Analyzing Soaps and Detergents: Dirt Removal, Ions, and Solubility
VerifiedAdded on 2023/06/15
|9
|1745
|494
Essay
AI Summary
This essay explores the chemistry of soaps and detergents, detailing how they function to remove dirt. It explains that soaps, composed of sodium or potassium salts with long-chain carboxylic acids, and detergents, made of mixed chemical compositions, work by breaking down oily dirt into water-soluble emulsions. The essay highlights the dual nature of these cleaning agents, with hydrophilic and hydrophobic ends that bridge water and dirt, reducing surface tension and enabling dirt removal. It also discusses how water hardness, caused by ions like calcium and magnesium, affects the solubility and effectiveness of soaps and detergents by forming scum, and emphasizes that detergents must neutralize these ions before cleaning, thus increasing the amount needed for effective cleaning. The document concludes by stating that while the attraction between the ionic parts of soaps/detergents and ions in hard water reduces the solubility of the cleaning agents, the chemical combination of their parts is essential for their cleaning action.

Name:
Course:
Professor:
Date:
Soaps and detergents
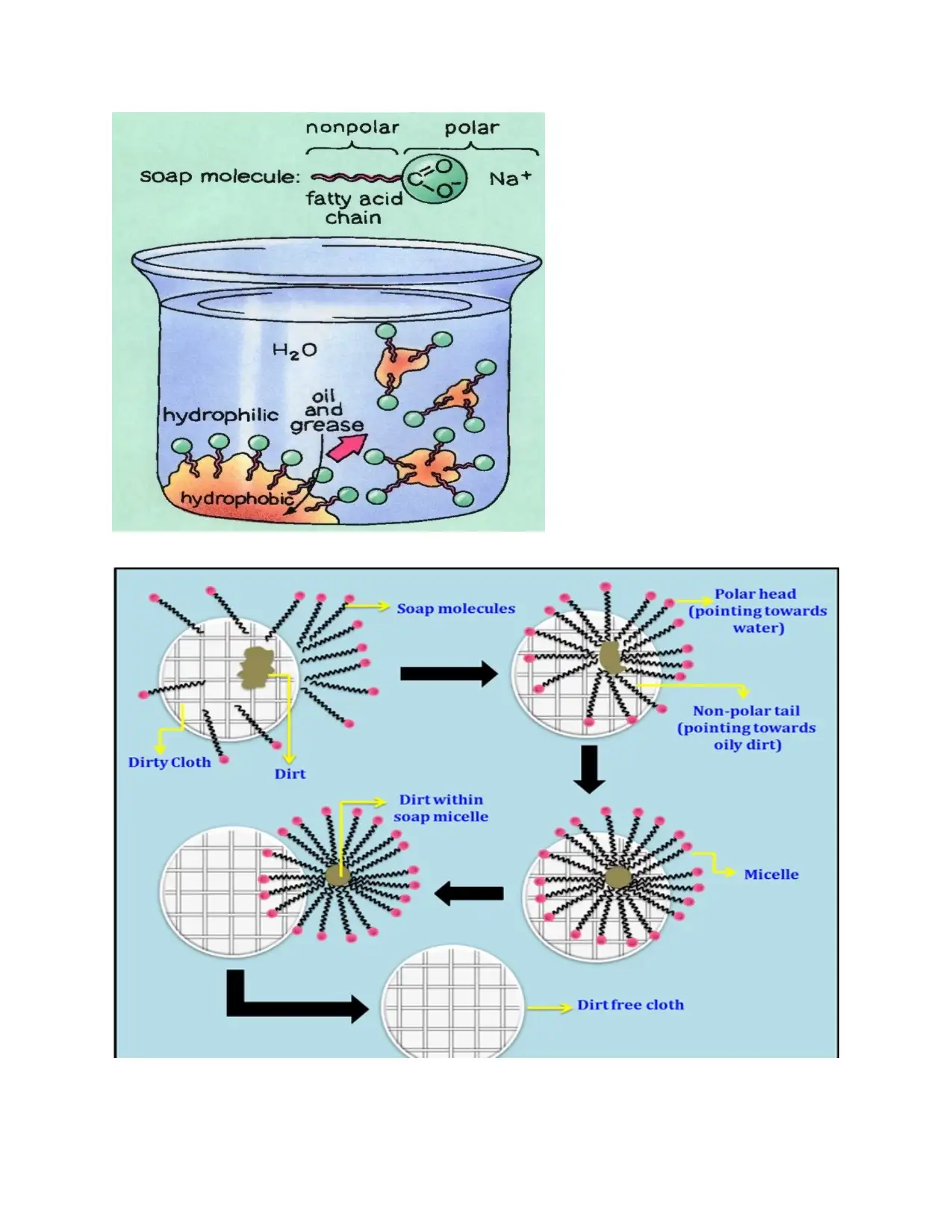
Soaps and detergents are used to remove dirt. Dirt is oily in nature and this characteristic mean
that it is insoluble in water. Most of dirts are considered to be oily (Raymond 55). The sodium or
potassium salts which have long chain carboxylic acids in soaps help in removal of dirt. The
soaps have a carboxylic acid end on one side which is bonded by a metal ion end. The two major
parts of the soaps and detergents are the non-ionic hydrocarbon group and the ionic group of
COO-Na+. The soap and detergents components help to decompose the oily parts therefore
making them insoluble in water. The chains of chemical compositions in soaps and detergents
are responsible to removal of the oily parts and making them soluble in water. Soaps are cleaning
agents which have salts of fatty acids (Birdi 62). Detergents on the other hand are composed of
mixed chemical compositions which aid the cleaning factor. The components of soaps and
detergents are represented as;
(a fatty acid end) : CH3-(CH2)n-COO-Na+ : (water soluble end)
Therefore soaps and detergents have two main parts, which include the ionic end which is
