BTEC L3 Applied Science: Solutions to Energy, Gas Laws & Algebra
VerifiedAdded on 2023/06/10
|7
|1291
|109
Homework Assignment
AI Summary
This assignment provides detailed solutions to a range of problems relevant to a BTEC Level 3 Extended Diploma in Applied Science. The problems cover topics including conduction, convection, and radiation, applying Stefan-Boltzmann's law and Fourier's Law to calculate heat transfer. It also includes the application of gas laws such as Boyle's Law, Charles's Law, and Gay-Lussac's Law to solve problems related to pressure, volume, and temperature changes in gases. Furthermore, the assignment addresses energy changes and efficiency, including calculations involving specific latent heat and specific heat capacity, as well as the use of logarithms and indices in scientific calculations related to gravitational constants and atomic speeds. The document also includes solutions for algebraic manipulations and calculus problems. Desklib offers a wealth of similar solved assignments and resources for students.

T4-: Conduction, Convection, Radiation
Task 2
1) Given-: Radius of Sun, r =6.69∗108 m
Temperature of Sun, T =5778 K
Solution-: According to Stefan- Boltzman, emissive power of black body-:
E=σA T 4 W ,W h ere σ=5.67∗10−8 W
m2 K 4
E=σ∗π r2∗T 4
E=5.67∗10−8∗π∗( 6.69∗108 )2
∗( 5778 )4
E=8.886∗1025 W
2) Given-: Room Temperature, T r=292 K
Outdoor Temperature, T 0=274 K
Area of glass window, A=6 m2
Uniform Thickness, t=4 mm=4∗10−3 m
Thermal Conductivity, k =0.8 W
mK
Solution-: From Fourier’s Law-:
Q=−kA T 0−T r
t
Q=−0.8∗6∗(274−292)
4∗10−3
Q=21.6∗103 W
3) Given-: Temperature difference, dT =20 K
Area of glass window, A=2 m2
Uniform Thickness, t=8∗10−3 m
Thermal Conductivity, k =0.8 W
mK
