SOP for Preparation of Iron Syrup Report 2022
VerifiedAdded on 2022/09/14
|10
|1462
|23
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head = SOP FOR PREPARATION OF IRON SYRUP 0
SOP for preparation of iron syrup
Scale up calculation
Student detail
[Pick the date]
SOP for preparation of iron syrup
Scale up calculation
Student detail
[Pick the date]
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

SOP for preparation of iron syrup
1
Contents
Introduction......................................................................................................................................2
Aim..................................................................................................................................................2
Objective..........................................................................................................................................2
Materials and Method......................................................................................................................2
Chemical:.....................................................................................................................................2
The apparatus used for the study:................................................................................................2
Experiments/ Methodology applied for the preparation of iron syrup.........................................3
Calculations.....................................................................................................................................4
Discussions......................................................................................................................................6
Conclusion.......................................................................................................................................7
Reference.........................................................................................................................................9
1
Contents
Introduction......................................................................................................................................2
Aim..................................................................................................................................................2
Objective..........................................................................................................................................2
Materials and Method......................................................................................................................2
Chemical:.....................................................................................................................................2
The apparatus used for the study:................................................................................................2
Experiments/ Methodology applied for the preparation of iron syrup.........................................3
Calculations.....................................................................................................................................4
Discussions......................................................................................................................................6
Conclusion.......................................................................................................................................7
Reference.........................................................................................................................................9

SOP for preparation of iron syrup
2
Introduction
The syrup is a thick highly dense liquid generally made in the presence of sugar and water, the
preparation of syrup is carried out with or without the presence of flavored additives. The liquid
nature of the syrup assists in the faster absorption of the medication in comparison to the tablets;
therefore it is more preferred over a tablet in some cases, where quick response is required,
(Drumond et al., 2017). The present work was carried out for the preparation of Iron syrup. The
iron syrup is highly applicable to the disorders related to the lack of iron in the body.
Aim
The lab experiment was conducted for the preparation of iron syrup using ferrous sulfate.
Objective
I. Ferrous sulfate of 99.7 % purity was used for the preparation of iron syrup.
II. Analysis of various aspect involved in the preparation of iron syrup was carried out.
Materials and Method
The materials that were used for the preparation of iron syrup were:
Chemical:
FeSO4, Citric acid, Ascorbic acid, Liquid Glucose, Sucrose Syrup (Conc 67% w/v), Methyl
Paraben, Propyl Paraben, Sodium saccharin, Glycerin, Raspberry Flavor, Raspberry Red Color
and distilled water.
The apparatus used for the study:
Beaker, Filter paper, Stirrer, Measuring cylinder, Balance, Pipette, Bottle with lid, Funnel, Glass
rod, labels.
2
Introduction
The syrup is a thick highly dense liquid generally made in the presence of sugar and water, the
preparation of syrup is carried out with or without the presence of flavored additives. The liquid
nature of the syrup assists in the faster absorption of the medication in comparison to the tablets;
therefore it is more preferred over a tablet in some cases, where quick response is required,
(Drumond et al., 2017). The present work was carried out for the preparation of Iron syrup. The
iron syrup is highly applicable to the disorders related to the lack of iron in the body.
Aim
The lab experiment was conducted for the preparation of iron syrup using ferrous sulfate.
Objective
I. Ferrous sulfate of 99.7 % purity was used for the preparation of iron syrup.
II. Analysis of various aspect involved in the preparation of iron syrup was carried out.
Materials and Method
The materials that were used for the preparation of iron syrup were:
Chemical:
FeSO4, Citric acid, Ascorbic acid, Liquid Glucose, Sucrose Syrup (Conc 67% w/v), Methyl
Paraben, Propyl Paraben, Sodium saccharin, Glycerin, Raspberry Flavor, Raspberry Red Color
and distilled water.
The apparatus used for the study:
Beaker, Filter paper, Stirrer, Measuring cylinder, Balance, Pipette, Bottle with lid, Funnel, Glass
rod, labels.
