorts to remove
the degeneracy and achieve a lower energy11.
The oxidation state of Cr in Na4[(Cr(CN)6].10H2O is +2 i.e. Cr+2 in a perfectly
octahedral N- ligand environment has the ground state electronic configuration
= t2 g
4
Which represents the electronic arrangements as follows d xy
2 , d xz
' , diyz
❑ , dixy
' , d xz
2 , d xz
' and
d xy
' , d xz
' , d xz
2 of equal energy, because of the Jahn-Teller effect, the octahedral
symmetry of the complex will be automatically lowered to tetragonal symmetry to
stabilize the complex8,9. This explains why in Na4[Cr(CN)6]·10H2O it was found that
there were four Cr-C bonds at 2.066 Å and two Cr-C bonds at 1.936 Å. This is an
example of axial compressions distortion. There is a strong field due to CN- ligands
To summarise, due to the two Cr-C bonds, which are axial, being shorter in length,
the type of distortion is tetragonal compression9.
1
1)
a)
The distortion in given chemical formula is due to Jahn-Teller effect. The Jahn-Teller
effect describes that if the ground electronic configuration of a nonlinear complex is
orbitally degenerate, and asymmetrically filled, then the complex distorts to remove
the degeneracy and achieve a lower energy11.
The oxidation state of Cr in Na4[(Cr(CN)6].10H2O is +2 i.e. Cr+2 in a perfectly
octahedral N- ligand environment has the ground state electronic configuration
= t2 g
4
Which represents the electronic arrangements as follows d xy
2 , d xz
' , diyz
❑ , dixy
' , d xz
2 , d xz
' and
d xy
' , d xz
' , d xz
2 of equal energy, because of the Jahn-Teller effect, the octahedral
symmetry of the complex will be automatically lowered to tetragonal symmetry to
stabilize the complex8,9. This explains why in Na4[Cr(CN)6]·10H2O it was found that
there were four Cr-C bonds at 2.066 Å and two Cr-C bonds at 1.936 Å. This is an
example of axial compressions distortion. There is a strong field due to CN- ligands
To summarise, due to the two Cr-C bonds, which are axial, being shorter in length,
the type of distortion is tetragonal compression9.
1
Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