hydrophilic. This is water attracting end and is soluble in water. The other end is non-polar
hydrocarbon chain which usually hydrophobic or water repelling end. This end is able to attract
dirt and get attached to them (Parent 18). In their working, these molecules are able to bridge
between water and dirt. They then break the dirt and form emulsions which consist of the dirt
Course:
Professor:
Date:
Soaps and detergents
Soaps and detergents are used to remove dirt. Dirt is oily in nature and this characteristic mean
that it is insoluble in water. Most of dirts are considered to be oily (Raymond 55). The sodium or
potassium salts which have long chain carboxylic acids in soaps help in removal of dirt. The
soaps have a carboxylic acid end on one side which is bonded by a metal ion end. The two major
parts of the soaps and detergents are the non-ionic hydrocarbon group and the ionic group of
COO-Na+. The soap and detergents components help to decompose the oily parts therefore
making them insoluble in water. The chains of chemical compositions in soaps and detergents
are responsible to removal of the oily parts and making them soluble in water. Soaps are cleaning
agents which have salts of fatty acids (Birdi 62). Detergents on the other hand are composed of
mixed chemical compositions which aid the cleaning factor. The components of soaps and
detergents are represented as;
(a fatty acid end) : CH3-(CH2)n-COO-Na+ : (water soluble end)
Therefore soaps and detergents have two main parts, which include the ionic end which is
hydrophilic. This is water attracting end and is soluble in water. The other end is non-polar
hydrocarbon chain which usually hydrophobic or water repelling end. This end is able to attract
dirt and get attached to them (Parent 18). In their working, these molecules are able to bridge
between water and dirt. They then break the dirt and form emulsions which consist of the dirt
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

droplets which are suspended in water. In their action, soaps and detergents chemical molecules
are able to reduce the surface tension of water and therefore reducing the interfacial tension
between dirt and water. This makes the dirt soluble in water and therefore be removed by water.
The ability of the soap parts to attract other molecules in this case that of dirt makes then brings
the cleaning effect (Ali, Ali and James 29).