Solution-: From Fourier’s Law-:
Q=kA dT
t
Q= 0.8∗2∗20
8∗10−3
Q=4∗103 W
4) Given-:Surface area of radiator, A=4 m2
Room Temperature, T r=20 ℃=293 K
Water Temperature, T w=60 ℃=323 K
Emissivity of radiator surface, ϵ=0.4
Solution-: According to Stefan- Boltzman, emissive power of non-black body-:
E=ϵσA T4 W , W h ere σ =5.67∗10−8 W
m2 K4
E=ϵ∗σ∗A∗(T w
4−T r
4 )
Task 2
1) Given-: Radius of Sun, r =6.69∗108 m
Temperature of Sun, T =5778 K
Solution-: According to Stefan- Boltzman, emissive power of black body-:
E=σA T 4 W ,W h ere σ=5.67∗10−8 W
m2 K 4
E=σ∗π r2∗T 4
E=5.67∗10−8∗π∗( 6.69∗108 )2
∗( 5778 )4
E=8.886∗1025 W
2) Given-: Room Temperature, T r=292 K
Outdoor Temperature, T 0=274 K
Area of glass window, A=6 m2
Uniform Thickness, t=4 mm=4∗10−3 m
Thermal Conductivity, k =0.8 W
mK
Solution-: From Fourier’s Law-:
Q=−kA T 0−T r
t
Q=−0.8∗6∗(274−292)
4∗10−3
Q=21.6∗103 W
3) Given-: Temperature difference, dT =20 K
Area of glass window, A=2 m2
Uniform Thickness, t=8∗10−3 m
Thermal Conductivity, k =0.8 W
mK
Solution-: From Fourier’s Law-:
Q=kA dT
t
Q= 0.8∗2∗20
8∗10−3
Q=4∗103 W
4) Given-:Surface area of radiator, A=4 m2
Room Temperature, T r=20 ℃=293 K
Water Temperature, T w=60 ℃=323 K
Emissivity of radiator surface, ϵ=0.4
Solution-: According to Stefan- Boltzman, emissive power of non-black body-:
E=ϵσA T4 W , W h ere σ =5.67∗10−8 W
m2 K4
E=ϵ∗σ∗A∗(T w
4−T r
4 )
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
E=0.4∗5.67∗10−8∗4∗(3234 −2934 )
E=318.83W
T6-: Energy Changes and Efficiency
Task 1
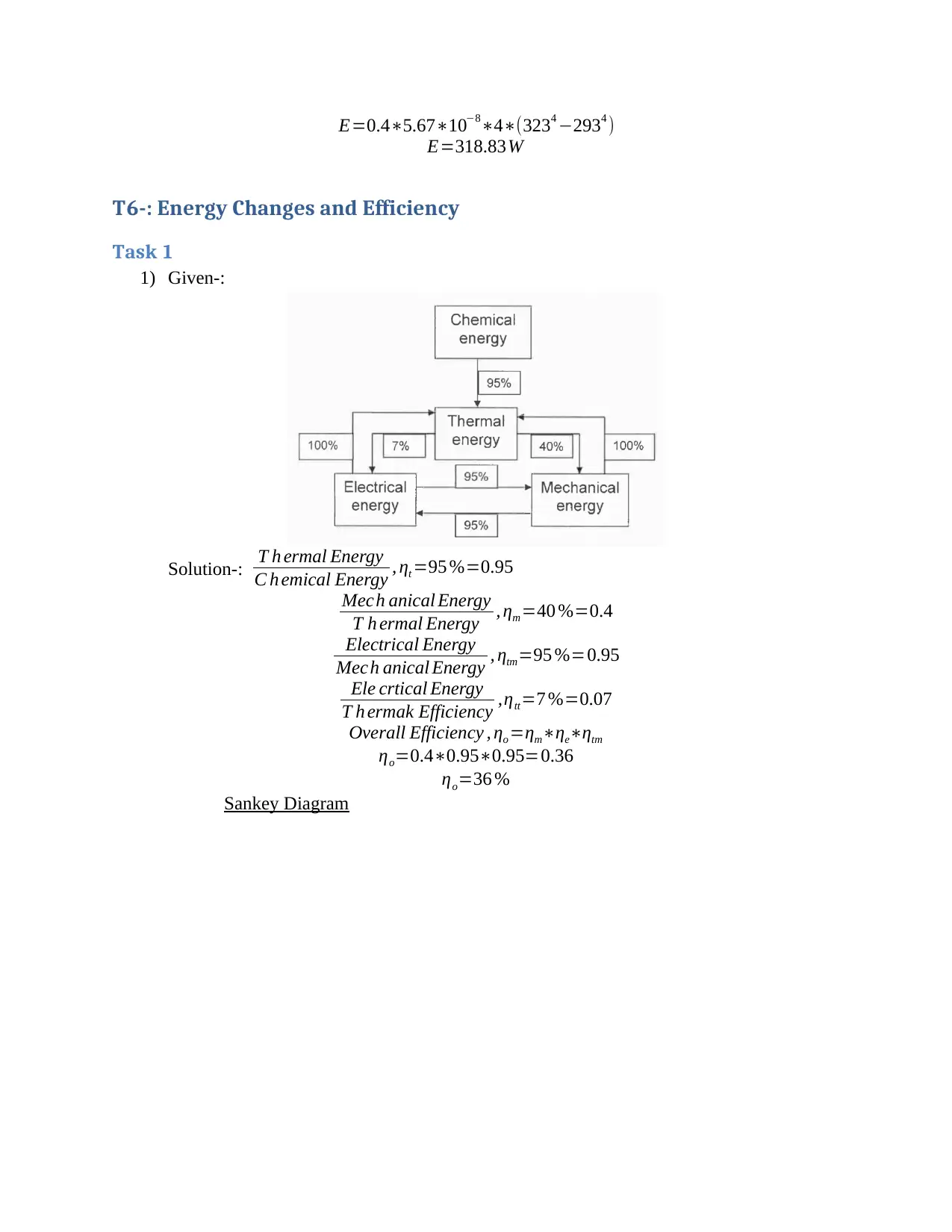
1) Given-:
Solution-: T h ermal Energy
C h emical Energy , ηt =95 %=0.95
Mech anical Energy
T h ermal Energy , ηm =40 %=0.4
Electrical Energy
Mech anical Energy , ηtm=95 %=0.95
Ele crtical Energy