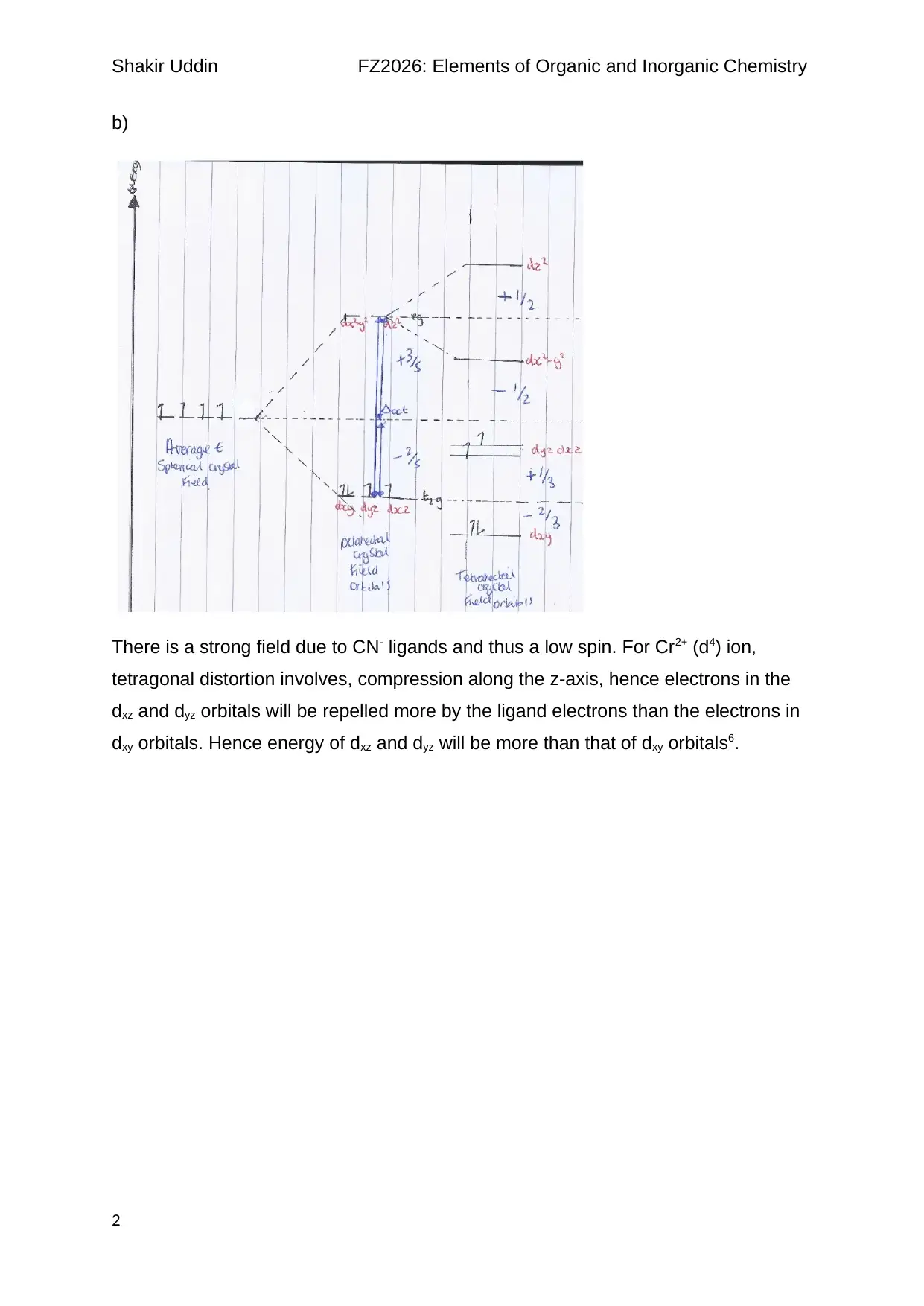
b)
There is a strong field due to CN- ligands and thus a low spin. For Cr2+ (d4) ion,
tetragonal distortion involves, compression along the z-axis, hence electrons in the
dxz and dyz orbitals will be repelled more by the ligand electrons than the electrons in
dxy orbitals. Hence energy of dxz and dyz will be more than that of dxy orbitals6.
2
b)
There is a strong field due to CN- ligands and thus a low spin. For Cr2+ (d4) ion,
tetragonal distortion involves, compression along the z-axis, hence electrons in the
dxz and dyz orbitals will be repelled more by the ligand electrons than the electrons in
dxy orbitals. Hence energy of dxz and dyz will be more than that of dxy orbitals6.
2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
c)
Normal Crystal field stabilisation energy, CFSE = 4(-2/5) = -8/5 + PE
Therefore, under the condition that this is distorted:
Distorted Crystal Field Stabilisation Energy, CFSE
= 2(-2/3) = -4/3
And
= 2(1/3) = 2/3
So, the distorted CFCE = -4/3 + 2/3 = -2/3
3
c)
Normal Crystal field stabilisation energy, CFSE = 4(-2/5) = -8/5 + PE
Therefore, under the condition that this is distorted:
Distorted Crystal Field Stabilisation Energy, CFSE
= 2(-2/3) = -4/3
And
= 2(1/3) = 2/3
So, the distorted CFCE = -4/3 + 2/3 = -2/3
3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
2)
a)
Looking at the molecule of Fe3O4, I can deduce that the oxygen account for -8 for the
total oxidation state within the compound. Since Iron mainly exists in the form of +2
and +3, to make the integer of +8 there would need to be two sets of Fe3+ and one
set of Fe2+ within the complex9.
Structures of the ions:
26F e ( II ) : [ Ar ] 3 d6 : F e ( III ) : [ Ar ] 3 d5 :
24Cr ( III ) : [ Ar ] 3 d3
To summarise:
FeCr2O4:
Fe2+ = d6 and Cr3+ = d3
Fe3O4:
Fe2+ = d6 and Fe3+ = d5
4
2)
a)
Looking at the molecule of Fe3O4, I can deduce that the oxygen account for -8 for the
total oxidation state within the compound. Since Iron mainly exists in the form of +2
and +3, to make the integer of +8 there would need to be two sets of Fe3+ and one
set of Fe2+ within the complex9.
