MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury
VerifiedAdded on 2022/10/04
|8
|8376
|207
AI Summary
This study investigates the correlation between matrix metalloprotein serum levels and remission after traumatic spinal cord injury (SCI) and suggests MMP-8 and MMP-9 serum levels as early markers for remission. The study was conducted on 115 patients and the results indicate that further studies with an enlarged collective are warranted. The study was approved by the ethics committee of the University of Heidelberg and the Landesärztekammer Rheinland-Pfalz, Germany.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

ORIGINAL ARTICLE
Exploratory study to suggest the possibility of MMP-8 a
MMP-9 serum levels as early markers for remission afte
traumatic spinal cord injury
A Moghaddam1, R Heller1, V Daniel2, T Swing1, M Akbar3, H-J Gerner1 and B Biglari4
Study design:A prospective observational study reporting the correlation between matrix metalloprotein serum levels and
after traumatic spinal cord injury (SCI).
Objectives:To investigate serum cytokine levels as predictive markers.
Setting: Germany, Rhineland-Palatinate (Rheinland-Pfalz).
Methods:Between 2010 and 2015, data sets from 115 patients (33 female, 82 male) after traumatic SCI were recorded a
Trauma Centre Ludwigshafen. We examined the serum levels of Matix metallopraoteinases (MMPs) MMP-2, MMP-8, MMP
and MMP-12 over a 12-week period, that is, at admission and 4, 9, 12 h, 1 and 3 days and 1, 2, 4, 8 and 12 weeks after t
Following the same match-pair procedure as in our previous studies, we selected 10 patients with SCI and neurologicalremission
(Group 1) and 10 patients with an initial American Spinal Injury Association (ASIA) A grade and no neurological remission
Ten patients with an isolated vertebralfracture without neurologicaldeficits served as a controlgroup (Group C).Our analysis was
performed using a Luminex Performance Human High Sensitivity Cytokine Panel. Multivariate logistic regression models
examine the predictive value of MMPs with respect to neurological remission vs no neurological remission.
Results: MMP-8 and MMP-9 provided significantly different values. The favoured predictive modelallows to differentiate between
neurological remission and no neurological remission in 97% of cases.
Conclusions:The results indicate thatfurtherstudies with an enlarged collective are warranted in orderto investigate current
monitoring, prognostic and tracking techniques as well as scoring systems.
Spinal Cord (2017) 55, 8–15; doi:10.1038/sc.2016.104; published online 5 July 2016
INTRODUCTION
Spinalcord injury (SCI) impairs patients’quality oflife greatly and
causes immense financial consequences for them and their families.1,2
Current studies report an average annual SCI incidence of 40 cases per
million in the United States.3 Despite substantialresearch on SCI,no
ground breaking step hasbeen madetowardsunderstanding the
mechanisms ofSCI and exploring new therapies.New therapies for
reversing neurologicaldeficits are also lacking.2 Currenttreatments
such as medications or surgical treatment are limited in their success
and results are very poor.4–6Furthermore, most therapeutic strategies
lack convincing evidence for their beneficialeffects.6,7There exist no
valid markers specifying the potentialfor remission up to this point.
The pathophysiological process is characterised by a primary and a
secondary phase ofinjury.Although the firstphase ismarked by
mechanical trauma, the second phase is more complex due to a variety
of pathophysiologicalprocesses.2 The early inflammatory response
involvesan initial waveof infiltratingneutrophils,followed by
migration of monocytes and macrophages into the injured segment.
Each of these inflammatory cells expresses MMPs including MMP-2
(gelatinase A), MMP-8 (neutrophil collagenase), MMP-9 (gelatin
MMP-11 (stromelysin-3)and MMP-12 (metalloelastase).8 Further-
more, MMP-10 is suspected to have a critical role in controlling
remodelling in macrophagesand moderating scartissue formation
during wound repair.9
Currently,thereare still few reportsfocusedon diagnostic
biomarkers in SCI.10,11In view ofdevastating consequences ofSCI
and the poor therapeutic solutions, there is an urgent need for
effortsto innovatereliablebiomarkersfor remission afterSCI.11
Because oftheir relevance in processes after traumatic injuries su
as traumaticspinalcord injuries,as well as differingcatalytic
mechanisms,we decided to investigate MMP-2,MMP-8, MMP-9,
MMP-10 and MMP-12.8,9,12Our goalwas to discover a prognostic
biomarker for remission potentialafter SCI by investigating matrix-
metalloproteines in peripheralserum and therewith determine neu-
rologicaloutcome.Animalstudies investigating possible markers ar
limited in terms of transferability.13 The human modelin this study
investigated the following:first,if markers can be found in serum to
predict rehabilitation post SCI; second, if there is an effective m
1HTRG Heidelberg Trauma Research Group,Centerfor Orthopedics,Trauma Surgeryand SpinalCord Injury,Heidelberg UniversityHospital,Heidelberg,Germany;
2Transplantation Immunology, Institute of Immunology, University of Heidelberg, Heidelberg, Germany;3Spine Center, Center for Orthopedics, Trauma Surgery and SpinalCord
Injury, Heidelberg University Hospital, Heidelberg, Germany and4Department of Paraplegiology, Berufsgenossenschaftliche Unfallklinik Ludwigshafen, Ludwigshafen, G
Correspondence:Dr Professor Arash Moghaddam,HTRG Heidelberg Trauma Research Group,Center for Orthopedics,Trauma Surgery and SpinalCord Injury,Heidelberg
University Hospital, Schlierbacher Landstrabe 200a, Heidelberg 69118, Germany.
