Standard Operating Procedure for Ear Swab Analysis in Microbiology Lab
VerifiedAdded on 2022/11/01
|10
|1780
|368
Practical Assignment
AI Summary
This assignment presents a comprehensive Standard Operating Procedure (SOP) for the analysis of ear swabs in a microbiology laboratory. The SOP outlines the aim, which is to describe the analysis of ear swabs, and the principle, which discusses the various microorganisms causing ear infections, including Otitis externa and Otitis media. It details the methods, including apparatus and reagents, specimen collection, storage and transport, and sample rejection criteria. The procedure covers microscopic examination, culture methods, and interpretation of results, including antimicrobial susceptibility testing. The SOP emphasizes quality assurance, limitations, and reporting requirements, providing a complete guide for accurate ear swab analysis. The document also includes risk assessments, emergency procedures, and waste disposal protocols. This document is a valuable resource for understanding and performing ear swab analysis in a clinical setting.

Running head: SOP FOR EAR SWABS ANALSIS 1
Standard Operating Procedure for Ear Swab Analysis
Name of Author
Institution
Date of Submission
Standard Operating Procedure for Ear Swab Analysis
Name of Author
Institution
Date of Submission
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SOP FOR EAR SWAB DOCUMENT VERSION/NO: 2
MICROBIOLOGY STANDARD OPERATING PROCEDURE
Analysis of Ear Swabs
Document Version/No:
Replaces Document:
Applies to:
Modified By:
Reviewed and Approved by:
Created on:
Date of Revision:
Date of Review:
1. Aim
To describe the Analysis of ear swabs
2. Principle
There is a wide range of microorganisms that can cause infections to both the middle and
external ear. Infections of the external auditory canal are referred to as Otitis externa.
Notably, most of the pathogens causing ear infections are similar to those causing skin
and soft tissue infections (Geyer et al., 2011). A localized Acute Otitis externa is caused
by either Staphylococcus aureus or Group A Streptococcus on the other hand a diffuse
acute externa is caused by either Steptococccus aureus or Pseudomonas aeruginosa.
Chronic Otitis externa develops due to colonization of the ear with coliforms and fungi
like Aspergillus niger (Khattak, Sheikh & Aleem, 2017). Diabetic patients and those with
compromised immune system suffer the risk of malignant Otitis externa caused by
invasive P. aeruginosa. Consequently, Acute Otitis Media is the infection and
inflammation of the middle ear caused by migration of pathogens from the upper
MICROBIOLOGY STANDARD OPERATING PROCEDURE
Analysis of Ear Swabs
Document Version/No:
Replaces Document:
Applies to:
Modified By:
Reviewed and Approved by:
Created on:
Date of Revision:
Date of Review:
1. Aim
To describe the Analysis of ear swabs
2. Principle
There is a wide range of microorganisms that can cause infections to both the middle and
external ear. Infections of the external auditory canal are referred to as Otitis externa.
Notably, most of the pathogens causing ear infections are similar to those causing skin
and soft tissue infections (Geyer et al., 2011). A localized Acute Otitis externa is caused
by either Staphylococcus aureus or Group A Streptococcus on the other hand a diffuse
acute externa is caused by either Steptococccus aureus or Pseudomonas aeruginosa.
Chronic Otitis externa develops due to colonization of the ear with coliforms and fungi
like Aspergillus niger (Khattak, Sheikh & Aleem, 2017). Diabetic patients and those with
compromised immune system suffer the risk of malignant Otitis externa caused by
invasive P. aeruginosa. Consequently, Acute Otitis Media is the infection and
inflammation of the middle ear caused by migration of pathogens from the upper

SOP FOR EAR SWAB DOCUMENT VERSION/NO: 3
respiratory tract flora like Moraxella catarrhalis. In addition, chronic suppurative otitis is
caused by P. aeruginosa and S.aureus (Khan et al., 2019).
3. Methods
3.1. Apparatus and Reagents
Amies transport medium
MacConkey Media
Sterile Swabs
Clean glass slides
Innoculating Loop
Bunsen Burner
Microscope
18-24 hour cultured organisms
Immersion oil and distilled water.
Safranin, ethyl Alcohol, Grams Iodine, and Crystal violet stain.
3.2. Collection of Specimen
The optimal time for the ear swab specimen collection is before any antimicrobial
therapy administration and when pus can be visualized. The swab is placed on the outer
ear and then rotated gently to avoid any trauma to the ear to collect the secretions. The
respiratory tract flora like Moraxella catarrhalis. In addition, chronic suppurative otitis is
caused by P. aeruginosa and S.aureus (Khan et al., 2019).
