Deakin University SLE212 Biochemistry T1 2019: Worksheet B Analysis
VerifiedAdded on 2023/01/20
|10
|2416
|94
Homework Assignment
AI Summary
This document provides a comprehensive solution for SLE212 Biochemistry Worksheet B, focusing on the preparation of buffers and the titration of amino acids. It begins with calculations related to a phosphoric acid buffer, including determining the ratio of base to acid at a specific pH and calculati...

SLE212 Biochemistry T1 2019 Assessment Worksheet B
Worksheet Worksheet B
Preparation of buffers and titration of amino acid
Name
Prac Partners
Prac date
Due date
1) Electronic Format
The worksheet must be produced entirely in an electronic fashion, however, we do not accept ePens! Hand-
written or hand-drawn components, be it as original or as scanned image, will not be accepted/not receive any
marks! Submit electronically as either a Word or PDF file via the designated CloudDeakin dropbox.
2) Plagiarism and Collusion
Before submitting your worksheet to the dropbox submit it to the Check Your Work: Turnitin Assignment Folder
available on CloudDeakin. In case that we find that work is the result of plagiarism (from foreign source like
internet or fellow student) or collusion we report this to the Faculty Academic Progress and Discipline Committee.
Note: It is NOT sufficient to submit your worksheet only to the Turnitin Folder! Your worksheet will be marked only
if you submit it to the appropriate Dropbox!
3) Due dates for worksheet submission:
Worksheet A is due 5.00 pm two teaching weeks after practical session 1, this is the day BEFORE your prac 2
Worksheet B is due 5.00 pm two teaching weeks after practical session 2, this is the day BEFORE your prac 3
Worksheet C is due 5.00 pm two teaching weeks after practical session 4 (!), this is the day BEFORE your prac 5
Worksheet D is due 5.00 pm ONE (!) teaching week after practical session 5 (!)
4) Referencing
All foreign sources of information, i.e. information that was not provided in this unit, must
be cited. The citation style to be used is Harvard style, for more information about this style and referencing in
general see: https://www.deakin.edu.au/students/studying/study-support/referencing. Citations will be
considered only when they are placed as in-text citations such that it is clear which statement they do support.
Furthermore, bibliographic details of a citation must be listed within the question where they are used, reference
lists at the end of the worksheet will not be accepted! The citation needs to be a quality journal article, book or
refereed website. No marks are awarded if Wikipedia or poor-quality websites (eg. the website of a course at
another institution) are cited.
The practical manual, the prac script, OLMs and lecture notes can NOT be cited! Citations of text
books are often most appropriate for the level of this unit and therefore encouraged! If you do cite a
textbook you MUST indicate relevant page numbers!
5) Learning Outcomes:
Q1, 3 and 4: Quantitation skills. Application of mathematical equation and discipline specific knowledge
to quantitatively describe a system.
Q2: Written communication. Presentation of experimental data.
Q5: Critical thinking & problem solving. Use of discipline specific knowledge and logic to predict the
chemical behaviour of a biochemical compound.
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Worksheet Worksheet B
Preparation of buffers and titration of amino acid
Name
Prac Partners
Prac date
Due date
1) Electronic Format
The worksheet must be produced entirely in an electronic fashion, however, we do not accept ePens! Hand-
written or hand-drawn components, be it as original or as scanned image, will not be accepted/not receive any
marks! Submit electronically as either a Word or PDF file via the designated CloudDeakin dropbox.
2) Plagiarism and Collusion
Before submitting your worksheet to the dropbox submit it to the Check Your Work: Turnitin Assignment Folder
available on CloudDeakin. In case that we find that work is the result of plagiarism (from foreign source like
internet or fellow student) or collusion we report this to the Faculty Academic Progress and Discipline Committee.
Note: It is NOT sufficient to submit your worksheet only to the Turnitin Folder! Your worksheet will be marked only
if you submit it to the appropriate Dropbox!
3) Due dates for worksheet submission:
Worksheet A is due 5.00 pm two teaching weeks after practical session 1, this is the day BEFORE your prac 2
Worksheet B is due 5.00 pm two teaching weeks after practical session 2, this is the day BEFORE your prac 3
Worksheet C is due 5.00 pm two teaching weeks after practical session 4 (!), this is the day BEFORE your prac 5
Worksheet D is due 5.00 pm ONE (!) teaching week after practical session 5 (!)