SOP for preparation of iron syrup
3
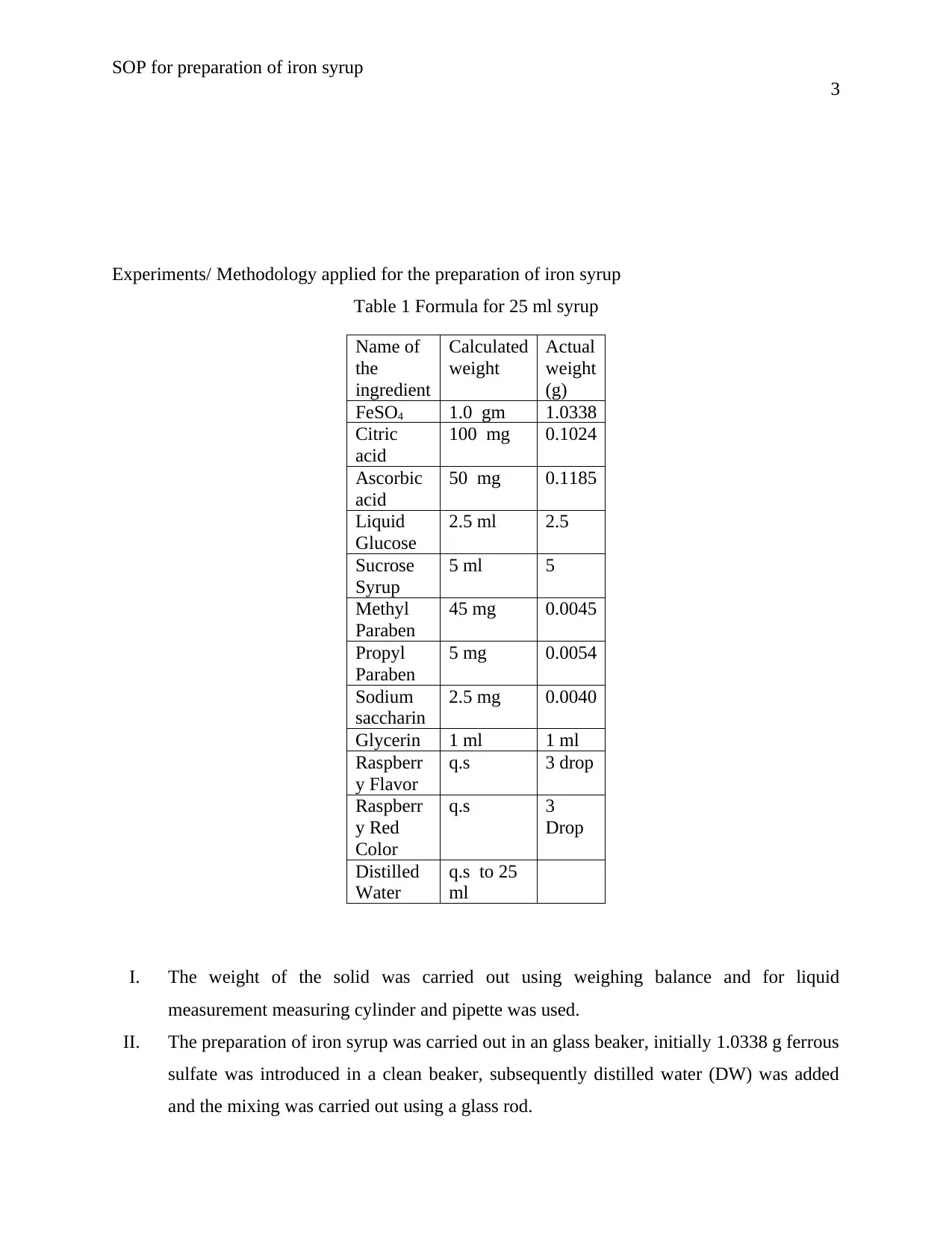
Experiments/ Methodology applied for the preparation of iron syrup
Table 1 Formula for 25 ml syrup
I. The weight of the solid was carried out using weighing balance and for liquid
measurement measuring cylinder and pipette was used.
II. The preparation of iron syrup was carried out in an glass beaker, initially 1.0338 g ferrous
sulfate was introduced in a clean beaker, subsequently distilled water (DW) was added
and the mixing was carried out using a glass rod.