3. Methods
3.1. Apparatus and Reagents
Amies transport medium
MacConkey Media
Sterile Swabs
Clean glass slides
Innoculating Loop
Bunsen Burner
Microscope
18-24 hour cultured organisms
Immersion oil and distilled water.
Safranin, ethyl Alcohol, Grams Iodine, and Crystal violet stain.
3.2. Collection of Specimen
The optimal time for the ear swab specimen collection is before any antimicrobial
therapy administration and when pus can be visualized. The swab is placed on the outer
ear and then rotated gently to avoid any trauma to the ear to collect the secretions. The
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

SOP FOR EAR SWAB DOCUMENT VERSION/NO: 4
entire sample specimen should be collected using sterile swabs and placed in Amies
transport medium with charcoal. Any swab or exudates is swabbed during the specimen
collection (International Standard, ISO 6887-1, 2017).
3.3. Storage and transport of Specimen
The collected specimen is transported using the Amies transport medium with charcoal.
The entire collected specimen should be stored and transported in sealed plastic bags to
the microbiology laboratory. The laboratory procedures should be undertaken as soon as
possible upon collection of the specimen. The collected samples are refrigerated if any
delays of over 2 hours are foreseeable (International Standard, ISO 6887-1, 2017).
3.4. Sample Rejection
The following samples are rejected by the laboratory and cannot proceed to processing.
These include improperly labeled samples and samples that have taken more than 2 hours
in transit after collection and were not refrigerated. Further, any specimen that is not
submitted in the appropriate Amies transport media, and any sample with detectable
external contamination would be rejected and the physician or clinician duly informed to
take another sample for analysis (Pereira, 2017).
3.5. Processing of the Specimen
3.5.1. Reception
The samples are received and record in the laboratory specimen book and then get
assigned a unique identity according to the laboratory protocols. The labels should be
associated with technician’s initials for accountability purposes. Further, the date of
entire sample specimen should be collected using sterile swabs and placed in Amies
transport medium with charcoal. Any swab or exudates is swabbed during the specimen
collection (International Standard, ISO 6887-1, 2017).
3.3. Storage and transport of Specimen
The collected specimen is transported using the Amies transport medium with charcoal.
The entire collected specimen should be stored and transported in sealed plastic bags to
the microbiology laboratory. The laboratory procedures should be undertaken as soon as
possible upon collection of the specimen. The collected samples are refrigerated if any
delays of over 2 hours are foreseeable (International Standard, ISO 6887-1, 2017).
3.4. Sample Rejection
The following samples are rejected by the laboratory and cannot proceed to processing.
These include improperly labeled samples and samples that have taken more than 2 hours
in transit after collection and were not refrigerated. Further, any specimen that is not
submitted in the appropriate Amies transport media, and any sample with detectable
external contamination would be rejected and the physician or clinician duly informed to
take another sample for analysis (Pereira, 2017).
3.5. Processing of the Specimen
3.5.1. Reception
The samples are received and record in the laboratory specimen book and then get
assigned a unique identity according to the laboratory protocols. The labels should be
associated with technician’s initials for accountability purposes. Further, the date of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
SOP FOR EAR SWAB DOCUMENT VERSION/NO: 5
specimen collection, as well as the location of collection, is recorded at the reception
(Hailu et al., 2016; Pacholewicz et al., 2013).
3.5.2. Microscopic Examination
Using a sterile pipette, one drop of the fluid containing specimen is placed on a clean
microscope slide. A drop of the specimen is then spread using a sterile loop for the gram
staining procedure (Guzel & Guner, 2009).