E-mail: arash.moghaddam@med.uni-heidelberg.de or email@arash.de
Received 7 April2016; revised 2 May 2016;accepted 9 May 2016;published online 5 July 2016
SpinalCord (2017) 55,8–15
& 2017 InternationalSpinalCord Society Allrights reserved 1362-4393/17
www.nature.com/sc
Exploratory study to suggest the possibility of MMP-8 a
MMP-9 serum levels as early markers for remission afte
traumatic spinal cord injury
A Moghaddam1, R Heller1, V Daniel2, T Swing1, M Akbar3, H-J Gerner1 and B Biglari4
Study design:A prospective observational study reporting the correlation between matrix metalloprotein serum levels and
after traumatic spinal cord injury (SCI).
Objectives:To investigate serum cytokine levels as predictive markers.
Setting: Germany, Rhineland-Palatinate (Rheinland-Pfalz).
Methods:Between 2010 and 2015, data sets from 115 patients (33 female, 82 male) after traumatic SCI were recorded a
Trauma Centre Ludwigshafen. We examined the serum levels of Matix metallopraoteinases (MMPs) MMP-2, MMP-8, MMP
and MMP-12 over a 12-week period, that is, at admission and 4, 9, 12 h, 1 and 3 days and 1, 2, 4, 8 and 12 weeks after t
Following the same match-pair procedure as in our previous studies, we selected 10 patients with SCI and neurologicalremission
(Group 1) and 10 patients with an initial American Spinal Injury Association (ASIA) A grade and no neurological remission
Ten patients with an isolated vertebralfracture without neurologicaldeficits served as a controlgroup (Group C).Our analysis was
performed using a Luminex Performance Human High Sensitivity Cytokine Panel. Multivariate logistic regression models
examine the predictive value of MMPs with respect to neurological remission vs no neurological remission.
Results: MMP-8 and MMP-9 provided significantly different values. The favoured predictive modelallows to differentiate between
neurological remission and no neurological remission in 97% of cases.
Conclusions:The results indicate thatfurtherstudies with an enlarged collective are warranted in orderto investigate current
monitoring, prognostic and tracking techniques as well as scoring systems.
Spinal Cord (2017) 55, 8–15; doi:10.1038/sc.2016.104; published online 5 July 2016
INTRODUCTION
Spinalcord injury (SCI) impairs patients’quality oflife greatly and
causes immense financial consequences for them and their families.1,2
Current studies report an average annual SCI incidence of 40 cases per
million in the United States.3 Despite substantialresearch on SCI,no
ground breaking step hasbeen madetowardsunderstanding the
mechanisms ofSCI and exploring new therapies.New therapies for
reversing neurologicaldeficits are also lacking.2 Currenttreatments
such as medications or surgical treatment are limited in their success
and results are very poor.4–6Furthermore, most therapeutic strategies
lack convincing evidence for their beneficialeffects.6,7There exist no
valid markers specifying the potentialfor remission up to this point.
The pathophysiological process is characterised by a primary and a
secondary phase ofinjury.Although the firstphase ismarked by
mechanical trauma, the second phase is more complex due to a variety
of pathophysiologicalprocesses.2 The early inflammatory response
involvesan initial waveof infiltratingneutrophils,followed by
migration of monocytes and macrophages into the injured segment.