In addition, being part of the surfactant category, the polar and non-polar ends are able to aid the
attraction of the dirt parts and lifting the stains off the fabrics (Anderson and David 73). This
allows the soaps and detergents to bring out the washing and rinsing effect. In addition, in their
actions, when dirty fabric is placed on water which has soap, the hydrocarbon ends of the soaps
molecules in the micelle attach to the dirt. The soap micelles are therefore able to entrap the dirt
particles using the hydrocarbon end. The other end which contains the ionic end remains
attached to water. Due to the action of the dirt particles on the surface of the fabric get dispersed
in the water and this enhances the cleaning effect. Therefore the actions of the soaps and
detergents are enhanced by the two ends, which include the hydrophilic and hydrophobic ends.
In addition, during the action, the two ends create a force which is opposing and therefore
loosening the dirt (Solway 51). Applying force on the fabric is able to agitate the freeing of the
dirt.
are able to reduce the surface tension of water and therefore reducing the interfacial tension
between dirt and water. This makes the dirt soluble in water and therefore be removed by water.
The ability of the soap parts to attract other molecules in this case that of dirt makes then brings
the cleaning effect (Ali, Ali and James 29).
In addition, being part of the surfactant category, the polar and non-polar ends are able to aid the
attraction of the dirt parts and lifting the stains off the fabrics (Anderson and David 73). This
allows the soaps and detergents to bring out the washing and rinsing effect. In addition, in their
actions, when dirty fabric is placed on water which has soap, the hydrocarbon ends of the soaps
molecules in the micelle attach to the dirt. The soap micelles are therefore able to entrap the dirt
particles using the hydrocarbon end. The other end which contains the ionic end remains
attached to water. Due to the action of the dirt particles on the surface of the fabric get dispersed
in the water and this enhances the cleaning effect. Therefore the actions of the soaps and
detergents are enhanced by the two ends, which include the hydrophilic and hydrophobic ends.
In addition, during the action, the two ends create a force which is opposing and therefore
loosening the dirt (Solway 51). Applying force on the fabric is able to agitate the freeing of the
dirt.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Introduction of ions in water makes water hard and this affected the action of soaps and
detergents in water. Ions such as calcium ions Ca2+ iron ions Fe2+, manganese Mn2+ and
magnesium Mg2+ among other may find their way into water and make it hard (William 25 and
Pugh 35). Their presence affects the functionality and action of soaps and detergents. In many
cases, the ions find their way when water flows through rocks and therefore extract them. The
cleaning effect of water is affected and soaps and detergent have to combine with ions first
before starting the cleaning effects of dirt. First, the chemical composition of the soaps and
detergents combine with the ions to form scum. The soap film or scum is insoluble in water and
it does not rinse away easily. Due to this action, the soap available for cleaning is reduced. In
other word, it is clear that the soap will clear the ions first before it starts cleaning out the dirt.
Therefore the presence of ions is able to affect the solubility rate of the soaps and detergents
(Satyanarayana 76). The main reasons are because the chemical components of the soaps and
detergents have to act on the ions first by neutralizing them and then present their cleaning
effects. In addition, introduction of ions in water increases the amount of soaps and detergents
which has to be used to bring out the required effect.
Since the detergents and soaps have a part which is water attracted, they can be considered to
have a particular degree of solubility. The water loving end will get attracted to water molecules
and therefore soaps and detergent combine with water. The presence of ions reduces the cleaning
effects of soaps and detergent (Brown, Brent, Eric and Christopher 73). In particular, their
solubility and compatibility with water molecules is affected by the presence of the ions. In
particular, soaps are either salts of long chains fatty acids of sodium or potassium. When the soap
is added to hard water which has the ions, the reaction of soaps and ions takes place first. The
detergents in water. Ions such as calcium ions Ca2+ iron ions Fe2+, manganese Mn2+ and
magnesium Mg2+ among other may find their way into water and make it hard (William 25 and
Pugh 35). Their presence affects the functionality and action of soaps and detergents. In many
cases, the ions find their way when water flows through rocks and therefore extract them. The
cleaning effect of water is affected and soaps and detergent have to combine with ions first
before starting the cleaning effects of dirt. First, the chemical composition of the soaps and
detergents combine with the ions to form scum. The soap film or scum is insoluble in water and
it does not rinse away easily. Due to this action, the soap available for cleaning is reduced. In
other word, it is clear that the soap will clear the ions first before it starts cleaning out the dirt.