3.5.3. Culture
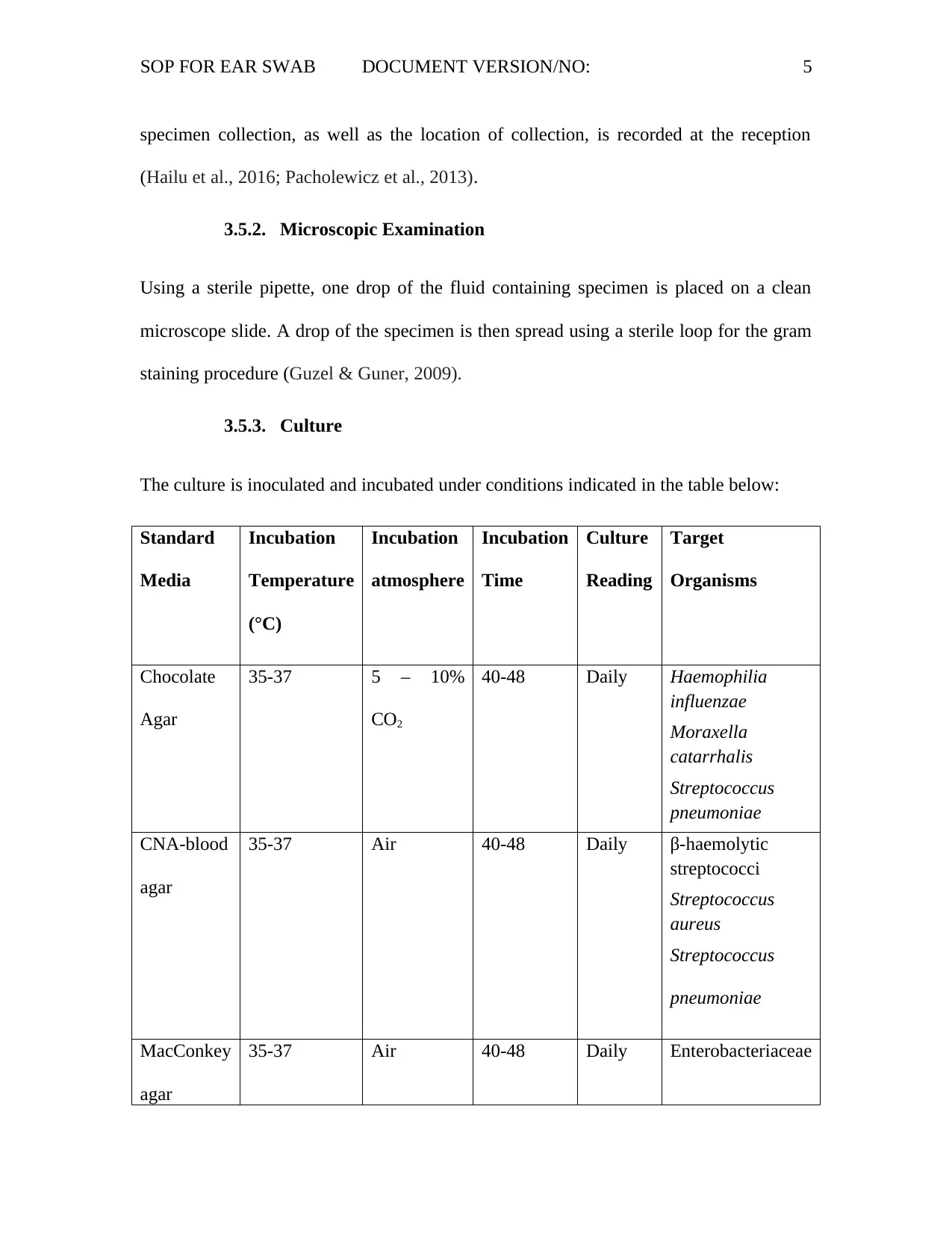
The culture is inoculated and incubated under conditions indicated in the table below:
Standard
Media
Incubation
Temperature
(°C)
Incubation
atmosphere
Incubation
Time
Culture
Reading
Target
Organisms
Chocolate
Agar
35-37 5 – 10%
CO2
40-48 Daily Haemophilia
influenzae
Moraxella
catarrhalis
Streptococcus
pneumoniae
CNA-blood
agar
35-37 Air 40-48 Daily β-haemolytic
streptococci
Streptococcus
aureus
Streptococcus
pneumoniae
MacConkey
agar
35-37 Air 40-48 Daily Enterobacteriaceae
specimen collection, as well as the location of collection, is recorded at the reception
(Hailu et al., 2016; Pacholewicz et al., 2013).
3.5.2. Microscopic Examination
Using a sterile pipette, one drop of the fluid containing specimen is placed on a clean
microscope slide. A drop of the specimen is then spread using a sterile loop for the gram
staining procedure (Guzel & Guner, 2009).