Each of these inflammatory cells expresses MMPs including MMP-2
(gelatinase A), MMP-8 (neutrophil collagenase), MMP-9 (gelatin
MMP-11 (stromelysin-3)and MMP-12 (metalloelastase).8 Further-
more, MMP-10 is suspected to have a critical role in controlling
remodelling in macrophagesand moderating scartissue formation
during wound repair.9
Currently,thereare still few reportsfocusedon diagnostic
biomarkers in SCI.10,11In view ofdevastating consequences ofSCI
and the poor therapeutic solutions, there is an urgent need for
effortsto innovatereliablebiomarkersfor remission afterSCI.11
Because oftheir relevance in processes after traumatic injuries su
as traumaticspinalcord injuries,as well as differingcatalytic
mechanisms,we decided to investigate MMP-2,MMP-8, MMP-9,
MMP-10 and MMP-12.8,9,12Our goalwas to discover a prognostic
biomarker for remission potentialafter SCI by investigating matrix-
metalloproteines in peripheralserum and therewith determine neu-
rologicaloutcome.Animalstudies investigating possible markers ar
limited in terms of transferability.13 The human modelin this study
investigated the following:first,if markers can be found in serum to
predict rehabilitation post SCI; second, if there is an effective m
1HTRG Heidelberg Trauma Research Group,Centerfor Orthopedics,Trauma Surgeryand SpinalCord Injury,Heidelberg UniversityHospital,Heidelberg,Germany;
2Transplantation Immunology, Institute of Immunology, University of Heidelberg, Heidelberg, Germany;3Spine Center, Center for Orthopedics, Trauma Surgery and SpinalCord
Injury, Heidelberg University Hospital, Heidelberg, Germany and4Department of Paraplegiology, Berufsgenossenschaftliche Unfallklinik Ludwigshafen, Ludwigshafen, G
Correspondence:Dr Professor Arash Moghaddam,HTRG Heidelberg Trauma Research Group,Center for Orthopedics,Trauma Surgery and SpinalCord Injury,Heidelberg
University Hospital, Schlierbacher Landstrabe 200a, Heidelberg 69118, Germany.
E-mail: arash.moghaddam@med.uni-heidelberg.de or email@arash.de
Received 7 April2016; revised 2 May 2016;accepted 9 May 2016;published online 5 July 2016
SpinalCord (2017) 55,8–15
& 2017 InternationalSpinalCord Society Allrights reserved 1362-4393/17
www.nature.com/sc
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

of monitoring neurologicalremission for future treatmentstrategies
for SCI;third,if results can be used to generate an improved animal
modelfor investigating SCI.14
The answers to these questions willhelp us find a way to monitor
improvement,as wellas help us develop a usefulobjective score.
MATERIALS AND METHODS
Between 2010 and 2015,data sets of 115 patients (33 females and 82 males)
were recorded after traumatic SCI in the Berufsgenossenschaftliche Unfallklinik
Ludwigshafen (BG Trauma Centre). Following the same match-pair procedure
as in our previous studies,15we selected 10 patients with SCI and neurological
remission (Group 1 = G1) and 10 patientswith an initialAmerican Spinal
Injury Association (ASIA)A grade and no neurologicalremission (Group
0 = G0).Furthermore,we chose 10 patients with an isolated vertebralfracture
without neurological deficits to serve as a control group (Group C = C). Blood
was drawn at the same time points in the studies for both groups. Four vials of
serum (each 7.5 ml) were obtained with a standard procedure at different time
points,that is,4,9,12 h,1 and 3 days and 1,2,4,8 and 12 weeks after SCI.
After 20 min ofcoagulation,blood was centrifuged at3000 r.p.m.,aliquoted
and stored at − 80 °C until analysis. Serum samples were obtained at the same
time points in both groups. The method of sample collection is the same as in
our previous cytokine analysis.16–18The ASIA impairment scale (AIS) grades
were conducted in awake and responsive patients at the time of admission and
12 weeks according to the InternationalStandards for NeurologicalClassifica-
tion ofSCI (ISNCSCI;Table 1).19,20Because BG Ludwigshafen is a primary
trauma centre with itsown helicopterpatientsincluded in the study were
admitted within 2 h after trauma.16 Surgicalstabilisation and decompression
was carried out3.52 ± 1.35 h after trauma.Quantification was carried out in
accordance with the GLP (good laboratory practice)provisions.A clinical
examination of the patients took place parallelto blood sampling.Therefore,
the recovery process was determined by the parameters of the clinicalcourse
(ASIA score).The quantitative measurementof MMP-2, MMP-8, MMP-9,
MMP-10 and MMP-12 from patient serum was conducted using the Luminex
PerformanceHuman High SensitivityCytokinePanel accordingto the
manufacturer’s instructions (Catalogue Number FCST07-05,Kit Lot Number
1415263).The kits were provided by R&D Systems (Minneapolis,MN, USA).
The lab technician performing the test was blinded to allpatients and clinical
information and alllab work was carried outin the Heidelberg University
Hospital.16 Storagetook placeuntil analysisat − 80 °C.Our prospective
observationalstudyhas been approved bythe ethicscommitteeof the
Universityof Heidelberg(S-514/2011)and the Landesärztekammer
Rheinland-Pfalz (837.188.12 / 8289-F),Germany.All study participants signed
and dated consentforms willingly and could voluntarily choose to leave the
study atany time and for any reason.Exclusion criteria were the following:
non-traumatic SCI, traumatic brain injury, severe abdominal trauma, traumatic
amputation of extremities and coma and all patients with an additional major
trauma apartfrom the SCI.Participants were notgiven methylprednisolone
sodium succinate during study participation.