Structures of the ions:
26F e ( II ) : [ Ar ] 3 d6 : F e ( III ) : [ Ar ] 3 d5 :
24Cr ( III ) : [ Ar ] 3 d3
To summarise:
FeCr2O4:
Fe2+ = d6 and Cr3+ = d3
Fe3O4:
Fe2+ = d6 and Fe3+ = d5
4

Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
b)
FeCr2O4:
Fe(II): [Ar]3d6 and Cr(III): [Ar]3d3
For the case of Fe(II) where Fe(II) will be high spin:
CFSE = 4(-0.4ΔO) + 2(0.6ΔO)
CFSE = -0.4ΔO
For the case of Cr(III) where Cr(III) will be high spin:
CFSE = 3(-0.4ΔO)
CFSE = -1.2ΔO
I can now deduce that Cr(III) has the greatest CFSE for octahedral coordination. This
means that Cr(III) will occupy the octahedral sites and Fe(II) the tetrahedral sites and
it will be a normal spinel. To summarise, in this condition Chromium(III) will occupy
the octahedral site and so the structure will be normal spinel7.
F etet
III [ Croct
III Croct
III ] O4
5
b)
FeCr2O4:
Fe(II): [Ar]3d6 and Cr(III): [Ar]3d3
For the case of Fe(II) where Fe(II) will be high spin:
CFSE = 4(-0.4ΔO) + 2(0.6ΔO)
CFSE = -0.4ΔO
For the case of Cr(III) where Cr(III) will be high spin:
CFSE = 3(-0.4ΔO)
CFSE = -1.2ΔO
I can now deduce that Cr(III) has the greatest CFSE for octahedral coordination. This
means that Cr(III) will occupy the octahedral sites and Fe(II) the tetrahedral sites and
it will be a normal spinel. To summarise, in this condition Chromium(III) will occupy
the octahedral site and so the structure will be normal spinel7.
F etet
III [ Croct
III Croct
III ] O4
5
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
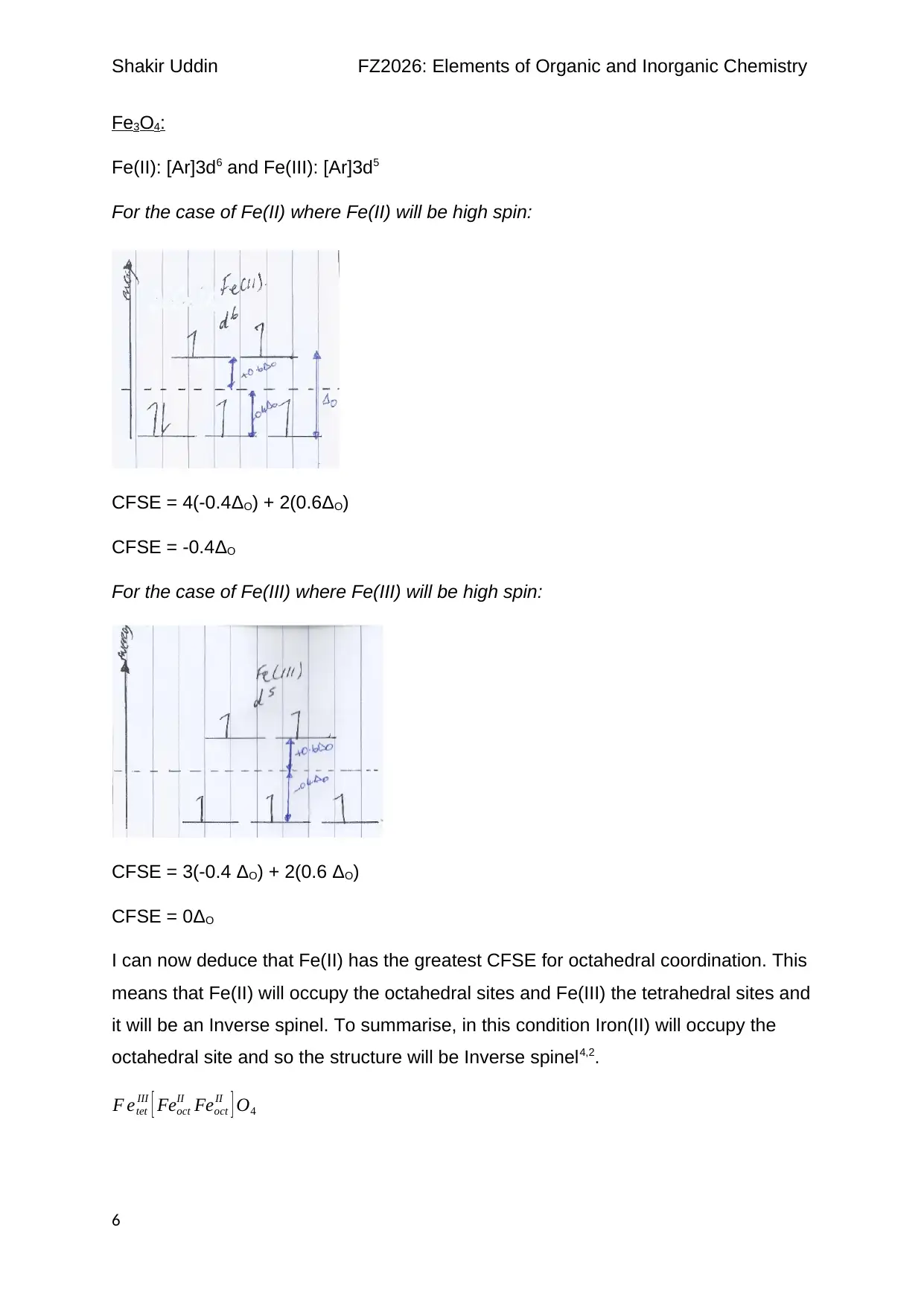
Fe3O4:
Fe(II): [Ar]3d6 and Fe(III): [Ar]3d5
For the case of Fe(II) where Fe(II) will be high spin:
CFSE = 4(-0.4ΔO) + 2(0.6ΔO)
CFSE = -0.4ΔO
For the case of Fe(III) where Fe(III) will be high spin:
CFSE = 3(-0.4 ΔO) + 2(0.6 ΔO)
CFSE = 0ΔO
I can now deduce that Fe(II) has the greatest CFSE for octahedral coordination. This
means that Fe(II) will occupy the octahedral sites and Fe(III) the tetrahedral sites and
it will be an Inverse spinel. To summarise, in this condition Iron(II) will occupy the
octahedral site and so the structure will be Inverse spinel4,2.
F etet
III [ Feoct
II Feoct
II ] O4
6
Fe3O4:
Fe(II): [Ar]3d6 and Fe(III): [Ar]3d5
For the case of Fe(II) where Fe(II) will be high spin:
CFSE = 4(-0.4ΔO) + 2(0.6ΔO)
CFSE = -0.4ΔO
For the case of Fe(III) where Fe(III) will be high spin:
CFSE = 3(-0.4 ΔO) + 2(0.6 ΔO)
CFSE = 0ΔO
I can now deduce that Fe(II) has the greatest CFSE for octahedral coordination. This