3.5.3. Culture
The culture is inoculated and incubated under conditions indicated in the table below:
Standard
Media
Incubation
Temperature
(°C)
Incubation
atmosphere
Incubation
Time
Culture
Reading
Target
Organisms
Chocolate
Agar
35-37 5 – 10%
CO2
40-48 Daily Haemophilia
influenzae
Moraxella
catarrhalis
Streptococcus
pneumoniae
CNA-blood
agar
35-37 Air 40-48 Daily β-haemolytic
streptococci
Streptococcus
aureus
Streptococcus
pneumoniae
MacConkey
agar
35-37 Air 40-48 Daily Enterobacteriaceae
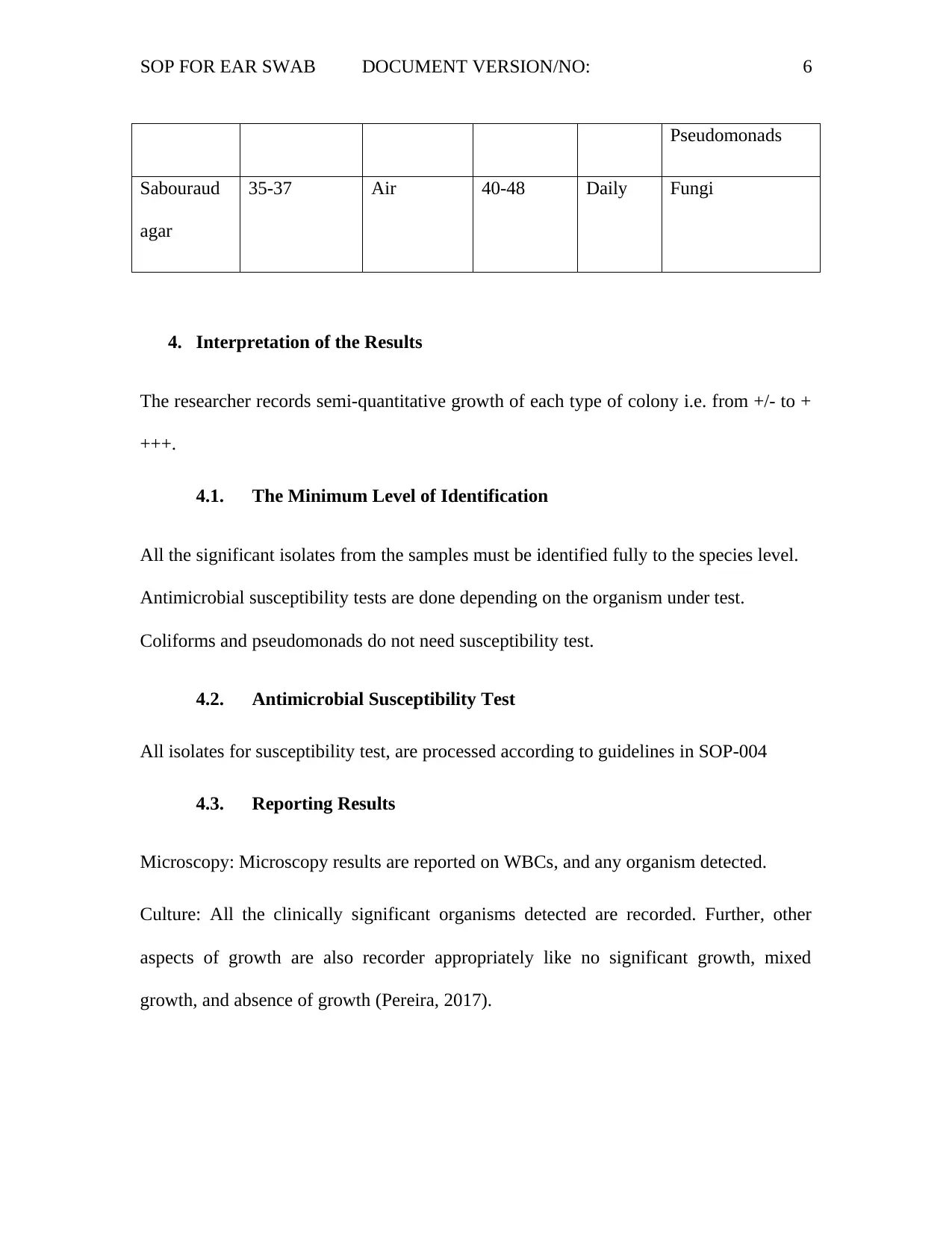
SOP FOR EAR SWAB DOCUMENT VERSION/NO: 6
Pseudomonads
Sabouraud
agar
35-37 Air 40-48 Daily Fungi
4. Interpretation of the Results
The researcher records semi-quantitative growth of each type of colony i.e. from +/- to +
+++.
4.1. The Minimum Level of Identification
All the significant isolates from the samples must be identified fully to the species level.
Antimicrobial susceptibility tests are done depending on the organism under test.
Coliforms and pseudomonads do not need susceptibility test.
4.2. Antimicrobial Susceptibility Test
All isolates for susceptibility test, are processed according to guidelines in SOP-004
4.3. Reporting Results
Microscopy: Microscopy results are reported on WBCs, and any organism detected.