Matching
We compared three groups of patients as in our previous studies:15
Patientswith traumaticSCI without neurologicalremission
(Group 0 = G0)
Patients with traumatic SCI with neurologicalremission
(Group 1 = G1)
Fracture patientswithout neurologicalimpairment(Control
Group = C)
Patients with traumatic SCI who showed no neurologicalremission within
3 monthswereassigned to G0.Thosewith traumaticSCI who showed
neurological remission within 3 months were assigned to G1. We matched t
two groups with a third group of patients with vertebral fractures that prese
no neurological impairment. Patients were enroled in Group C and served a
controlgroup.Patients were matched on the basis offour criteria:age,sex,
aetiology and AO classification. If more than one match was found for a non
remission, then patients with the most similar clinical profile were chosen a
vice versa.According to matching criteria,three groups (each group n = 10)
could be formed of the above-mentioned total of 115 study patients (Table
Statistical analysis
All statisticalcalculationswere performed eitherwith SPSS (SPSS Statistics
for Windows 2012,version 21.0,IBM Corp,Armonk, NY,USA) or R version
3.2.321 using the package ‘pROC’22for receiver operator characteristics (ROC)
analysis.Figureswerecreated by using GraphPad Prism version 5.00 for
Windows,GraphPad Software,CA, USA, www.graphpad.com.Explorative
correlation analyses were conducted between allvariables.In order to detect
location shifts between groups,the non-parametric Mann–Whitney U-test for
independent samples was used.To determine location shifts within one group
at different time points,the Wilcoxon signed-rank test for dependent samples
was used.The Χ2-test was used to assess statistically significant differences in
sex, aetiology of accident, AO Classification, AIS, the type of paralysis and G
Comparison of more than two independent samples was conducted using th
Kruskal–Wallistest.Logisticregression modelswereused to assessthe
predictive powerof variablesfor improvementin AIS while adjusting for
potentially clinically relevant covariates.Modelselection for logistic regression
was based on AIC,23and clinical relevance of covariates was taken into account
When appropriate,we investigatedclinicallyjustifiableinteractionsand
moderation effects.The primary measure for the predictive performance of
any logistic regression modelwas area under the curve (AUC) ofthe ROC
curve.All P-values quoted are to be interpreted in a descriptive way as they
were notadjusted formultiple testing,and thisis an exploratory posthoc
analysis.
RESULTS
This study was designed to be a prospective,explorative study with
matched pairs and no randomisation.18Criteria for matching included
the patient’s sex, age, aetiology and AO classification (Table 2). Pa
demographics were documented,and analysis of the entire collective
and comparison of groups was performed as in our previous study16
Patients demographics
In our match-pair analysis,the collective consisted of 30 patients (9
females and 21 males).On average,subjects were 42.03 ± 17.23 years
of age. Twenty patients were affected by traumatic SCI and serve
study group (Group S = S).Ten patientshad a traumaticinjury
withoutneurologicalimpairmentand serveas the controlgroup
(Group C = C).In the study group,there were 10 patients with AIS
improvement (remission) and 10 AIS A grades with no improvemen
(no remission).There were no lesions of the spinal cord.The clinical
characteristics of the entire collective are given in Table 2.
Table 1 ASIA impairment scale (AIS) grade and functional
impairment (clinical state) due to SCI
AIS grade Clinical state
A Complete—no motor or sensory function is preserved in the sacral
segments S4–S5
B Incomplete—sensory but not motor function is preserved below the NLI
and includes the sacral segments S4–S5
C Incomplete—motor function is preserved below the NLI, and more than
half of the key muscles below the NLI have a muscle grade o3
D Incomplete—motor function is preserved below the NLI, and at least half
of the key muscles below the NLI have a muscle grade of 3 or more
E Normal—motor and sensory function is normal
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
9
Spinal Cord
for SCI;third,if results can be used to generate an improved animal
modelfor investigating SCI.14
The answers to these questions willhelp us find a way to monitor
improvement,as wellas help us develop a usefulobjective score.