Name of
the
ingredient
Calculated
weight
Actual
weight
(g)
FeSO4 1.0 gm 1.0338
Citric
acid
100 mg 0.1024
Ascorbic
acid
50 mg 0.1185
Liquid
Glucose
2.5 ml 2.5
Sucrose
Syrup
5 ml 5
Methyl
Paraben
45 mg 0.0045
Propyl
Paraben
5 mg 0.0054
Sodium
saccharin
2.5 mg 0.0040
Glycerin 1 ml 1 ml
Raspberr
y Flavor
q.s 3 drop
Raspberr
y Red
Color
q.s 3
Drop
Distilled
Water
q.s to 25
ml
3
Experiments/ Methodology applied for the preparation of iron syrup
Table 1 Formula for 25 ml syrup
I. The weight of the solid was carried out using weighing balance and for liquid
measurement measuring cylinder and pipette was used.
II. The preparation of iron syrup was carried out in an glass beaker, initially 1.0338 g ferrous
sulfate was introduced in a clean beaker, subsequently distilled water (DW) was added
and the mixing was carried out using a glass rod.
Name of
the
ingredient
Calculated
weight
Actual
weight
(g)
FeSO4 1.0 gm 1.0338
Citric
acid
100 mg 0.1024
Ascorbic
acid
50 mg 0.1185
Liquid
Glucose
2.5 ml 2.5
Sucrose
Syrup
5 ml 5
Methyl
Paraben
45 mg 0.0045
Propyl
Paraben
5 mg 0.0054
Sodium
saccharin
2.5 mg 0.0040
Glycerin 1 ml 1 ml
Raspberr
y Flavor
q.s 3 drop
Raspberr
y Red
Color
q.s 3
Drop
Distilled
Water
q.s to 25
ml
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

SOP for preparation of iron syrup
4
III. To the above mixture 0.1024 g citric acid and 0.1185 g ascorbic acid was added and
subsequently mixed with the help of glass rod, the final solution was stored.
IV. In an separate beaker 0.0045 g methyl paraben, 0.0054 g propyl paraben and 0.0040 g
sodium saccharin was added along with 5 ml of sucrose syrup, subsequently liquid
glucose 2.5 mL and glycerin 1 ml was added and stirred with the help of glass rod.
V. The mixture prepared in the step four was added to the mixture in step three and vigorous
stirring was carried out using glass rod until the homogenous solution was not obtained.
VI. To the homogenous solution 3 drops each of Raspberry flavor and color was added and
stirred.
VII. The mixture was filtered to remove any particulate matter in the homogenous mixture in
an a measuring cylinder, and the final volume 25 ml mas make up using DW.
VIII. The filtered solution was transferred in a bottle and labeled accordingly.
Calculations
An assumption was made that the initial calculation given for the ferrous sulfate was for the solid
with 100 % purity. Therefor for the solid with 99.7 % purity, the amount of ferrous sulfate
required for will be:
Total amount of 100 % pure ferrous sulfate required for the preparation of 25 ml solution
is = 1g
In case of the solid with 99.7 % purity x g of ferrous sulfate is required
x= 1
99.7 X 100
x = 1.003 g
1.003 g of ferrous sulfate was required for the preparation of 25 ml iron syrup
For the preparation of 25 L (25000 ml)syrup
25000 ml will require = 25000 X 1.003
25
=1003 g
4
III. To the above mixture 0.1024 g citric acid and 0.1185 g ascorbic acid was added and
subsequently mixed with the help of glass rod, the final solution was stored.
IV. In an separate beaker 0.0045 g methyl paraben, 0.0054 g propyl paraben and 0.0040 g
sodium saccharin was added along with 5 ml of sucrose syrup, subsequently liquid
glucose 2.5 mL and glycerin 1 ml was added and stirred with the help of glass rod.
V. The mixture prepared in the step four was added to the mixture in step three and vigorous
stirring was carried out using glass rod until the homogenous solution was not obtained.
VI. To the homogenous solution 3 drops each of Raspberry flavor and color was added and
stirred.
VII. The mixture was filtered to remove any particulate matter in the homogenous mixture in
an a measuring cylinder, and the final volume 25 ml mas make up using DW.