Culture: All the clinically significant organisms detected are recorded. Further, other
aspects of growth are also recorder appropriately like no significant growth, mixed
growth, and absence of growth (Pereira, 2017).
Pseudomonads
Sabouraud
agar
35-37 Air 40-48 Daily Fungi
4. Interpretation of the Results
The researcher records semi-quantitative growth of each type of colony i.e. from +/- to +
+++.
4.1. The Minimum Level of Identification
All the significant isolates from the samples must be identified fully to the species level.
Antimicrobial susceptibility tests are done depending on the organism under test.
Coliforms and pseudomonads do not need susceptibility test.
4.2. Antimicrobial Susceptibility Test
All isolates for susceptibility test, are processed according to guidelines in SOP-004
4.3. Reporting Results
Microscopy: Microscopy results are reported on WBCs, and any organism detected.
Culture: All the clinically significant organisms detected are recorded. Further, other
aspects of growth are also recorder appropriately like no significant growth, mixed
growth, and absence of growth (Pereira, 2017).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

SOP FOR EAR SWAB DOCUMENT VERSION/NO: 7
5. Quality Assurance
The quality controls for the laboratory are negative controls of media with no inoculum
while the positive control is media inoculated with a commercial pure strain of the
organism for (Pereira, 2017). There is a plan to contact and external laboratory for
reference purposes and quality control (Guzel & Guner, 2009).
6. Limitations
It should be noted that the use of an antimicrobial agent prior to sample collection could
lead to negative results.
7. Reporting Requirements
Pseudomonas species are often reported. However, their antibiotic susceptibility can only
be reported in case of confirmed severity.
8. References
Geyer, M., Howell-Jones, R., Cunningham, R., & McNulty, C. (2011). Consensus of
microbiology reporting of ear swab results to primary care clinicians in patients
with otitis externa. British journal of biomedical science, 68(4), 174-180.
Guzel, O., & Guner, E. I. (2009). ISO 15189 accreditation: Requirements for quality and
competence of medical laboratories, experience of a laboratory I. Clinical
biochemistry, 42(4-5), 274-278.
5. Quality Assurance
The quality controls for the laboratory are negative controls of media with no inoculum
while the positive control is media inoculated with a commercial pure strain of the
organism for (Pereira, 2017). There is a plan to contact and external laboratory for
reference purposes and quality control (Guzel & Guner, 2009).
6. Limitations
It should be noted that the use of an antimicrobial agent prior to sample collection could
lead to negative results.
7. Reporting Requirements
Pseudomonas species are often reported. However, their antibiotic susceptibility can only
be reported in case of confirmed severity.
8. References
Geyer, M., Howell-Jones, R., Cunningham, R., & McNulty, C. (2011). Consensus of
microbiology reporting of ear swab results to primary care clinicians in patients
with otitis externa. British journal of biomedical science, 68(4), 174-180.
Guzel, O., & Guner, E. I. (2009). ISO 15189 accreditation: Requirements for quality and
competence of medical laboratories, experience of a laboratory I. Clinical
biochemistry, 42(4-5), 274-278.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

SOP FOR EAR SWAB DOCUMENT VERSION/NO: 8
Hailu, D., Mekonnen, D., Derbie, A., Mulu, W., & Abera, B. (2016). Pathogenic bacteria
profile and antimicrobial susceptibility patterns of ear infection at Bahir Dar
Regional Health Research Laboratory Center, Ethiopia. SpringerPlus, 5(1), 466.
Hutchison, M. L., Gittins, J., Walker, A., Sparks, N., Humphrey, T. J., Burton, C., &
Moore, A. (2004). An assessment of the microbiological risks involved with egg
washing under commercial conditions. Journal of food protection, 67(1), 4-11.
International Standards Organization (ISO). (2017). International Standard, ISO 6887-
1 [Ebook] (2nd ed., pp. 3-11). Geneva, Switzerland. Retrieved from
https://www.sis.se/api/document/preview/921626/
Khan, S. A., Khan, N., Iqbal, M., Khan, S., & Hussain, G. (2019). Bacteriological Study
of Discharging Ear in Patients of Active Mucosal Chronic Otitis Media Attending
a Tertiary Care Hospital. Journal of Saidu Medical College, 9(1).
Khattak, S. F., Sheikh, N. A., & Aleem, A. (2017). Microbiological profile from middle
ear and nasopharynx in patients suffering from chronic active mucosal otitis
media. Journal of Ayub Medical College Abbottabad, 29(4), 610-613.
Pacholewicz, E., Swart, A., Lipman, L. J., Wagenaar, J. A., Havelaar, A. H., & Duim, B.