means that Fe(II) will occupy the octahedral sites and Fe(III) the tetrahedral sites and
it will be an Inverse spinel. To summarise, in this condition Iron(II) will occupy the
octahedral site and so the structure will be Inverse spinel4,2.
F etet
III [ Feoct
II Feoct
II ] O4
6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
3)
a)
The likely position of bromine is as given above, this is due to carbonyl group being
electron withdrawing (deactivating) group, hence electrophilic substitution will not
have preferred on carbonyl attacked Aromatic ring on the left1,2. Instead, the bromine
would favour the right ring as there is a methyl group which donates electron density
to the ring, which makes it a weak activator. Alkyl groups are ortho-para directing.
There are two ortho positions over one possible para positions, however the bromine
is likely to join at the para region rather than the ortho regions. This is due to there
being steric hindrance with the other bulky substituent on the benzene ring5.
b)
Looking at this separately, the alkylamino group is activating whereas the acyl group
is deactivating. This means that the right benzene ring is more reactive and so ortho-
para positions here are favoured as alkylamino groups are ortho-para directing.
There are two ortho positions over one possible para positions, however the bromine
is likely to join at the para region rather than the ortho regions. This is due to there
being steric hindrance with the other bulky substituent on the benzene ring3.
c)
7
3)
a)
The likely position of bromine is as given above, this is due to carbonyl group being
electron withdrawing (deactivating) group, hence electrophilic substitution will not
have preferred on carbonyl attacked Aromatic ring on the left1,2. Instead, the bromine
would favour the right ring as there is a methyl group which donates electron density
to the ring, which makes it a weak activator. Alkyl groups are ortho-para directing.
There are two ortho positions over one possible para positions, however the bromine
is likely to join at the para region rather than the ortho regions. This is due to there
being steric hindrance with the other bulky substituent on the benzene ring5.
b)
Looking at this separately, the alkylamino group is activating whereas the acyl group
is deactivating. This means that the right benzene ring is more reactive and so ortho-
para positions here are favoured as alkylamino groups are ortho-para directing.