MATERIALS AND METHODS
Between 2010 and 2015,data sets of 115 patients (33 females and 82 males)
were recorded after traumatic SCI in the Berufsgenossenschaftliche Unfallklinik
Ludwigshafen (BG Trauma Centre). Following the same match-pair procedure
as in our previous studies,15we selected 10 patients with SCI and neurological
remission (Group 1 = G1) and 10 patientswith an initialAmerican Spinal
Injury Association (ASIA)A grade and no neurologicalremission (Group
0 = G0).Furthermore,we chose 10 patients with an isolated vertebralfracture
without neurological deficits to serve as a control group (Group C = C). Blood
was drawn at the same time points in the studies for both groups. Four vials of
serum (each 7.5 ml) were obtained with a standard procedure at different time
points,that is,4,9,12 h,1 and 3 days and 1,2,4,8 and 12 weeks after SCI.
After 20 min ofcoagulation,blood was centrifuged at3000 r.p.m.,aliquoted
and stored at − 80 °C until analysis. Serum samples were obtained at the same
time points in both groups. The method of sample collection is the same as in
our previous cytokine analysis.16–18The ASIA impairment scale (AIS) grades
were conducted in awake and responsive patients at the time of admission and
12 weeks according to the InternationalStandards for NeurologicalClassifica-
tion ofSCI (ISNCSCI;Table 1).19,20Because BG Ludwigshafen is a primary
trauma centre with itsown helicopterpatientsincluded in the study were
admitted within 2 h after trauma.16 Surgicalstabilisation and decompression
was carried out3.52 ± 1.35 h after trauma.Quantification was carried out in
accordance with the GLP (good laboratory practice)provisions.A clinical
examination of the patients took place parallelto blood sampling.Therefore,
the recovery process was determined by the parameters of the clinicalcourse
(ASIA score).The quantitative measurementof MMP-2, MMP-8, MMP-9,
MMP-10 and MMP-12 from patient serum was conducted using the Luminex
PerformanceHuman High SensitivityCytokinePanel accordingto the
manufacturer’s instructions (Catalogue Number FCST07-05,Kit Lot Number
1415263).The kits were provided by R&D Systems (Minneapolis,MN, USA).
The lab technician performing the test was blinded to allpatients and clinical
information and alllab work was carried outin the Heidelberg University
Hospital.16 Storagetook placeuntil analysisat − 80 °C.Our prospective
observationalstudyhas been approved bythe ethicscommitteeof the
Universityof Heidelberg(S-514/2011)and the Landesärztekammer
Rheinland-Pfalz (837.188.12 / 8289-F),Germany.All study participants signed
and dated consentforms willingly and could voluntarily choose to leave the
study atany time and for any reason.Exclusion criteria were the following:
non-traumatic SCI, traumatic brain injury, severe abdominal trauma, traumatic
amputation of extremities and coma and all patients with an additional major
trauma apartfrom the SCI.Participants were notgiven methylprednisolone
sodium succinate during study participation.
Matching
We compared three groups of patients as in our previous studies:15
Patientswith traumaticSCI without neurologicalremission
(Group 0 = G0)
Patients with traumatic SCI with neurologicalremission
(Group 1 = G1)
Fracture patientswithout neurologicalimpairment(Control
Group = C)
Patients with traumatic SCI who showed no neurologicalremission within
3 monthswereassigned to G0.Thosewith traumaticSCI who showed
neurological remission within 3 months were assigned to G1. We matched t
two groups with a third group of patients with vertebral fractures that prese
no neurological impairment. Patients were enroled in Group C and served a
controlgroup.Patients were matched on the basis offour criteria:age,sex,
aetiology and AO classification. If more than one match was found for a non
remission, then patients with the most similar clinical profile were chosen a
vice versa.According to matching criteria,three groups (each group n = 10)
could be formed of the above-mentioned total of 115 study patients (Table
Statistical analysis
All statisticalcalculationswere performed eitherwith SPSS (SPSS Statistics
for Windows 2012,version 21.0,IBM Corp,Armonk, NY,USA) or R version
3.2.321 using the package ‘pROC’22for receiver operator characteristics (ROC)
analysis.Figureswerecreated by using GraphPad Prism version 5.00 for
Windows,GraphPad Software,CA, USA, www.graphpad.com.Explorative
correlation analyses were conducted between allvariables.In order to detect
location shifts between groups,the non-parametric Mann–Whitney U-test for
independent samples was used.To determine location shifts within one group
at different time points,the Wilcoxon signed-rank test for dependent samples
was used.The Χ2-test was used to assess statistically significant differences in
sex, aetiology of accident, AO Classification, AIS, the type of paralysis and G
Comparison of more than two independent samples was conducted using th
Kruskal–Wallistest.Logisticregression modelswereused to assessthe
predictive powerof variablesfor improvementin AIS while adjusting for
potentially clinically relevant covariates.Modelselection for logistic regression
was based on AIC,23and clinical relevance of covariates was taken into account
When appropriate,we investigatedclinicallyjustifiableinteractionsand
moderation effects.The primary measure for the predictive performance of
any logistic regression modelwas area under the curve (AUC) ofthe ROC
curve.All P-values quoted are to be interpreted in a descriptive way as they
were notadjusted formultiple testing,and thisis an exploratory posthoc
analysis.