4) Referencing
All foreign sources of information, i.e. information that was not provided in this unit, must
be cited. The citation style to be used is Harvard style, for more information about this style and referencing in
general see: https://www.deakin.edu.au/students/studying/study-support/referencing. Citations will be
considered only when they are placed as in-text citations such that it is clear which statement they do support.
Furthermore, bibliographic details of a citation must be listed within the question where they are used, reference
lists at the end of the worksheet will not be accepted! The citation needs to be a quality journal article, book or
refereed website. No marks are awarded if Wikipedia or poor-quality websites (eg. the website of a course at
another institution) are cited.
The practical manual, the prac script, OLMs and lecture notes can NOT be cited! Citations of text
books are often most appropriate for the level of this unit and therefore encouraged! If you do cite a
textbook you MUST indicate relevant page numbers!
5) Learning Outcomes:
Q1, 3 and 4: Quantitation skills. Application of mathematical equation and discipline specific knowledge
to quantitatively describe a system.
Q2: Written communication. Presentation of experimental data.
Q5: Critical thinking & problem solving. Use of discipline specific knowledge and logic to predict the
chemical behaviour of a biochemical compound.
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SLE212 Biochemistry T1 2019 Assessment Worksheet B
1. Phosphoric acid buffer.
Phosphoric acid is a tri-protic acid, which can dissociate three different protons at three different pka’s.
These pka values are 2.13, 7.20 and 12.36.
a) Provide the chemical equation that describes the phosphoric buffer system that exists at pH 8!
Indicate the weak acid and it’s conjugate base, and the names of the components.
(1 mark)
b) Calculate to 2 decimal places, the ratio of base/acid at this pH. Show all details of your working out.
Include (!) in your working out the chemical formulas of the weak acid and conjugate base.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
H2PO-4(aq)+ HPO42- H+
Dihydrogen phosphate Hydrogen phosphate Hydrogen ion
Weak acid Conjugate bace
By using the Henderson Hasalblach equation:
Which is Ph=pka+loq[base]/[acid]
8.0=7.20+log[HPO42- ]/[H2PO4-]
8.0-7.20=log[HPO42- ]/[H2PO4-]
0.8=log[HPO42- ]/[H2PO4-]
By multiplying each side by log
100.8 = [HPO42- ]/[H2PO4-]
= 6.309 or
6.31=[HPO42- ][H2PO4-]
6.31[HPO42- ]=[H2PO4-]
1. Phosphoric acid buffer.
Phosphoric acid is a tri-protic acid, which can dissociate three different protons at three different pka’s.
These pka values are 2.13, 7.20 and 12.36.
a) Provide the chemical equation that describes the phosphoric buffer system that exists at pH 8!
Indicate the weak acid and it’s conjugate base, and the names of the components.
(1 mark)
b) Calculate to 2 decimal places, the ratio of base/acid at this pH. Show all details of your working out.
Include (!) in your working out the chemical formulas of the weak acid and conjugate base.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
H2PO-4(aq)+ HPO42- H+
Dihydrogen phosphate Hydrogen phosphate Hydrogen ion
Weak acid Conjugate bace
By using the Henderson Hasalblach equation:
Which is Ph=pka+loq[base]/[acid]
8.0=7.20+log[HPO42- ]/[H2PO4-]
8.0-7.20=log[HPO42- ]/[H2PO4-]
0.8=log[HPO42- ]/[H2PO4-]
By multiplying each side by log
100.8 = [HPO42- ]/[H2PO4-]
= 6.309 or
6.31=[HPO42- ][H2PO4-]
6.31[HPO42- ]=[H2PO4-]

SLE212 Biochemistry T1 2019 Assessment Worksheet B
1c. Calculate to 2 decimal places the concentration of the conjugate acid and conjugate base for a total
buffer concentration of 200 mM. Show all details of your working out, including (!) the chemical
formula of the acid and base, include all units and state the answer in a complete sentence.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Buffer concentration =[H2PO4-]+[HPO32-]= 200mM bu converting it =0.2M
1) From equation2:
[H2PO4-]+[HPO32-]=0.2M
2)From equation1(from part b)
6.31[H2PO4-]=[HPO32-]
- By combining (1)and (2) :
[H2PO4-]+6.31[H2PO4-]=0.2 M
7.31 [H2PO4-]=0.2 M
[H2PO4-]=(0.2/7.31M
Gives [H2PO4-]=0.03M
- using the concentration of acid into equation 2 gives :
0.03M +[ HPO32-]=0.2 M
[ HPO32-]=0.2-0.03M
[ HPO32-]=0.17 M
This means that the consentration on a conjugated acid and the conjugated base in this buffer
is 0.03 M and 0.17 M respectively.