There are two ortho positions over one possible para positions, however the bromine
is likely to join at the para region rather than the ortho regions. This is due to there
being steric hindrance with the other bulky substituent on the benzene ring3.
c)
7

Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
This is the position which has the most likelihood of forming due to the electron
donating nature of oxygen on the alkoxy group, therefore it directs electrophile to
ortho-para position,4. The alkoxy group would equally activate both rings however,
the position of the acyl group would make monobromination on the right-hand side
difficult as it is a deactivator. This means the benzene ring will be less nucleophilic.
Overall, the product is more likely to form on the more activated ring, the ring on the
left-hand side. There are two ortho positions over one possible para positions,
however the bromine is likely to join at the para region rather than the ortho regions.
This is due to there being steric hindrance with the other substituent on the benzene
ring. Although there is not as much steric hindrance as with other examples, this
steric hindrance factor dominates1.
4)
8
This is the position which has the most likelihood of forming due to the electron
donating nature of oxygen on the alkoxy group, therefore it directs electrophile to
ortho-para position,4. The alkoxy group would equally activate both rings however,
the position of the acyl group would make monobromination on the right-hand side
difficult as it is a deactivator. This means the benzene ring will be less nucleophilic.
Overall, the product is more likely to form on the more activated ring, the ring on the
left-hand side. There are two ortho positions over one possible para positions,
however the bromine is likely to join at the para region rather than the ortho regions.
This is due to there being steric hindrance with the other substituent on the benzene
ring. Although there is not as much steric hindrance as with other examples, this
steric hindrance factor dominates1.
4)
8
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
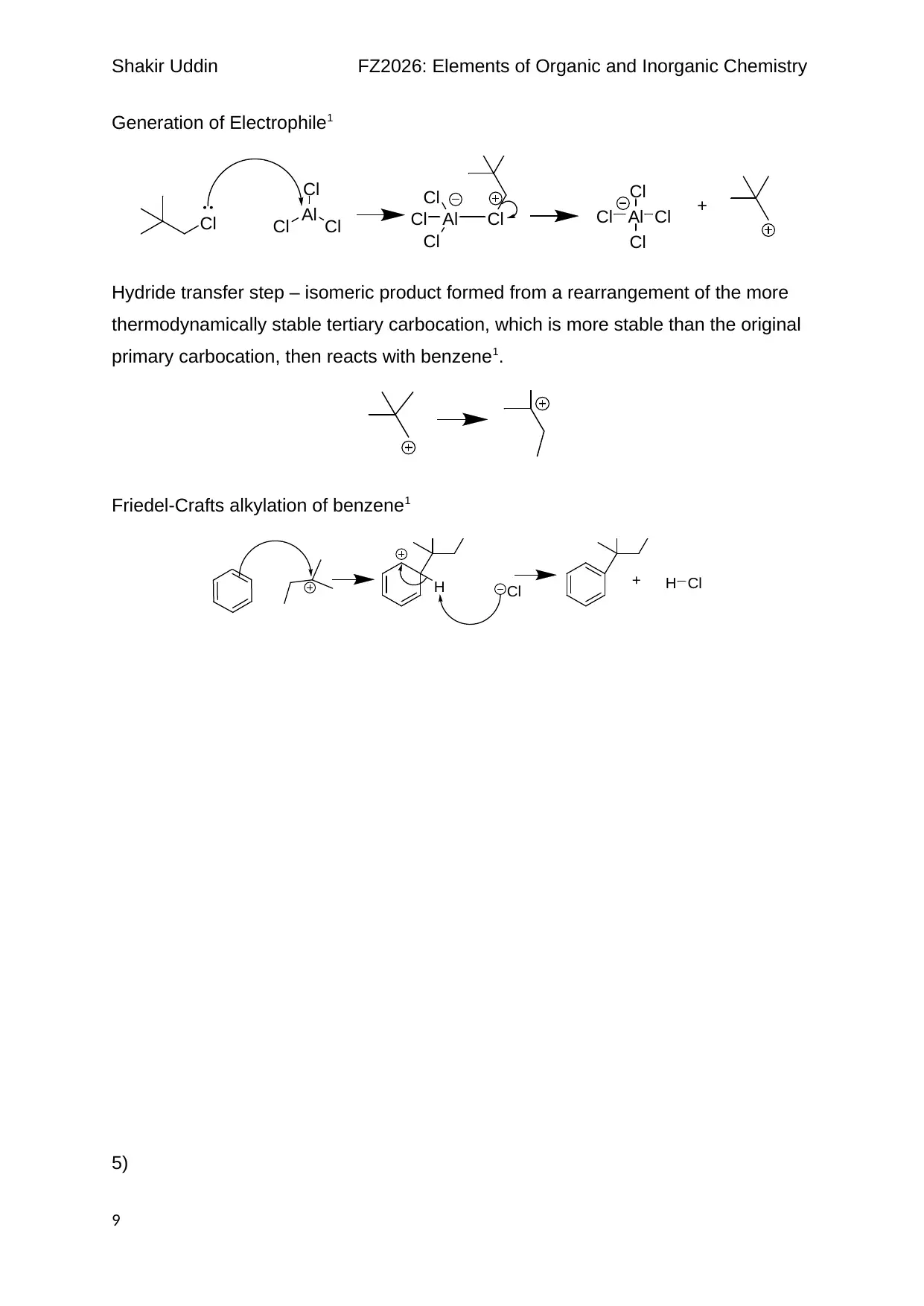
Generation of Electrophile1
Hydride transfer step – isomeric product formed from a rearrangement of the more
thermodynamically stable tertiary carbocation, which is more stable than the original
primary carbocation, then reacts with benzene1.