RESULTS
This study was designed to be a prospective,explorative study with
matched pairs and no randomisation.18Criteria for matching included
the patient’s sex, age, aetiology and AO classification (Table 2). Pa
demographics were documented,and analysis of the entire collective
and comparison of groups was performed as in our previous study16
Patients demographics
In our match-pair analysis,the collective consisted of 30 patients (9
females and 21 males).On average,subjects were 42.03 ± 17.23 years
of age. Twenty patients were affected by traumatic SCI and serve
study group (Group S = S).Ten patientshad a traumaticinjury
withoutneurologicalimpairmentand serveas the controlgroup
(Group C = C).In the study group,there were 10 patients with AIS
improvement (remission) and 10 AIS A grades with no improvemen
(no remission).There were no lesions of the spinal cord.The clinical
characteristics of the entire collective are given in Table 2.
Table 1 ASIA impairment scale (AIS) grade and functional
impairment (clinical state) due to SCI
AIS grade Clinical state
A Complete—no motor or sensory function is preserved in the sacral
segments S4–S5
B Incomplete—sensory but not motor function is preserved below the NLI
and includes the sacral segments S4–S5
C Incomplete—motor function is preserved below the NLI, and more than
half of the key muscles below the NLI have a muscle grade o3
D Incomplete—motor function is preserved below the NLI, and at least half
of the key muscles below the NLI have a muscle grade of 3 or more
E Normal—motor and sensory function is normal
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
9
Spinal Cord
Therewasno significantdifferencein the distribution ofage,
gender,aetiology and AO classification between patientswith and
withoutneurologicalremission (referred to asG1 and G0 in the
following text,respectively).All 20 patients with traumatic SCI (G0
and G1) received surgery (9 ventral45%;11 dorsal55%);14 were
treated with spondylodesis (70.0%).All 30 patients included in the
collective suffered vertebral fractures. The AIS grades at admission and
discharge as well as the NLI and the type of paralysis were significantly
different in G1 and G0 (Po0.05).
Analysis of the entire patient collective
For exact MMP concentrations (pg ml− 1) and a graphic comparison
of the groups,seeFigures1 and 2. Therewereno significant
differences in cytokine serum levels in regard to gender, age, p
AO classification,aetiology or NLI.