(2013). Propidium monoazide does not fully inhibit the detection of dead
Campylobacter on broiler chicken carcasses by qPCR. Journal of microbiological
methods, 95(1), 32-3
Hailu, D., Mekonnen, D., Derbie, A., Mulu, W., & Abera, B. (2016). Pathogenic bacteria
profile and antimicrobial susceptibility patterns of ear infection at Bahir Dar
Regional Health Research Laboratory Center, Ethiopia. SpringerPlus, 5(1), 466.
Hutchison, M. L., Gittins, J., Walker, A., Sparks, N., Humphrey, T. J., Burton, C., &
Moore, A. (2004). An assessment of the microbiological risks involved with egg
washing under commercial conditions. Journal of food protection, 67(1), 4-11.
International Standards Organization (ISO). (2017). International Standard, ISO 6887-
1 [Ebook] (2nd ed., pp. 3-11). Geneva, Switzerland. Retrieved from
https://www.sis.se/api/document/preview/921626/
Khan, S. A., Khan, N., Iqbal, M., Khan, S., & Hussain, G. (2019). Bacteriological Study
of Discharging Ear in Patients of Active Mucosal Chronic Otitis Media Attending
a Tertiary Care Hospital. Journal of Saidu Medical College, 9(1).
Khattak, S. F., Sheikh, N. A., & Aleem, A. (2017). Microbiological profile from middle
ear and nasopharynx in patients suffering from chronic active mucosal otitis
media. Journal of Ayub Medical College Abbottabad, 29(4), 610-613.
Pacholewicz, E., Swart, A., Lipman, L. J., Wagenaar, J. A., Havelaar, A. H., & Duim, B.
(2013). Propidium monoazide does not fully inhibit the detection of dead
Campylobacter on broiler chicken carcasses by qPCR. Journal of microbiological
methods, 95(1), 32-3
Pereira, P. (2017). ISO 15189:2012 Medical laboratories - Requirements for quality and
competence-Westgard. Retrieved 19 September 2019, from
https://www.westgard.com/iso-15189-2012-requirements-1.html
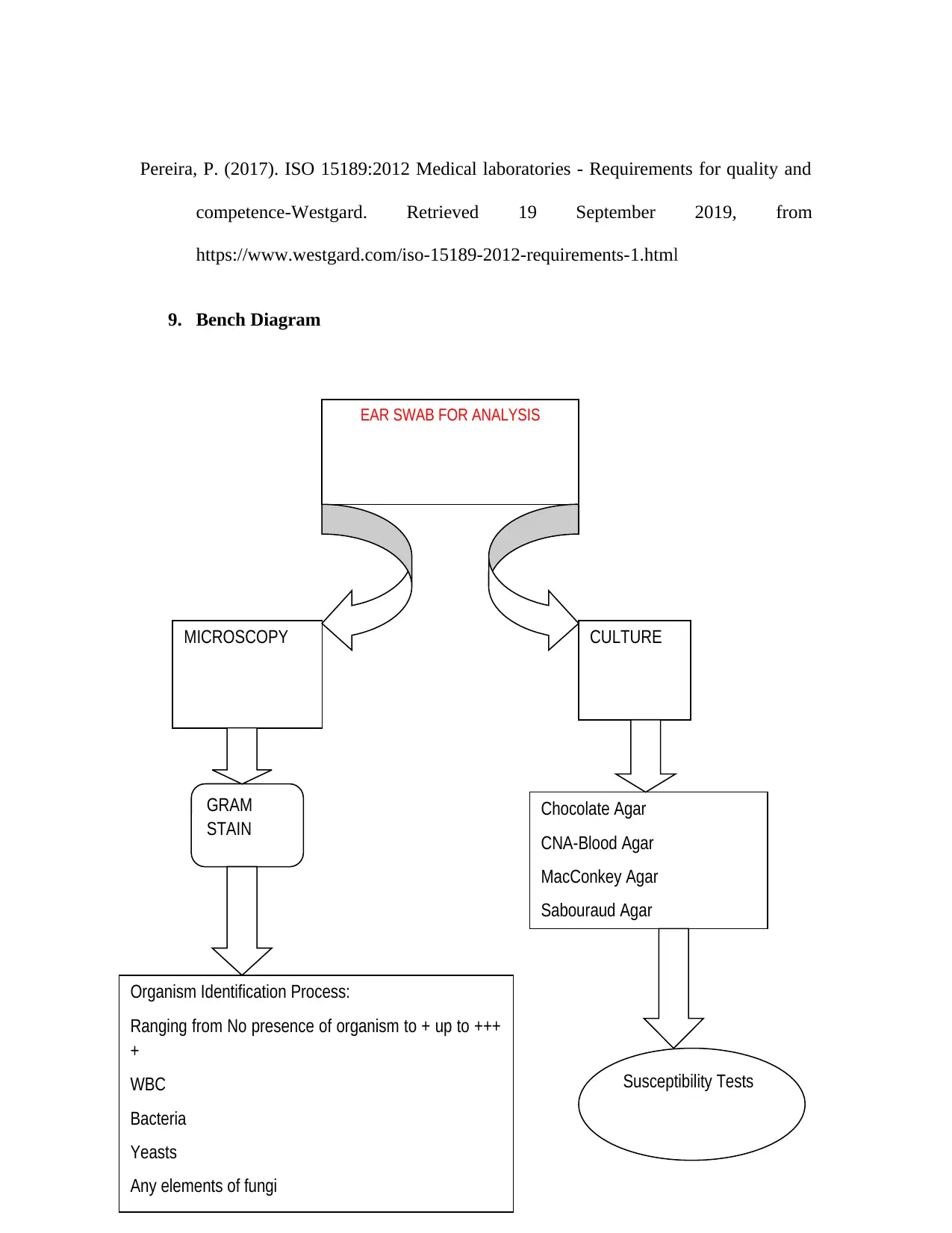
9. Bench Diagram
EAR SWAB FOR ANALYSIS
MICROSCOPY CULTURE
GRAM
STAIN
Organism Identification Process:
Ranging from No presence of organism to + up to +++
+
WBC
Bacteria
Yeasts
Any elements of fungi
Chocolate Agar
CNA-Blood Agar
MacConkey Agar
Sabouraud Agar
Susceptibility Tests
competence-Westgard. Retrieved 19 September 2019, from
https://www.westgard.com/iso-15189-2012-requirements-1.html
9. Bench Diagram
EAR SWAB FOR ANALYSIS
MICROSCOPY CULTURE
GRAM
STAIN
Organism Identification Process:
Ranging from No presence of organism to + up to +++
+
WBC
Bacteria
Yeasts
Any elements of fungi
Chocolate Agar
CNA-Blood Agar
MacConkey Agar
Sabouraud Agar
Susceptibility Tests
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
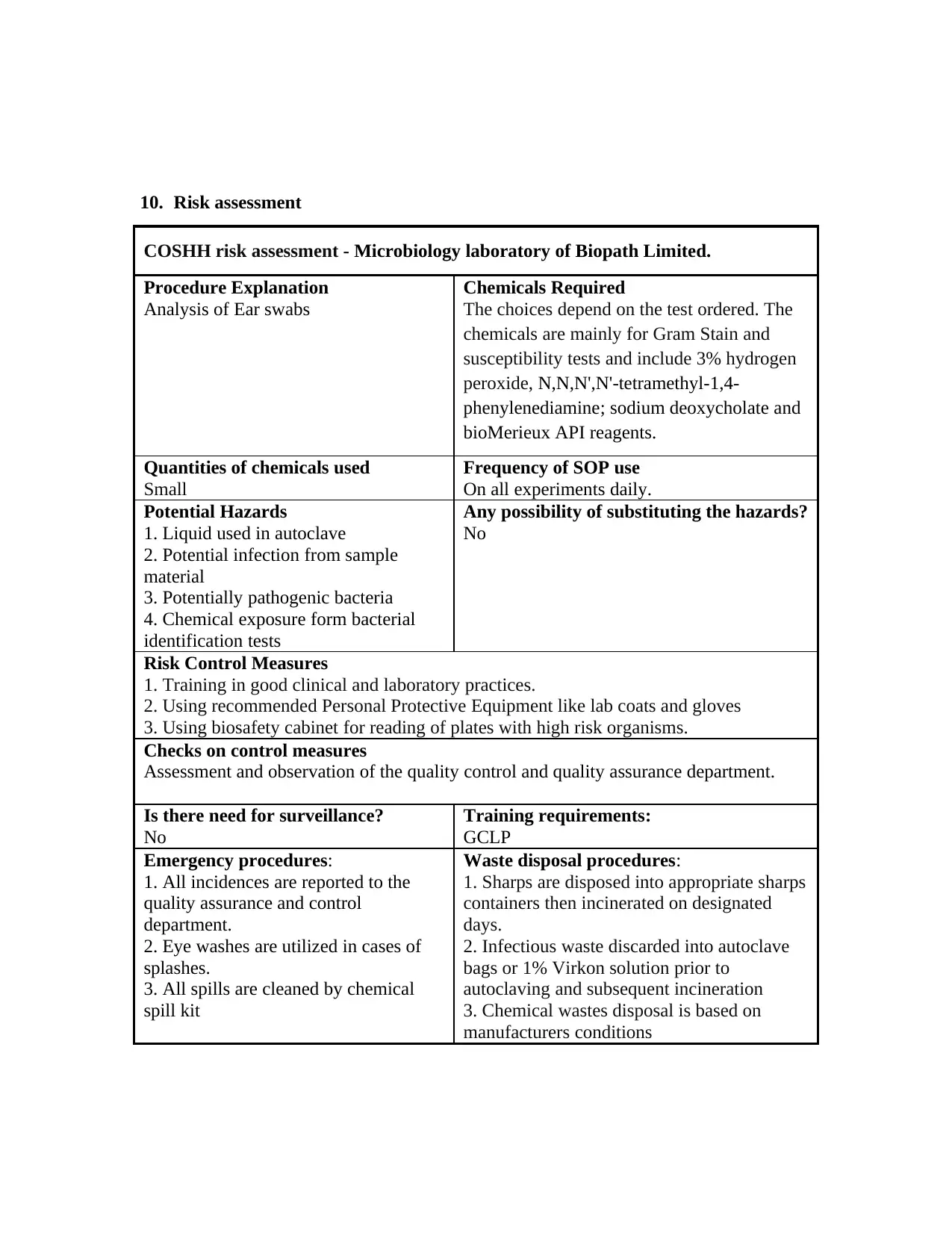
10. Risk assessment
COSHH risk assessment - Microbiology laboratory of Biopath Limited.
Procedure Explanation
Analysis of Ear swabs
Chemicals Required
The choices depend on the test ordered. The
chemicals are mainly for Gram Stain and
susceptibility tests and include 3% hydrogen
peroxide, N,N,N',N'-tetramethyl-1,4-
phenylenediamine; sodium deoxycholate and
bioMerieux API reagents.
Quantities of chemicals used
Small
Frequency of SOP use
On all experiments daily.
Potential Hazards
1. Liquid used in autoclave
2. Potential infection from sample
material
3. Potentially pathogenic bacteria
4. Chemical exposure form bacterial
identification tests
Any possibility of substituting the hazards?
No
Risk Control Measures
1. Training in good clinical and laboratory practices.
2. Using recommended Personal Protective Equipment like lab coats and gloves
3. Using biosafety cabinet for reading of plates with high risk organisms.
Checks on control measures
Assessment and observation of the quality control and quality assurance department.
Is there need for surveillance?
No
Training requirements:
GCLP
Emergency procedures:
1. All incidences are reported to the
quality assurance and control
department.
2. Eye washes are utilized in cases of
splashes.
3. All spills are cleaned by chemical
spill kit