VIII. The filtered solution was transferred in a bottle and labeled accordingly.
Calculations
An assumption was made that the initial calculation given for the ferrous sulfate was for the solid
with 100 % purity. Therefor for the solid with 99.7 % purity, the amount of ferrous sulfate
required for will be:
Total amount of 100 % pure ferrous sulfate required for the preparation of 25 ml solution
is = 1g
In case of the solid with 99.7 % purity x g of ferrous sulfate is required
x= 1
99.7 X 100
x = 1.003 g
1.003 g of ferrous sulfate was required for the preparation of 25 ml iron syrup
For the preparation of 25 L (25000 ml)syrup
25000 ml will require = 25000 X 1.003
25
=1003 g

SOP for preparation of iron syrup
5
Assumption was made regarding the purity of other materials and it was assumed that the purity
of the material remained the same therefore the amount of material required for 25 L.
For citric acid, the earlier requirement was of 100 mg for 25 ml, for the preparation of 25 L
25000 X 0.1
25
=100 g
For Ascorbic acid, the earlier requirement was of 50 mg for 25 ml, for the preparation of 25 L
25000 X 0.05
25
=50 g
For liquid glucose , the earlier requirement was of 2.5 ml for 25 ml, for the preparation of 25 L
25000 X 2.5
25
=2500 ml
For sucrose syrup , the earlier requirement was of 5 ml for 25 ml, for the preparation of 25 L
25000 X 5
25
= 5000 ml
For Methyl parabon , the earlier requirement was of 45 mg for 25 ml, for the preparation of 25 L
25000 X .045
25
= 45 g
For propyl parabon , the earlier requirement was of 5 mg for 25 ml, for the preparation of 25 L
25000 X 0.005
25
5
Assumption was made regarding the purity of other materials and it was assumed that the purity
of the material remained the same therefore the amount of material required for 25 L.
For citric acid, the earlier requirement was of 100 mg for 25 ml, for the preparation of 25 L
25000 X 0.1
25
=100 g
For Ascorbic acid, the earlier requirement was of 50 mg for 25 ml, for the preparation of 25 L
25000 X 0.05
25
=50 g
For liquid glucose , the earlier requirement was of 2.5 ml for 25 ml, for the preparation of 25 L
25000 X 2.5
25
=2500 ml
For sucrose syrup , the earlier requirement was of 5 ml for 25 ml, for the preparation of 25 L
25000 X 5
25
= 5000 ml
For Methyl parabon , the earlier requirement was of 45 mg for 25 ml, for the preparation of 25 L
25000 X .045
25
= 45 g
For propyl parabon , the earlier requirement was of 5 mg for 25 ml, for the preparation of 25 L
25000 X 0.005
25

SOP for preparation of iron syrup
6
= 5 g
For sodium saccharin, the earlier requirement was of 2.5 mg for 25 ml, for the preparation of 25
L
25000 X 0.0025
25
=2.5 g
For glycerin, the earlier requirement was of 1 ml for 25 ml, for the preparation of 25 L
25000 X 1
25
= 1000 ml
For distilled water, the earlier requirement was of 1 ml for 25 ml, for the preparation of 25 L
25000 X 25
25
= 25000 ml
Discussions
The preparation carried out in the lab was for small scale preparation of the volume of 25 ml, and
it was observed that the weighing of the sample should have been carried out with more
precision as errors were observed when the actual weight was compared with the actual weight
taken. The study conducted by (Poskurica et al., 2015), reported the application of iron-
containing syrup for an increase in hemoglobin concentration. The study conducted by (Nisar &
Michael, 2016), reported that the use of iron syrup at the time of pregnancy helped in a deduction
in a number of neonatal death.