T h ermak Efficiency ,ηtt=7 %=0.07
Overall Efficiency , ηo =ηm∗ηe∗ηtm
ηo=0.4∗0.95∗0.95=0.36
ηo=36 %
Sankey Diagram
E=318.83W
T6-: Energy Changes and Efficiency
Task 1
1) Given-:
Solution-: T h ermal Energy
C h emical Energy , ηt =95 %=0.95
Mech anical Energy
T h ermal Energy , ηm =40 %=0.4
Electrical Energy
Mech anical Energy , ηtm=95 %=0.95
Ele crtical Energy
T h ermak Efficiency ,ηtt=7 %=0.07
Overall Efficiency , ηo =ηm∗ηe∗ηtm
ηo=0.4∗0.95∗0.95=0.36
ηo=36 %
Sankey Diagram
Task 2
1) Given-:Specific heat capacity of water,hw=4.2 J
g ℃
Specific latent heat of water, Lw=2300 J
g
Specific heat capacity of ice, hi =2.0 J
g ℃
Specific latent heat of ice, Li=340 J
g
Solution-: a) As per given-:
Volume of water, V w=2liter=0.002 m3
mass of water,mw=Density∗Volume=ρ∗V w
mw=1000∗0.002=2 kg=2000 g
Energy to evaporate water at 100 ℃,
E=mw∗Lw
E=2000∗2300=4.6∗106 J
b) mass of water,mw=0.5 kg=500 g
Energy to freeze water at 0 ℃,
E=mw∗Li
E=500∗340=1.7∗105 J
2) Given-:Specific heat capacity of water,hw=4.2 J
g ℃
Specific latent heat of water, Lw=2300 J
g
Specific heat capacity of ice, hi =2.0 J
g ℃
Specific latent heat of ice, Li=340 J
g
1) Given-:Specific heat capacity of water,hw=4.2 J
g ℃
Specific latent heat of water, Lw=2300 J
g
Specific heat capacity of ice, hi =2.0 J
g ℃
Specific latent heat of ice, Li=340 J
g
Solution-: a) As per given-:
Volume of water, V w=2liter=0.002 m3
mass of water,mw=Density∗Volume=ρ∗V w
mw=1000∗0.002=2 kg=2000 g
Energy to evaporate water at 100 ℃,
E=mw∗Lw
E=2000∗2300=4.6∗106 J
b) mass of water,mw=0.5 kg=500 g
Energy to freeze water at 0 ℃,
E=mw∗Li
E=500∗340=1.7∗105 J
2) Given-:Specific heat capacity of water,hw=4.2 J
g ℃
Specific latent heat of water, Lw=2300 J
g
Specific heat capacity of ice, hi =2.0 J