1c. Calculate to 2 decimal places the concentration of the conjugate acid and conjugate base for a total
buffer concentration of 200 mM. Show all details of your working out, including (!) the chemical
formula of the acid and base, include all units and state the answer in a complete sentence.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Buffer concentration =[H2PO4-]+[HPO32-]= 200mM bu converting it =0.2M
1) From equation2:
[H2PO4-]+[HPO32-]=0.2M
2)From equation1(from part b)
6.31[H2PO4-]=[HPO32-]
- By combining (1)and (2) :
[H2PO4-]+6.31[H2PO4-]=0.2 M
7.31 [H2PO4-]=0.2 M
[H2PO4-]=(0.2/7.31M
Gives [H2PO4-]=0.03M
- using the concentration of acid into equation 2 gives :
0.03M +[ HPO32-]=0.2 M
[ HPO32-]=0.2-0.03M
[ HPO32-]=0.17 M
This means that the consentration on a conjugated acid and the conjugated base in this buffer
is 0.03 M and 0.17 M respectively.
You're viewing a preview
Unlock full access by subscribing today!

SLE212 Biochemistry T1 2019 Assessment Worksheet B
2. Carbonic acid in the blood forms one of the most important buffer systems in our body. When our
tissues are burning metabolic fuel they produce H+ and CO2. The enzyme carbonic anhydrase use H2O
to convert most of the CO2 into carbonic acid (H2CO3) as indicated in the chemical equilibrium
shown:
If a person holds their breath and swims 25 m underwater i) What will happen to the pH of their
blood at the end of this swim? ii) explain the chemical reasoning behind this this change. (1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
H2O (l) + CO2 (g) H2CO3 (aq)
i)At the end of the swim the pH of the blood will be increasing by Hyperventilation
ii)At the end of this swim , the amount of breathing or the volume of inhalation are higher than
normal.During hyperventilation more CO2 are exhaled which leads to lowering the CO2 pressure
in the body. Using the Le Chatelier's Principle, the equation will shift to the left, i.e. the system
will favor the backward reaction where H2CO3(aq) will broken down into H2O(l) and CO2(g) ,Thus
increasing the pH.
2. Carbonic acid in the blood forms one of the most important buffer systems in our body. When our
tissues are burning metabolic fuel they produce H+ and CO2. The enzyme carbonic anhydrase use H2O
to convert most of the CO2 into carbonic acid (H2CO3) as indicated in the chemical equilibrium
shown:
If a person holds their breath and swims 25 m underwater i) What will happen to the pH of their
blood at the end of this swim? ii) explain the chemical reasoning behind this this change. (1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
H2O (l) + CO2 (g) H2CO3 (aq)
i)At the end of the swim the pH of the blood will be increasing by Hyperventilation
ii)At the end of this swim , the amount of breathing or the volume of inhalation are higher than
normal.During hyperventilation more CO2 are exhaled which leads to lowering the CO2 pressure
in the body. Using the Le Chatelier's Principle, the equation will shift to the left, i.e. the system
will favor the backward reaction where H2CO3(aq) will broken down into H2O(l) and CO2(g) ,Thus
increasing the pH.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SLE212 Biochemistry T1 2019 Assessment Worksheet B
3. Titration curve of an unknown amino acid
Using Excel, prepare a graph of your titration curve of the unknown amino acid. Depict your
experimental measurements as STRAIGHT MARKED SCATTER PLOT (ie data points are connected by
straight lines). Include a suitable descriptive title stating the identity of the unknown amino acid,
proper labelling of the axes and an appropriate figure legend. In your graph clearly label the regions
responsible for the titrations of alpha carboxyl, alpha amino and R functional groups. Also, indicate
within the graph all observable titration mid-points and all observable titration end-points and indicate
estimates of the pKa of the three functional groups (do not provide pka values from the literature, we
want you to read out the pka from your titration curve).