Therefore the presence of ions is able to affect the solubility rate of the soaps and detergents
(Satyanarayana 76). The main reasons are because the chemical components of the soaps and
detergents have to act on the ions first by neutralizing them and then present their cleaning
effects. In addition, introduction of ions in water increases the amount of soaps and detergents
which has to be used to bring out the required effect.
Since the detergents and soaps have a part which is water attracted, they can be considered to
have a particular degree of solubility. The water loving end will get attracted to water molecules
and therefore soaps and detergent combine with water. The presence of ions reduces the cleaning
effects of soaps and detergent (Brown, Brent, Eric and Christopher 73). In particular, their
solubility and compatibility with water molecules is affected by the presence of the ions. In
particular, soaps are either salts of long chains fatty acids of sodium or potassium. When the soap
is added to hard water which has the ions, the reaction of soaps and ions takes place first. The
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

salt from the salt combines with the particular ions to form the respective precipitation ass a
scum.
Ca2+ + RCOONa = RCOOCa + 2Na+
Ion soap calcium salt (scum)
The reaction with the ions forms an insoluble precipitate. It is clear that the solubility of the soap
or detergent is affected by the presence of the ions (Urban 23). The high attraction between the
soap or detergent molecules with the ions reduces the soap or detergent solubility rate. The
solubility of soaps in water depends on the ionic part, which is soluble in water. The attraction of
this part to ions is more than its attraction to the water molecules. Therefore the introduction of
the ions in water will mean that the soap ionic part will first bond with the ion part and not the
water molecule (Rudnick 107). Since the combination of the ions and ionic part of the soap
forms a scum which is insoluble, the solubility of soap is highly reduced when the ions are
present in water.
On the other hand, detergents a basically similar to soaps but only differs little on the water
soluble portion (Raymond 109). There are three types of detergent as shown below;
scum.
Ca2+ + RCOONa = RCOOCa + 2Na+
Ion soap calcium salt (scum)
The reaction with the ions forms an insoluble precipitate. It is clear that the solubility of the soap
or detergent is affected by the presence of the ions (Urban 23). The high attraction between the
soap or detergent molecules with the ions reduces the soap or detergent solubility rate. The
solubility of soaps in water depends on the ionic part, which is soluble in water. The attraction of
this part to ions is more than its attraction to the water molecules. Therefore the introduction of
the ions in water will mean that the soap ionic part will first bond with the ion part and not the
water molecule (Rudnick 107). Since the combination of the ions and ionic part of the soap
forms a scum which is insoluble, the solubility of soap is highly reduced when the ions are
present in water.