g ℃
Specific latent heat of ice, Li=340 J
g
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

mass, m=20 g
Solution-: Heat needed to change ice at 373 K to steam at 273 K
E=m Li +m hw (373−273 ) +m Lw
E=m( Li+ hw ( 100−0 ) +Lw)
E=20∗ (340+4.2∗100+ 2300 )
E=20∗3060
E=61200 J
3) Given-:Specific heat capacity of water,hw=4.2 J
g ℃
Specific heat capacity of ice, hi =2.0 J
g ℃
Specific latent heat of ice, Li=340 J
g
Specific heat capacity of aluminum, ha=0.9 J
g ℃
mass of aluminum, ma=100 g
mass of water, mw=200 g
Solution-: Heat needed to change ice at 373 K to steam at 273 K
E=ma ha ( 288−268 ) +mw hi ( 273−268 ) +mw Li+mw hw ( 288−273 )
E=ma ha∗20+ mw((hi∗5)+ Li +(hw∗15))
E= ( 100∗0.9∗20 ) +200∗( ( 2∗5 ) +340+(4.2∗15) )
E=1800+82600
E=84400 J
T1-: Logarithms and indices
Task 2
1) Given-:Gravitational Constant, G=6.67∗10−11 N m2 k g−2
Mass of earth, m=5.975∗1024 kg
Time taken to orbit the earth, t=27.3 days
t=27.3∗24∗60∗60=2358720 sec
Solution-: Distance from the centre of planet to centre of satellite, r-:
T = √ 4 π2 r3
GM
r =3
√ T 2 GM
4 π 2
r =3
√ ( 2358720)2∗6.67∗10−7∗5.575∗1024
4 π2
r =25.1∗1011 m
2) Given-:Pressure of gas, p=1∗105 Pa
Mass of atoms, m=6.6∗10−27 kg
Number of atoms, n=2∗1022
Solution-: Heat needed to change ice at 373 K to steam at 273 K
E=m Li +m hw (373−273 ) +m Lw
E=m( Li+ hw ( 100−0 ) +Lw)
E=20∗ (340+4.2∗100+ 2300 )
E=20∗3060
E=61200 J
3) Given-:Specific heat capacity of water,hw=4.2 J
g ℃
Specific heat capacity of ice, hi =2.0 J
g ℃
Specific latent heat of ice, Li=340 J
g
Specific heat capacity of aluminum, ha=0.9 J
g ℃
mass of aluminum, ma=100 g
mass of water, mw=200 g
Solution-: Heat needed to change ice at 373 K to steam at 273 K
E=ma ha ( 288−268 ) +mw hi ( 273−268 ) +mw Li+mw hw ( 288−273 )