Friedel-Crafts alkylation of benzene1
5)
9
Cl Al
Cl Cl
Cl
ClAl
Cl
Cl
Cl
Cl
Al Cl
Cl
Cl +
H Cl H Cl+
Generation of Electrophile1
Hydride transfer step – isomeric product formed from a rearrangement of the more
thermodynamically stable tertiary carbocation, which is more stable than the original
primary carbocation, then reacts with benzene1.
Friedel-Crafts alkylation of benzene1
5)
9
Cl Al
Cl Cl
Cl
ClAl
Cl
Cl
Cl
Cl
Al Cl
Cl
Cl +
H Cl H Cl+
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
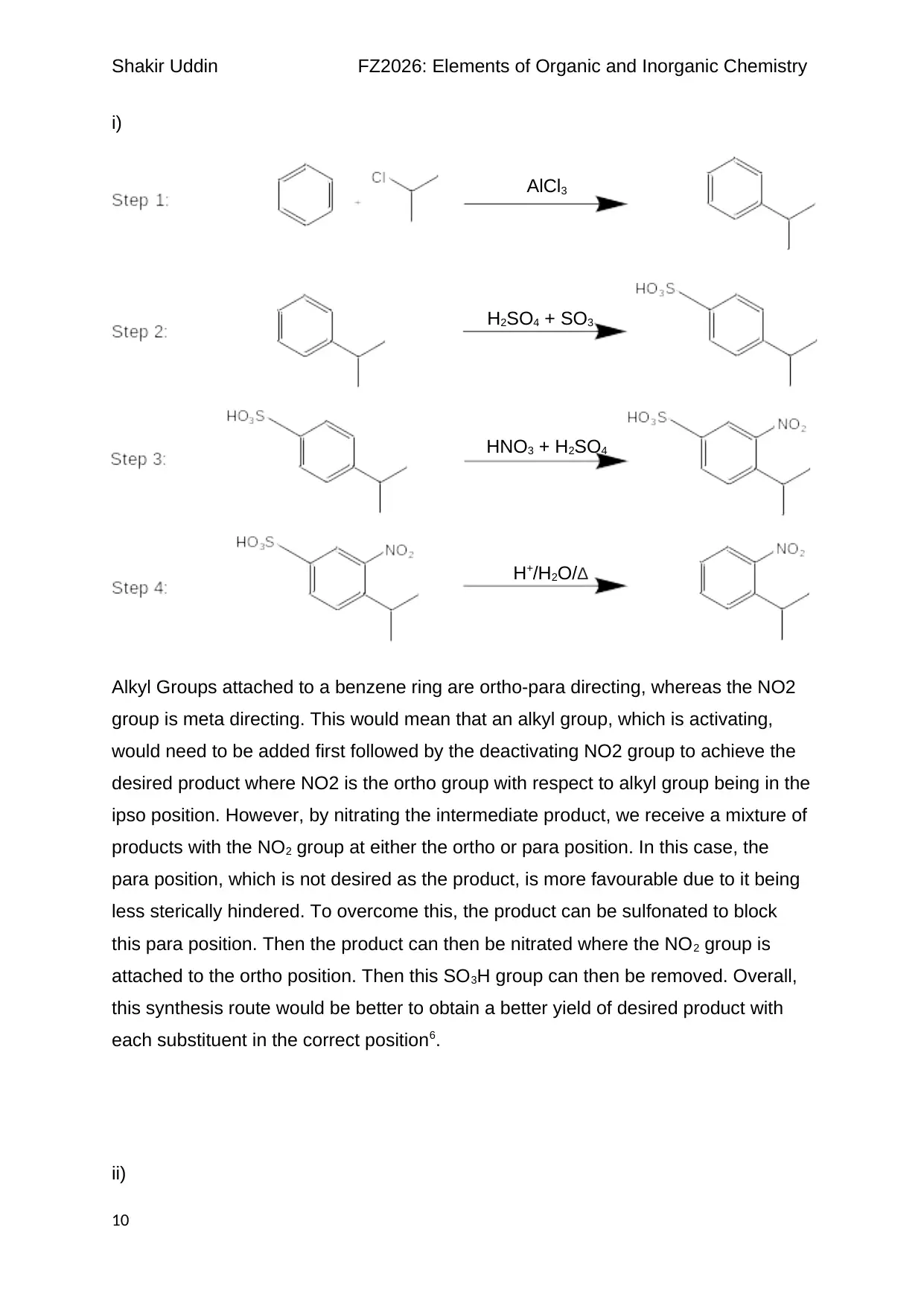
i)
Alkyl Groups attached to a benzene ring are ortho-para directing, whereas the NO2
group is meta directing. This would mean that an alkyl group, which is activating,
would need to be added first followed by the deactivating NO2 group to achieve the
desired product where NO2 is the ortho group with respect to alkyl group being in the
ipso position. However, by nitrating the intermediate product, we receive a mixture of
products with the NO2 group at either the ortho or para position. In this case, the
para position, which is not desired as the product, is more favourable due to it being
less sterically hindered. To overcome this, the product can be sulfonated to block
this para position. Then the product can then be nitrated where the NO2 group is
attached to the ortho position. Then this SO3H group can then be removed. Overall,
this synthesis route would be better to obtain a better yield of desired product with
each substituent in the correct position6.
ii)
10
AlCl3
HNO3 + H2SO4
H+/H2O/Δ
H2SO4 + SO3
i)
Alkyl Groups attached to a benzene ring are ortho-para directing, whereas the NO2