IMPORTANT: The color of the regression line and the data points MUST be BLUE (you will receive 0
marks for question 1 in case that a different colour is shown).
Explanatory note: The teaching team strictly enforce the above indicated color requirements in order to ensure the
originality of 2019 worksheet submissions.
(2 marks)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
3. Titration curve of an unknown amino acid
Using Excel, prepare a graph of your titration curve of the unknown amino acid. Depict your
experimental measurements as STRAIGHT MARKED SCATTER PLOT (ie data points are connected by
straight lines). Include a suitable descriptive title stating the identity of the unknown amino acid,
proper labelling of the axes and an appropriate figure legend. In your graph clearly label the regions
responsible for the titrations of alpha carboxyl, alpha amino and R functional groups. Also, indicate
within the graph all observable titration mid-points and all observable titration end-points and indicate
estimates of the pKa of the three functional groups (do not provide pka values from the literature, we
want you to read out the pka from your titration curve).
IMPORTANT: The color of the regression line and the data points MUST be BLUE (you will receive 0
marks for question 1 in case that a different colour is shown).
Explanatory note: The teaching team strictly enforce the above indicated color requirements in order to ensure the
originality of 2019 worksheet submissions.
(2 marks)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
SLE212 Biochemistry T1 2019 Assessment Worksheet B
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Carbolxy group
pka1=2
0
2
4
6
8
10
12
14
16
18
20
22
24
26
28
30
32
34
36
0
2
4
6
8
10
12
14
Titration curve for unknown amino
acid(Histidine)
PH
Volume(mL)of 0.5 M NaOH added
PH
R group-
Imdazone group
pkaR=6
amino acid
pka2=9
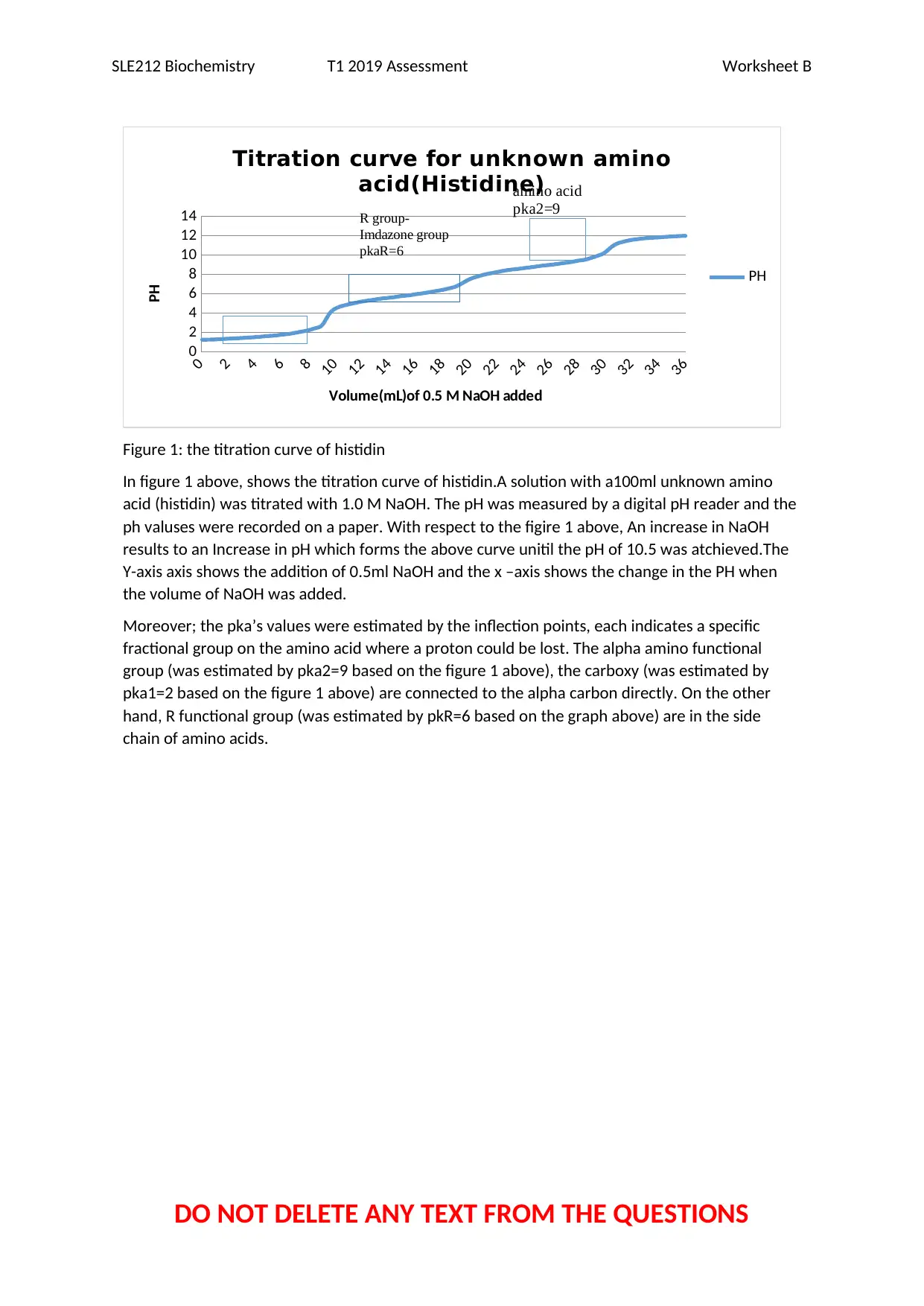
Figure 1: the titration curve of histidin
In figure 1 above, shows the titration curve of histidin.A solution with a100ml unknown amino
acid (histidin) was titrated with 1.0 M NaOH. The pH was measured by a digital pH reader and the