E=ma ha∗20+ mw((hi∗5)+ Li +(hw∗15))
E= ( 100∗0.9∗20 ) +200∗( ( 2∗5 ) +340+(4.2∗15) )
E=1800+82600
E=84400 J
T1-: Logarithms and indices
Task 2
1) Given-:Gravitational Constant, G=6.67∗10−11 N m2 k g−2
Mass of earth, m=5.975∗1024 kg
Time taken to orbit the earth, t=27.3 days
t=27.3∗24∗60∗60=2358720 sec
Solution-: Distance from the centre of planet to centre of satellite, r-:
T = √ 4 π2 r3
GM
r =3
√ T 2 GM
4 π 2
r =3
√ ( 2358720)2∗6.67∗10−7∗5.575∗1024
4 π2
r =25.1∗1011 m
2) Given-:Pressure of gas, p=1∗105 Pa
Mass of atoms, m=6.6∗10−27 kg
Number of atoms, n=2∗1022
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Volume of flask, V =7∗10−5 m3
Solution-:a) Mean square speed of atoms,
c2= 3∗pV
Nm
c2= 3∗105∗7∗10−5
2∗1022∗6.6∗10−27
c2=159090.9 m2
s2
b) r.m.s speed of a typical atoms,
r . m. s speed = √c2
r . m. s speed= √ 159090.9
r . m. s speed=398.86 m
s
T5-: The Gas Laws
Task 2 for M2
1) Given-:Initial volume of gas, ν1 =200 c m3=0.0002 m3
Initial Temperature, T 1=27 ℃=300 K
Initial Pressure, p1=1 atm .=101.3 kPa
Solution-: a) Final pressure is doubled at constant temperature
Final Pressure, p2=2 p1=202.6 kPa
According to Boyle’s law at constant Temperature
p1 ν1= p2 ν2
ν2= p1∗ν1
p2
ν2=101.3∗0.0002
202.6
ν2=0.0001 m3
b) Absolute temperature is double at constant pressure
Final Temperature, T 2=2 T1=600 K
According to Charles law at constant pressure
ν1
T1
= ν2
T2
ν2=T 2∗ν1
T 1
ν2=600∗0.0002
300
ν2=0.0004 m3
c) Final pressure, p2=1.5 atm .=¿151.95kPa
Final Temperature, T 2=127 ℃=400 K
Solution-:a) Mean square speed of atoms,
c2= 3∗pV
Nm
c2= 3∗105∗7∗10−5
2∗1022∗6.6∗10−27
c2=159090.9 m2
s2
b) r.m.s speed of a typical atoms,
r . m. s speed = √c2
r . m. s speed= √ 159090.9
r . m. s speed=398.86 m
s