group is meta directing. This would mean that an alkyl group, which is activating,
would need to be added first followed by the deactivating NO2 group to achieve the
desired product where NO2 is the ortho group with respect to alkyl group being in the
ipso position. However, by nitrating the intermediate product, we receive a mixture of
products with the NO2 group at either the ortho or para position. In this case, the
para position, which is not desired as the product, is more favourable due to it being
less sterically hindered. To overcome this, the product can be sulfonated to block
this para position. Then the product can then be nitrated where the NO2 group is
attached to the ortho position. Then this SO3H group can then be removed. Overall,
this synthesis route would be better to obtain a better yield of desired product with
each substituent in the correct position6.
ii)
10
AlCl3
HNO3 + H2SO4
H+/H2O/Δ
H2SO4 + SO3
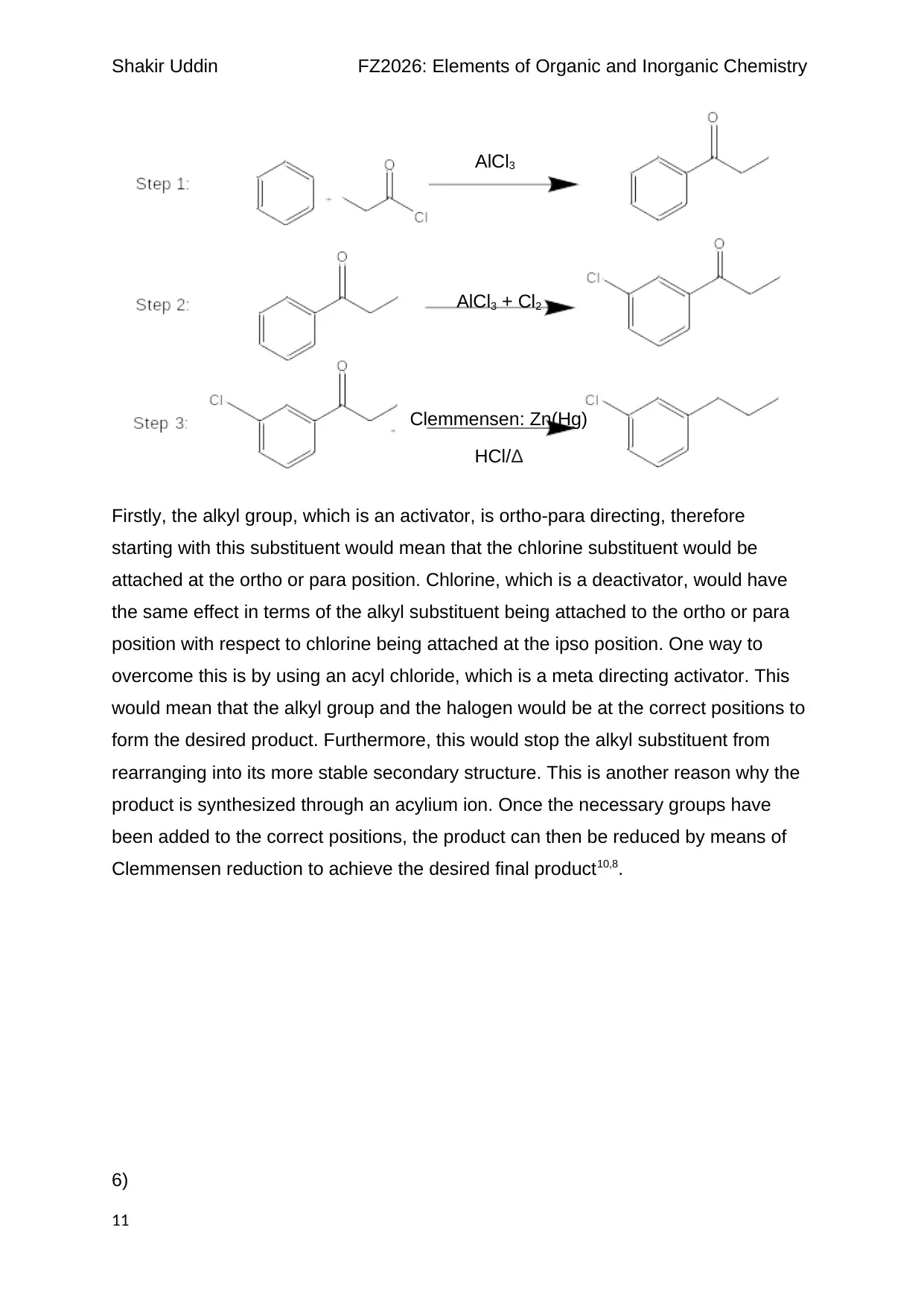
Shakir Uddin FZ2026: Elements of Organic and Inorganic Chemistry
Firstly, the alkyl group, which is an activator, is ortho-para directing, therefore
starting with this substituent would mean that the chlorine substituent would be
attached at the ortho or para position. Chlorine, which is a deactivator, would have
the same effect in terms of the alkyl substituent being attached to the ortho or para
position with respect to chlorine being attached at the ipso position. One way to
overcome this is by using an acyl chloride, which is a meta directing activator. This
would mean that the alkyl group and the halogen would be at the correct positions to