On the other hand, detergents a basically similar to soaps but only differs little on the water
soluble portion (Raymond 109). There are three types of detergent as shown below;
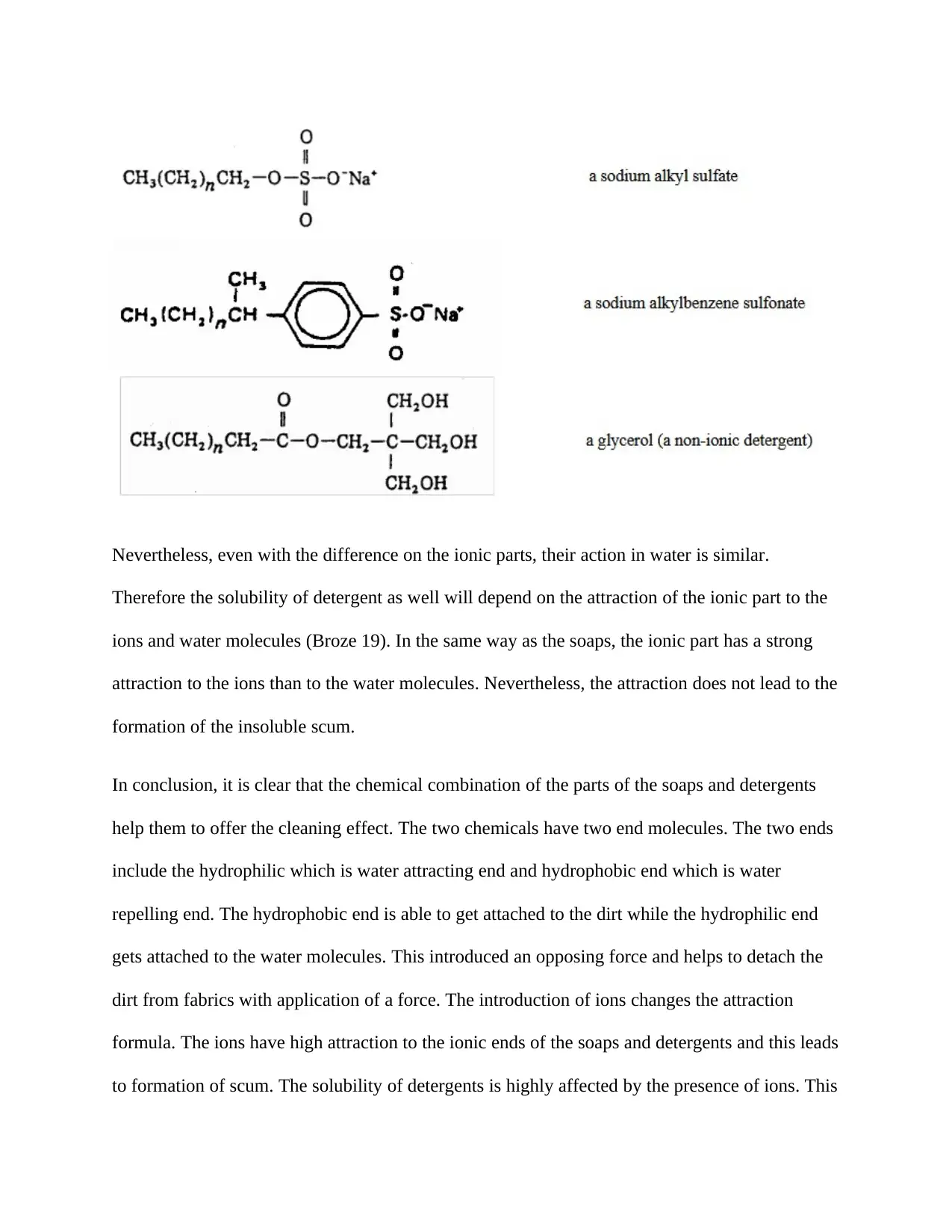
Nevertheless, even with the difference on the ionic parts, their action in water is similar.
Therefore the solubility of detergent as well will depend on the attraction of the ionic part to the
ions and water molecules (Broze 19). In the same way as the soaps, the ionic part has a strong
attraction to the ions than to the water molecules. Nevertheless, the attraction does not lead to the
formation of the insoluble scum.
In conclusion, it is clear that the chemical combination of the parts of the soaps and detergents
help them to offer the cleaning effect. The two chemicals have two end molecules. The two ends
include the hydrophilic which is water attracting end and hydrophobic end which is water
repelling end. The hydrophobic end is able to get attached to the dirt while the hydrophilic end
gets attached to the water molecules. This introduced an opposing force and helps to detach the
dirt from fabrics with application of a force. The introduction of ions changes the attraction
formula. The ions have high attraction to the ionic ends of the soaps and detergents and this leads
to formation of scum. The solubility of detergents is highly affected by the presence of ions. This
Therefore the solubility of detergent as well will depend on the attraction of the ionic part to the
ions and water molecules (Broze 19). In the same way as the soaps, the ionic part has a strong
attraction to the ions than to the water molecules. Nevertheless, the attraction does not lead to the
formation of the insoluble scum.