6
= 5 g
For sodium saccharin, the earlier requirement was of 2.5 mg for 25 ml, for the preparation of 25
L
25000 X 0.0025
25
=2.5 g
For glycerin, the earlier requirement was of 1 ml for 25 ml, for the preparation of 25 L
25000 X 1
25
= 1000 ml
For distilled water, the earlier requirement was of 1 ml for 25 ml, for the preparation of 25 L
25000 X 25
25
= 25000 ml
Discussions
The preparation carried out in the lab was for small scale preparation of the volume of 25 ml, and
it was observed that the weighing of the sample should have been carried out with more
precision as errors were observed when the actual weight was compared with the actual weight
taken. The study conducted by (Poskurica et al., 2015), reported the application of iron-
containing syrup for an increase in hemoglobin concentration. The study conducted by (Nisar &
Michael, 2016), reported that the use of iron syrup at the time of pregnancy helped in a deduction
in a number of neonatal death.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
SOP for preparation of iron syrup
7
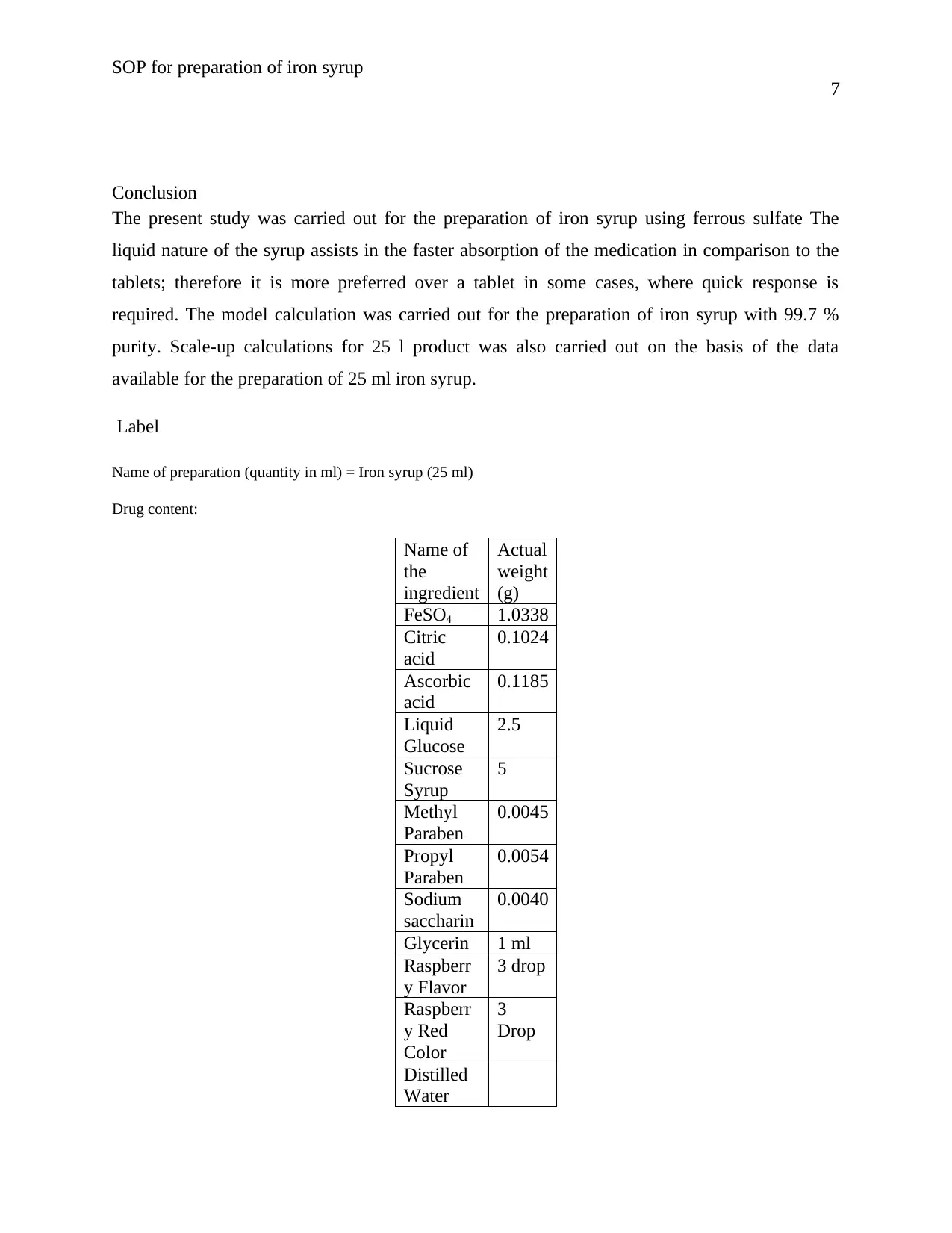
Conclusion
The present study was carried out for the preparation of iron syrup using ferrous sulfate The
liquid nature of the syrup assists in the faster absorption of the medication in comparison to the
tablets; therefore it is more preferred over a tablet in some cases, where quick response is
required. The model calculation was carried out for the preparation of iron syrup with 99.7 %
purity. Scale-up calculations for 25 l product was also carried out on the basis of the data
available for the preparation of 25 ml iron syrup.