ph valuses were recorded on a paper. With respect to the figire 1 above, An increase in NaOH
results to an Increase in pH which forms the above curve unitil the pH of 10.5 was atchieved.The
Y-axis axis shows the addition of 0.5ml NaOH and the x –axis shows the change in the PH when
the volume of NaOH was added.
Moreover; the pka’s values were estimated by the inflection points, each indicates a specific
fractional group on the amino acid where a proton could be lost. The alpha amino functional
group (was estimated by pka2=9 based on the figure 1 above), the carboxy (was estimated by
pka1=2 based on the figure 1 above) are connected to the alpha carbon directly. On the other
hand, R functional group (was estimated by pkR=6 based on the graph above) are in the side
chain of amino acids.
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Carbolxy group
pka1=2
0
2
4
6
8
10
12
14
16
18
20
22
24
26
28
30
32
34
36
0
2
4
6
8
10
12
14
Titration curve for unknown amino
acid(Histidine)
PH
Volume(mL)of 0.5 M NaOH added
PH
R group-
Imdazone group
pkaR=6
amino acid
pka2=9
Figure 1: the titration curve of histidin
In figure 1 above, shows the titration curve of histidin.A solution with a100ml unknown amino
acid (histidin) was titrated with 1.0 M NaOH. The pH was measured by a digital pH reader and the
ph valuses were recorded on a paper. With respect to the figire 1 above, An increase in NaOH
results to an Increase in pH which forms the above curve unitil the pH of 10.5 was atchieved.The
Y-axis axis shows the addition of 0.5ml NaOH and the x –axis shows the change in the PH when
the volume of NaOH was added.
Moreover; the pka’s values were estimated by the inflection points, each indicates a specific
fractional group on the amino acid where a proton could be lost. The alpha amino functional
group (was estimated by pka2=9 based on the figure 1 above), the carboxy (was estimated by
pka1=2 based on the figure 1 above) are connected to the alpha carbon directly. On the other
hand, R functional group (was estimated by pkR=6 based on the graph above) are in the side
chain of amino acids.
You're viewing a preview
Unlock full access by subscribing today!