T5-: The Gas Laws
Task 2 for M2
1) Given-:Initial volume of gas, ν1 =200 c m3=0.0002 m3
Initial Temperature, T 1=27 ℃=300 K
Initial Pressure, p1=1 atm .=101.3 kPa
Solution-: a) Final pressure is doubled at constant temperature
Final Pressure, p2=2 p1=202.6 kPa
According to Boyle’s law at constant Temperature
p1 ν1= p2 ν2
ν2= p1∗ν1
p2
ν2=101.3∗0.0002
202.6
ν2=0.0001 m3
b) Absolute temperature is double at constant pressure
Final Temperature, T 2=2 T1=600 K
According to Charles law at constant pressure
ν1
T1
= ν2
T2
ν2=T 2∗ν1
T 1
ν2=600∗0.0002
300
ν2=0.0004 m3
c) Final pressure, p2=1.5 atm .=¿151.95kPa
Final Temperature, T 2=127 ℃=400 K
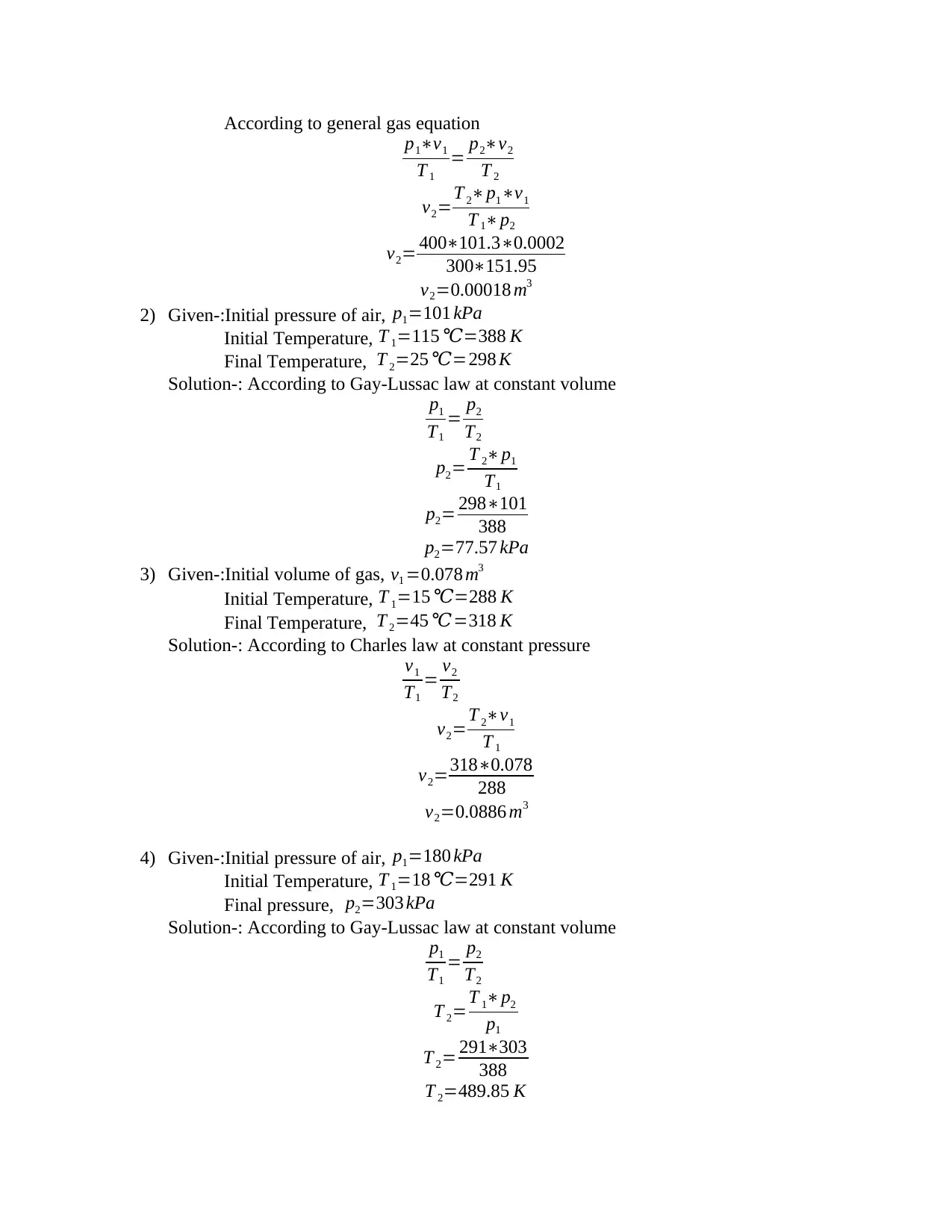
According to general gas equation
p1∗ν1
T 1
= p2∗ν2
T 2
ν2=T 2∗p1∗ν1
T 1∗p2
ν2= 400∗101.3∗0.0002
300∗151.95
ν2=0.00018 m3
2) Given-:Initial pressure of air, p1=101 kPa
Initial Temperature, T 1=115 ℃=388 K
Final Temperature, T 2=25 ℃=298 K
Solution-: According to Gay-Lussac law at constant volume
p1
T1
= p2
T2
p2= T 2∗p1
T1
p2= 298∗101
388
p2=77.57 kPa
3) Given-:Initial volume of gas, v1 =0.078 m3
Initial Temperature, T 1=15 ℃=288 K
Final Temperature, T 2=45 ℃=318 K
Solution-: According to Charles law at constant pressure
ν1
T1
= ν2
T2
ν2=T 2∗ν1
T 1
ν2=318∗0.078
288
ν2=0.0886 m3
4) Given-:Initial pressure of air, p1=180 kPa
Initial Temperature, T 1=18 ℃=291 K
Final pressure, p2=303 kPa
Solution-: According to Gay-Lussac law at constant volume
p1
T1
= p2
T2
T 2=T 1∗p2
p1
T 2= 291∗303
388
T 2=489.85 K
p1∗ν1
T 1
= p2∗ν2
T 2
ν2=T 2∗p1∗ν1
T 1∗p2
ν2= 400∗101.3∗0.0002
300∗151.95
ν2=0.00018 m3
2) Given-:Initial pressure of air, p1=101 kPa
Initial Temperature, T 1=115 ℃=388 K
Final Temperature, T 2=25 ℃=298 K
Solution-: According to Gay-Lussac law at constant volume