Label
Name of preparation (quantity in ml) = Iron syrup (25 ml)
Drug content:
Name of
the
ingredient
Actual
weight
(g)
FeSO4 1.0338
Citric
acid
0.1024
Ascorbic
acid
0.1185
Liquid
Glucose
2.5
Sucrose
Syrup
5
Methyl
Paraben
0.0045
Propyl
Paraben
0.0054
Sodium
saccharin
0.0040
Glycerin 1 ml
Raspberr
y Flavor
3 drop
Raspberr
y Red
Color
3
Drop
Distilled
Water
7
Conclusion
The present study was carried out for the preparation of iron syrup using ferrous sulfate The
liquid nature of the syrup assists in the faster absorption of the medication in comparison to the
tablets; therefore it is more preferred over a tablet in some cases, where quick response is
required. The model calculation was carried out for the preparation of iron syrup with 99.7 %
purity. Scale-up calculations for 25 l product was also carried out on the basis of the data
available for the preparation of 25 ml iron syrup.
Label
Name of preparation (quantity in ml) = Iron syrup (25 ml)
Drug content:
Name of
the
ingredient
Actual
weight
(g)
FeSO4 1.0338
Citric
acid
0.1024
Ascorbic
acid
0.1185
Liquid
Glucose
2.5
Sucrose
Syrup
5
Methyl
Paraben
0.0045
Propyl
Paraben
0.0054
Sodium
saccharin
0.0040
Glycerin 1 ml
Raspberr
y Flavor
3 drop
Raspberr
y Red
Color
3
Drop
Distilled
Water

SOP for preparation of iron syrup
8
Use of preparation= Mixing
Dosage: “as directed by physician”
Direction: Based on the SOP
Auxiliary label: Kindly consult medical professionals prior to the use of syrup
Storage condition: Room temperature in dark
Mfg date: 4/5/2020 Exp date:3/4/2022
Batch No.2123424321
Manufactured by: Lab
8
Use of preparation= Mixing
Dosage: “as directed by physician”
Direction: Based on the SOP
Auxiliary label: Kindly consult medical professionals prior to the use of syrup
Storage condition: Room temperature in dark
Mfg date: 4/5/2020 Exp date:3/4/2022
Batch No.2123424321
Manufactured by: Lab

SOP for preparation of iron syrup
9
Reference
Drumond, N., van Riet-Nales, D.A., Karapinar-Çarkit, F. & Stegemann, S., 2017. Patients’
appropriateness, acceptability, usability and preferences for pharmaceutical preparations: results
from a literature review on clinical evidence. International journal of pharmaceutics , 521(1-2),
pp.294-305.
Nisar, Y.B. & Michael, J.D., 2016. Iron/folic acid supplementation during pregnancy prevents
neonatal and under-five mortality in Pakistan: propensity score matched sample from two
Pakistan Demographic and Health Surveys. Global health action, 9(1), p.29621.
Poskurica, M., Petrović, & Poskurica, M., 2015. Administration of iron in renal anemia.
Vojnosanitetski pregled , 72(4), pp.361-67.
9
Reference
Drumond, N., van Riet-Nales, D.A., Karapinar-Çarkit, F. & Stegemann, S., 2017. Patients’
appropriateness, acceptability, usability and preferences for pharmaceutical preparations: results
from a literature review on clinical evidence. International journal of pharmaceutics , 521(1-2),
pp.294-305.
Nisar, Y.B. & Michael, J.D., 2016. Iron/folic acid supplementation during pregnancy prevents
neonatal and under-five mortality in Pakistan: propensity score matched sample from two
Pakistan Demographic and Health Surveys. Global health action, 9(1), p.29621.
Poskurica, M., Petrović, & Poskurica, M., 2015. Administration of iron in renal anemia.
Vojnosanitetski pregled , 72(4), pp.361-67.
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.