SLE212 Biochemistry T1 2019 Assessment Worksheet B
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
SLE212 Biochemistry T1 2019 Assessment Worksheet B
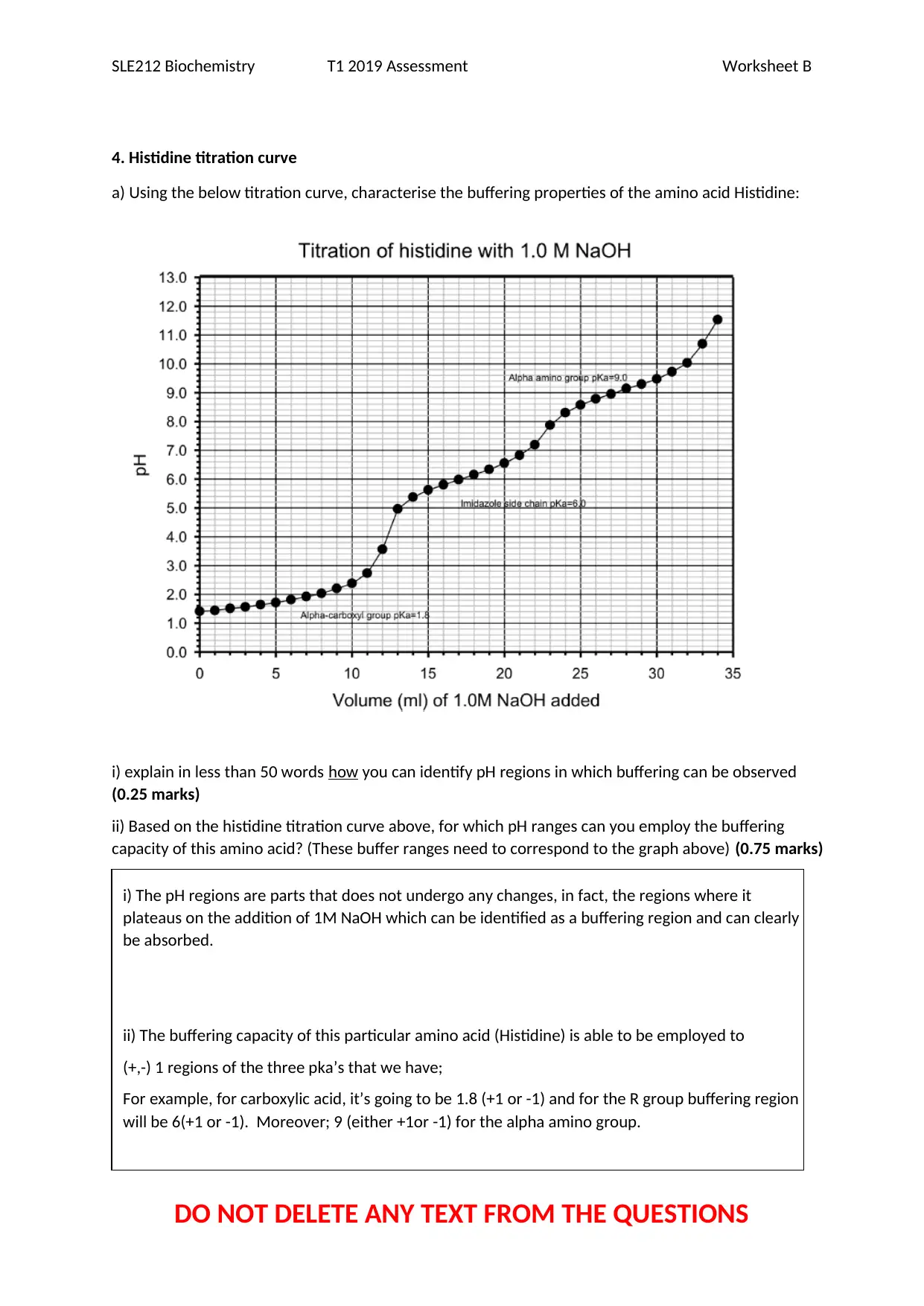
4. Histidine titration curve
a) Using the below titration curve, characterise the buffering properties of the amino acid Histidine:
i) explain in less than 50 words how you can identify pH regions in which buffering can be observed
(0.25 marks)
ii) Based on the histidine titration curve above, for which pH ranges can you employ the buffering
capacity of this amino acid? (These buffer ranges need to correspond to the graph above) (0.75 marks)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
i) The pH regions are parts that does not undergo any changes, in fact, the regions where it
plateaus on the addition of 1M NaOH which can be identified as a buffering region and can clearly
be absorbed.
ii) The buffering capacity of this particular amino acid (Histidine) is able to be employed to
(+,-) 1 regions of the three pka’s that we have;
For example, for carboxylic acid, it’s going to be 1.8 (+1 or -1) and for the R group buffering region
will be 6(+1 or -1). Moreover; 9 (either +1or -1) for the alpha amino group.