form the desired product. Furthermore, this would stop the alkyl substituent from
rearranging into its more stable secondary structure. This is another reason why the
product is synthesized through an acylium ion. Once the necessary groups have
been added to the correct positions, the product can then be reduced by means of
Clemmensen reduction to achieve the desired final product10,8.
6)
11
AlCl3
Clemmensen: Zn(Hg)
HCl/Δ
AlCl3 + Cl2
Firstly, the alkyl group, which is an activator, is ortho-para directing, therefore
starting with this substituent would mean that the chlorine substituent would be
attached at the ortho or para position. Chlorine, which is a deactivator, would have
the same effect in terms of the alkyl substituent being attached to the ortho or para
position with respect to chlorine being attached at the ipso position. One way to
overcome this is by using an acyl chloride, which is a meta directing activator. This
would mean that the alkyl group and the halogen would be at the correct positions to
form the desired product. Furthermore, this would stop the alkyl substituent from
rearranging into its more stable secondary structure. This is another reason why the
product is synthesized through an acylium ion. Once the necessary groups have
been added to the correct positions, the product can then be reduced by means of
Clemmensen reduction to achieve the desired final product10,8.
6)
11
AlCl3
Clemmensen: Zn(Hg)
HCl/Δ
AlCl3 + Cl2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 17
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.