Serum values of MMP-10 and MMP-12 remained undetectable
Comparison of SCI patients vs control group
We investigated how MMP values reflectthe biochemicalprocesses
after SCI by comparing patients with (S) with patients without(C)
neurologicalimpairment.
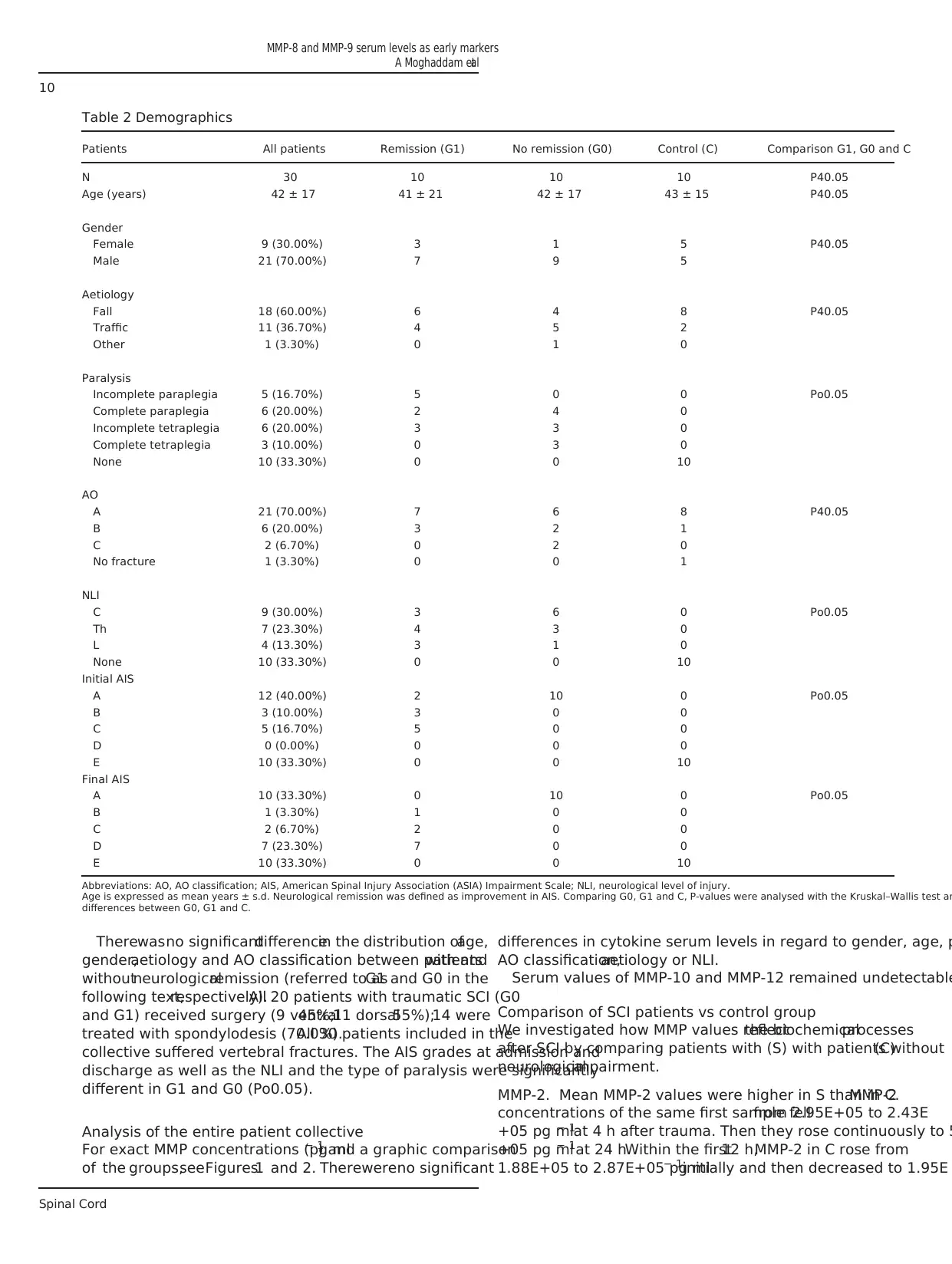
MMP-2. Mean MMP-2 values were higher in S than in C.MMP-2
concentrations of the same first sample fellfrom 2.95E+05 to 2.43E
+05 pg ml− 1at 4 h after trauma. Then they rose continuously to 5
+05 pg ml− 1at 24 h.Within the first12 h,MMP-2 in C rose from
1.88E+05 to 2.87E+05 pg ml− 1initially and then decreased to 1.95E
Table 2 Demographics
Patients All patients Remission (G1) No remission (G0) Control (C) Comparison G1, G0 and C
N 30 10 10 10 P40.05
Age (years) 42 ± 17 41 ± 21 42 ± 17 43 ± 15 P40.05
Gender
Female 9 (30.00%) 3 1 5 P40.05
Male 21 (70.00%) 7 9 5
Aetiology
Fall 18 (60.00%) 6 4 8 P40.05
Traffic 11 (36.70%) 4 5 2
Other 1 (3.30%) 0 1 0
Paralysis
Incomplete paraplegia 5 (16.70%) 5 0 0 Po0.05
Complete paraplegia 6 (20.00%) 2 4 0
Incomplete tetraplegia 6 (20.00%) 3 3 0
Complete tetraplegia 3 (10.00%) 0 3 0
None 10 (33.30%) 0 0 10
AO
A 21 (70.00%) 7 6 8 P40.05
B 6 (20.00%) 3 2 1
C 2 (6.70%) 0 2 0
No fracture 1 (3.30%) 0 0 1
NLI
C 9 (30.00%) 3 6 0 Po0.05
Th 7 (23.30%) 4 3 0
L 4 (13.30%) 3 1 0
None 10 (33.30%) 0 0 10
Initial AIS
A 12 (40.00%) 2 10 0 Po0.05
B 3 (10.00%) 3 0 0
C 5 (16.70%) 5 0 0
D 0 (0.00%) 0 0 0
E 10 (33.30%) 0 0 10
Final AIS
A 10 (33.30%) 0 10 0 Po0.05
B 1 (3.30%) 1 0 0
C 2 (6.70%) 2 0 0
D 7 (23.30%) 7 0 0
E 10 (33.30%) 0 0 10
Abbreviations: AO, AO classification; AIS, American Spinal Injury Association (ASIA) Impairment Scale; NLI, neurological level of injury.
Age is expressed as mean years ± s.d. Neurological remission was defined as improvement in AIS. Comparing G0, G1 and C, P-values were analysed with the Kruskal–Wallis test an
differences between G0, G1 and C.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
10
Spinal Cord
gender,aetiology and AO classification between patientswith and
withoutneurologicalremission (referred to asG1 and G0 in the
following text,respectively).All 20 patients with traumatic SCI (G0
and G1) received surgery (9 ventral45%;11 dorsal55%);14 were
treated with spondylodesis (70.0%).All 30 patients included in the
collective suffered vertebral fractures. The AIS grades at admission and
discharge as well as the NLI and the type of paralysis were significantly
different in G1 and G0 (Po0.05).