4. Histidine titration curve
a) Using the below titration curve, characterise the buffering properties of the amino acid Histidine:
i) explain in less than 50 words how you can identify pH regions in which buffering can be observed
(0.25 marks)
ii) Based on the histidine titration curve above, for which pH ranges can you employ the buffering
capacity of this amino acid? (These buffer ranges need to correspond to the graph above) (0.75 marks)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
i) The pH regions are parts that does not undergo any changes, in fact, the regions where it
plateaus on the addition of 1M NaOH which can be identified as a buffering region and can clearly
be absorbed.
ii) The buffering capacity of this particular amino acid (Histidine) is able to be employed to
(+,-) 1 regions of the three pka’s that we have;
For example, for carboxylic acid, it’s going to be 1.8 (+1 or -1) and for the R group buffering region
will be 6(+1 or -1). Moreover; 9 (either +1or -1) for the alpha amino group.

SLE212 Biochemistry T1 2019 Assessment Worksheet B
4b) From the histidine titration curve above, how does the form of the amino acid at the end of the
second equivalence point differ from that at the beginning of the titration? i) Describe this change in
terms of the names and forms of the functional groups ii) Deduce with reasoning (!) how the overall
charge of the amino acid changes from the beginning of the titration to the end of the second
equivalence point (1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
i) When the 2ed equivalence point gets at the end, the imidazole side chain is
going to be deprotonated, whereas; protonated at the start or beginning of the
titration, and carries no charge. The Alpha –carboxyl group is going to be
protonated at the beginning. However, deprotonated when it’s reached the
second point with a negative charge and the amino group is going to be +1.
ii) The amino acid charge is going to be +2, whereas, after passing the second
equivalence point, it’s going to be nutral, with +1 on the amino group and -1
on the carboxyl group.
4b) From the histidine titration curve above, how does the form of the amino acid at the end of the
second equivalence point differ from that at the beginning of the titration? i) Describe this change in
terms of the names and forms of the functional groups ii) Deduce with reasoning (!) how the overall
charge of the amino acid changes from the beginning of the titration to the end of the second
equivalence point (1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
i) When the 2ed equivalence point gets at the end, the imidazole side chain is
going to be deprotonated, whereas; protonated at the start or beginning of the
titration, and carries no charge. The Alpha –carboxyl group is going to be
protonated at the beginning. However, deprotonated when it’s reached the
second point with a negative charge and the amino group is going to be +1.
ii) The amino acid charge is going to be +2, whereas, after passing the second
equivalence point, it’s going to be nutral, with +1 on the amino group and -1
on the carboxyl group.
You're viewing a preview
Unlock full access by subscribing today!

SLE212 Biochemistry T1 2019 Assessment Worksheet B
5. Tyrosine has a side-chain with an ionisable phenol group. i) explain in less than 20 words the general
definition of pI; ii) state in a complete sentence the pI of tyrosine; and iii) explain in about 50 words
how the involved chemicals group determine the pI of tyrosine (in your explanation name the charges
that are present on the ionisable groups)!
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
i) PI or iso electric point of an amino acid means the point at which there is no net
charge.
ii) The PI of Tyrosin is 5.66.
iii) When the charge of Tyrosine is at it’s lowest pH value (less than 2.2) it will be +1 on
the amino group, whereas the other groups are uncharged which provides a total
charge of +. By increasing the pH will deprotonates carboxyl group and give a
negative charge, as this specific pH and the net charge becomes zero .The side chain
remain with no charge in it, also. The Iso-electric point or (PI) can be found by
calculation where the average of the two pka’s are tken( pk1=2.2,pk2=9.1) which
5.66.
5. Tyrosine has a side-chain with an ionisable phenol group. i) explain in less than 20 words the general
definition of pI; ii) state in a complete sentence the pI of tyrosine; and iii) explain in about 50 words
how the involved chemicals group determine the pI of tyrosine (in your explanation name the charges
that are present on the ionisable groups)!
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
i) PI or iso electric point of an amino acid means the point at which there is no net
charge.
ii) The PI of Tyrosin is 5.66.
iii) When the charge of Tyrosine is at it’s lowest pH value (less than 2.2) it will be +1 on
the amino group, whereas the other groups are uncharged which provides a total
charge of +. By increasing the pH will deprotonates carboxyl group and give a
negative charge, as this specific pH and the net charge becomes zero .The side chain
remain with no charge in it, also. The Iso-electric point or (PI) can be found by
calculation where the average of the two pka’s are tken( pk1=2.2,pk2=9.1) which
5.66.
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