Waste disposal procedures:
1. Sharps are disposed into appropriate sharps
containers then incinerated on designated
days.
2. Infectious waste discarded into autoclave
bags or 1% Virkon solution prior to
autoclaving and subsequent incineration
3. Chemical wastes disposal is based on
manufacturers conditions
COSHH risk assessment - Microbiology laboratory of Biopath Limited.
Procedure Explanation
Analysis of Ear swabs
Chemicals Required
The choices depend on the test ordered. The
chemicals are mainly for Gram Stain and
susceptibility tests and include 3% hydrogen
peroxide, N,N,N',N'-tetramethyl-1,4-
phenylenediamine; sodium deoxycholate and
bioMerieux API reagents.
Quantities of chemicals used
Small
Frequency of SOP use
On all experiments daily.
Potential Hazards
1. Liquid used in autoclave
2. Potential infection from sample
material
3. Potentially pathogenic bacteria
4. Chemical exposure form bacterial
identification tests
Any possibility of substituting the hazards?
No
Risk Control Measures
1. Training in good clinical and laboratory practices.
2. Using recommended Personal Protective Equipment like lab coats and gloves
3. Using biosafety cabinet for reading of plates with high risk organisms.
Checks on control measures
Assessment and observation of the quality control and quality assurance department.
Is there need for surveillance?
No
Training requirements:
GCLP
Emergency procedures:
1. All incidences are reported to the
quality assurance and control
department.
2. Eye washes are utilized in cases of
splashes.
3. All spills are cleaned by chemical
spill kit
Waste disposal procedures:
1. Sharps are disposed into appropriate sharps
containers then incinerated on designated
days.
2. Infectious waste discarded into autoclave
bags or 1% Virkon solution prior to
autoclaving and subsequent incineration
3. Chemical wastes disposal is based on
manufacturers conditions
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.