In conclusion, it is clear that the chemical combination of the parts of the soaps and detergents
help them to offer the cleaning effect. The two chemicals have two end molecules. The two ends
include the hydrophilic which is water attracting end and hydrophobic end which is water
repelling end. The hydrophobic end is able to get attached to the dirt while the hydrophilic end
gets attached to the water molecules. This introduced an opposing force and helps to detach the
dirt from fabrics with application of a force. The introduction of ions changes the attraction
formula. The ions have high attraction to the ionic ends of the soaps and detergents and this leads
to formation of scum. The solubility of detergents is highly affected by the presence of ions. This
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

is because the ionic parts of the soaps must first get attached to the ions presence before they
dissolve into water.
Bibliography
Ali, Mohammad F, Ali B. M. El, and James G. Speight. Handbook of Industrial Chemistry:
Organic Chemicals. New York: McGraw-Hill, 2014. Internet resource.
Anderson, John, and David Calder. Higher Chemistry. London: Hodder Education Group, 2016.
Internet resource.
Birdi, K Samul, Surface Chemistry Essentials. Boca Raton, FL: CRC Press, 2014. Print.
dissolve into water.
Bibliography
Ali, Mohammad F, Ali B. M. El, and James G. Speight. Handbook of Industrial Chemistry:
Organic Chemicals. New York: McGraw-Hill, 2014. Internet resource.
Anderson, John, and David Calder. Higher Chemistry. London: Hodder Education Group, 2016.
Internet resource.
Birdi, K Samul, Surface Chemistry Essentials. Boca Raton, FL: CRC Press, 2014. Print.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Brown, William H, Brent L. Iverson, Eric V. Anslyn, and Christopher S. Foote. Organic
Chemistry. , 2014. Print.
Broze, Guy, (ed.). Handbook of Detergents, Part A: Properties. Surfactant Science Series. New
York: M. Dekker, 2009.
Parent, K., Building a Better Bleach: A Green Chemistry Challenge. ChemMatters, 22 (2), 2010.
17–19.
Pugh, Robert J. Bubble and Foam Chemistry. Cambridge: Cambridge university Press, 2016.
Print.
Raymond G. Bistline, Jr., "Anionic and Related Lime Soap Dispersants," in Anionic surfactants:
organic chemistry, Helmut Stache, (ed.) CRC Press, 2011
Rudnick, Leslie R. Lubricant Additives: Chemistry and Applications, Third Edition. Portland:
CRC Press, 2017. Print.
Satyanarayana, Usha. Lipids. Elsevier Health Sciences APAC, 2014. Internet resource.
Solway, Andrew. Chemistry. , 2014. Internet resource.
Urban, David G. How to Formulate & Compound Industrial Detergents. BookSurge Publishing,
2013.
William Reusch, Application of Solubility: Soap, Virtual Textbook of Organic Chemistry.
Michigan State U. April 24, 2015, Print.
Chemistry. , 2014. Print.
Broze, Guy, (ed.). Handbook of Detergents, Part A: Properties. Surfactant Science Series. New
York: M. Dekker, 2009.
Parent, K., Building a Better Bleach: A Green Chemistry Challenge. ChemMatters, 22 (2), 2010.
17–19.
Pugh, Robert J. Bubble and Foam Chemistry. Cambridge: Cambridge university Press, 2016.
Print.
Raymond G. Bistline, Jr., "Anionic and Related Lime Soap Dispersants," in Anionic surfactants:
organic chemistry, Helmut Stache, (ed.) CRC Press, 2011
Rudnick, Leslie R. Lubricant Additives: Chemistry and Applications, Third Edition. Portland:
CRC Press, 2017. Print.
Satyanarayana, Usha. Lipids. Elsevier Health Sciences APAC, 2014. Internet resource.
Solway, Andrew. Chemistry. , 2014. Internet resource.
Urban, David G. How to Formulate & Compound Industrial Detergents. BookSurge Publishing,
2013.
William Reusch, Application of Solubility: Soap, Virtual Textbook of Organic Chemistry.
Michigan State U. April 24, 2015, Print.

⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