p1
T1
= p2
T2
p2= T 2∗p1
T1
p2= 298∗101
388
p2=77.57 kPa
3) Given-:Initial volume of gas, v1 =0.078 m3
Initial Temperature, T 1=15 ℃=288 K
Final Temperature, T 2=45 ℃=318 K
Solution-: According to Charles law at constant pressure
ν1
T1
= ν2
T2
ν2=T 2∗ν1
T 1
ν2=318∗0.078
288
ν2=0.0886 m3
4) Given-:Initial pressure of air, p1=180 kPa
Initial Temperature, T 1=18 ℃=291 K
Final pressure, p2=303 kPa
Solution-: According to Gay-Lussac law at constant volume
p1
T1
= p2
T2
T 2=T 1∗p2
p1
T 2= 291∗303
388
T 2=489.85 K
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
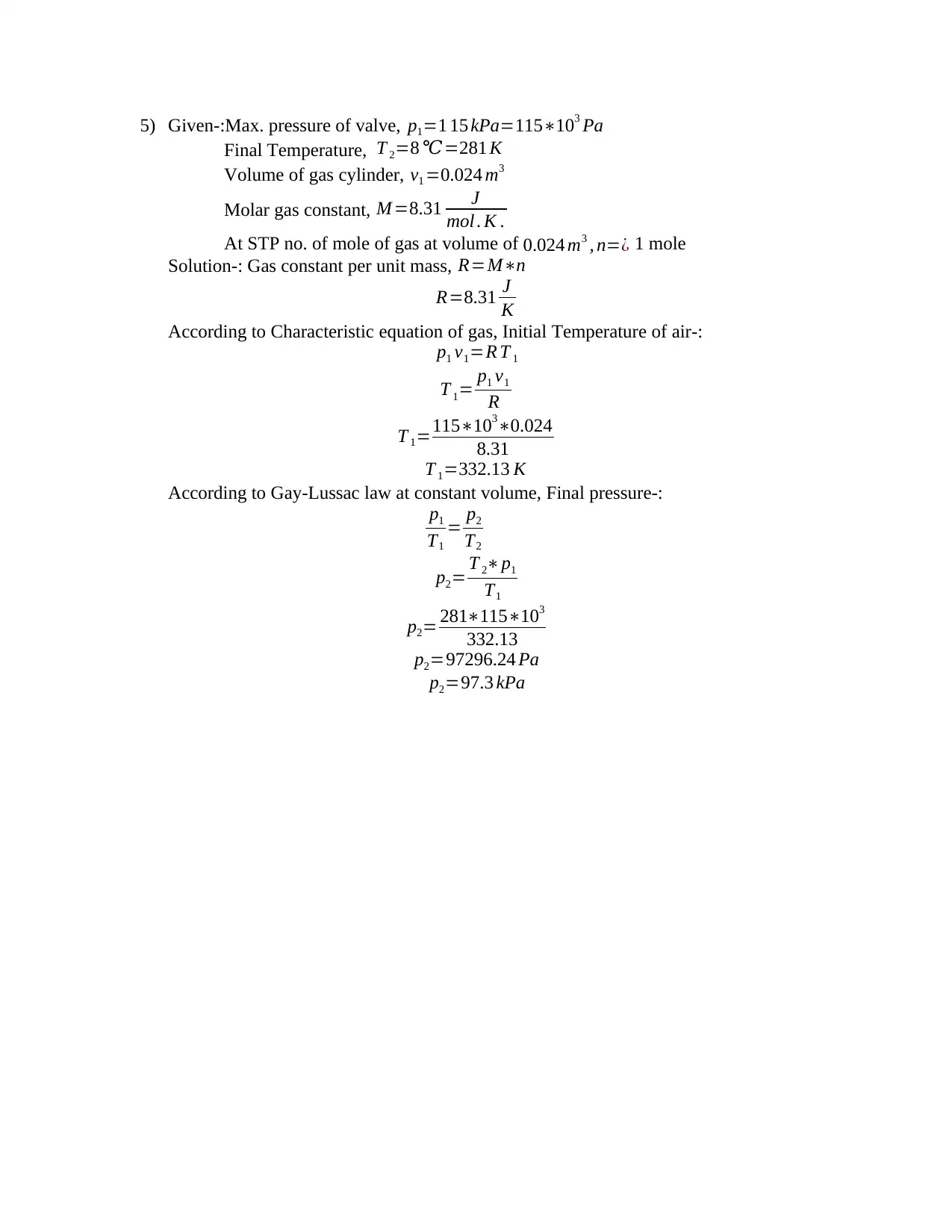
5) Given-:Max. pressure of valve, p1=1 15 kPa=115∗103 Pa
Final Temperature, T 2=8 ℃=281 K
Volume of gas cylinder, v1 =0.024 m3
Molar gas constant, M =8.31 J
mol . K .
At STP no. of mole of gas at volume of 0.024 m3 , n=¿ 1 mole
Solution-: Gas constant per unit mass, R=M∗n
R=8.31 J
K
According to Characteristic equation of gas, Initial Temperature of air-:
p1 v1=R T 1
T 1= p1 v1
R
T 1=115∗103∗0.024
8.31
T 1=332.13 K
According to Gay-Lussac law at constant volume, Final pressure-:
p1
T1
= p2
T2
p2= T 2∗p1
T1
p2= 281∗115∗103
332.13
p2=97296.24 Pa
p2=97.3 kPa
Final Temperature, T 2=8 ℃=281 K
Volume of gas cylinder, v1 =0.024 m3
Molar gas constant, M =8.31 J
mol . K .
At STP no. of mole of gas at volume of 0.024 m3 , n=¿ 1 mole
Solution-: Gas constant per unit mass, R=M∗n
R=8.31 J
K
According to Characteristic equation of gas, Initial Temperature of air-:
p1 v1=R T 1
T 1= p1 v1
R
T 1=115∗103∗0.024
8.31
T 1=332.13 K
According to Gay-Lussac law at constant volume, Final pressure-:
p1
T1
= p2
T2
p2= T 2∗p1
T1
p2= 281∗115∗103
332.13
p2=97296.24 Pa
p2=97.3 kPa
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