Analysis of the entire patient collective
For exact MMP concentrations (pg ml− 1) and a graphic comparison
of the groups,seeFigures1 and 2. Therewereno significant
differences in cytokine serum levels in regard to gender, age, p
AO classification,aetiology or NLI.
Serum values of MMP-10 and MMP-12 remained undetectable
Comparison of SCI patients vs control group
We investigated how MMP values reflectthe biochemicalprocesses
after SCI by comparing patients with (S) with patients without(C)
neurologicalimpairment.
MMP-2. Mean MMP-2 values were higher in S than in C.MMP-2
concentrations of the same first sample fellfrom 2.95E+05 to 2.43E
+05 pg ml− 1at 4 h after trauma. Then they rose continuously to 5
+05 pg ml− 1at 24 h.Within the first12 h,MMP-2 in C rose from
1.88E+05 to 2.87E+05 pg ml− 1initially and then decreased to 1.95E
Table 2 Demographics
Patients All patients Remission (G1) No remission (G0) Control (C) Comparison G1, G0 and C
N 30 10 10 10 P40.05
Age (years) 42 ± 17 41 ± 21 42 ± 17 43 ± 15 P40.05
Gender
Female 9 (30.00%) 3 1 5 P40.05
Male 21 (70.00%) 7 9 5
Aetiology
Fall 18 (60.00%) 6 4 8 P40.05
Traffic 11 (36.70%) 4 5 2
Other 1 (3.30%) 0 1 0
Paralysis
Incomplete paraplegia 5 (16.70%) 5 0 0 Po0.05
Complete paraplegia 6 (20.00%) 2 4 0
Incomplete tetraplegia 6 (20.00%) 3 3 0
Complete tetraplegia 3 (10.00%) 0 3 0
None 10 (33.30%) 0 0 10
AO
A 21 (70.00%) 7 6 8 P40.05
B 6 (20.00%) 3 2 1
C 2 (6.70%) 0 2 0
No fracture 1 (3.30%) 0 0 1
NLI
C 9 (30.00%) 3 6 0 Po0.05
Th 7 (23.30%) 4 3 0
L 4 (13.30%) 3 1 0
None 10 (33.30%) 0 0 10
Initial AIS
A 12 (40.00%) 2 10 0 Po0.05
B 3 (10.00%) 3 0 0
C 5 (16.70%) 5 0 0
D 0 (0.00%) 0 0 0
E 10 (33.30%) 0 0 10
Final AIS
A 10 (33.30%) 0 10 0 Po0.05
B 1 (3.30%) 1 0 0
C 2 (6.70%) 2 0 0
D 7 (23.30%) 7 0 0
E 10 (33.30%) 0 0 10
Abbreviations: AO, AO classification; AIS, American Spinal Injury Association (ASIA) Impairment Scale; NLI, neurological level of injury.
Age is expressed as mean years ± s.d. Neurological remission was defined as improvement in AIS. Comparing G0, G1 and C, P-values were analysed with the Kruskal–Wallis test an
differences between G0, G1 and C.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
10
Spinal Cord
+05 pg ml− 1. After 24 h,S and C adjusted to each other at a mean
level of 2.41E+05 ± 1.40E+04 pg ml− 1. No significant difference from
admission level has been detected. The Man–Whitney U-test assigned
no significant difference at every time point.
MMP-8. Mean MMP-8 values were 1.73E+04 ± 1.85E+03 pg ml− 1
for the first 24 h.MMP-8 concentrations of S increased after 3 days
from 1.42E+04 to 3.58E+04 pg ml− 1at 1 week after trauma.In C,
MMP-8 fell after 3 days from 1.55E+04 to 1.32E+04 pg ml− 1and then
rose to 2.59E+04 pg ml− 1. One week aftertrauma,we detected a
significant difference in the levelin S on admission (P = 0.016).The
Mann–Whitney U-test showed no significant differences at any time
point.Afterwards,the levels in both groups adjusted again to each
other at a mean levelof 1.73E+04 ± 2.17E+03 pg ml− 1.
MMP-9. Mean MMP-9 values for S and C showed the same pattern
for the whole period of time.In S,we detected significant differences
from the admissionlevel3 days(P = 0.002),1 (P = 0.011),2
(P = 0.017)and 3 months(P = 0.011)aftertrauma.The Mann–
Whitney U-testassigned no significant difference atany time point.
The globalMMP-9 trend is decreasing from a mean value of8.05E
+05 ± 7.21E+04 pg ml− 1 in the beginningto 3.81E+05 ± 5.03E
+04 pg ml− 13 months after trauma.
Comparison of patients with neurological remission vs patients
without neurological remission
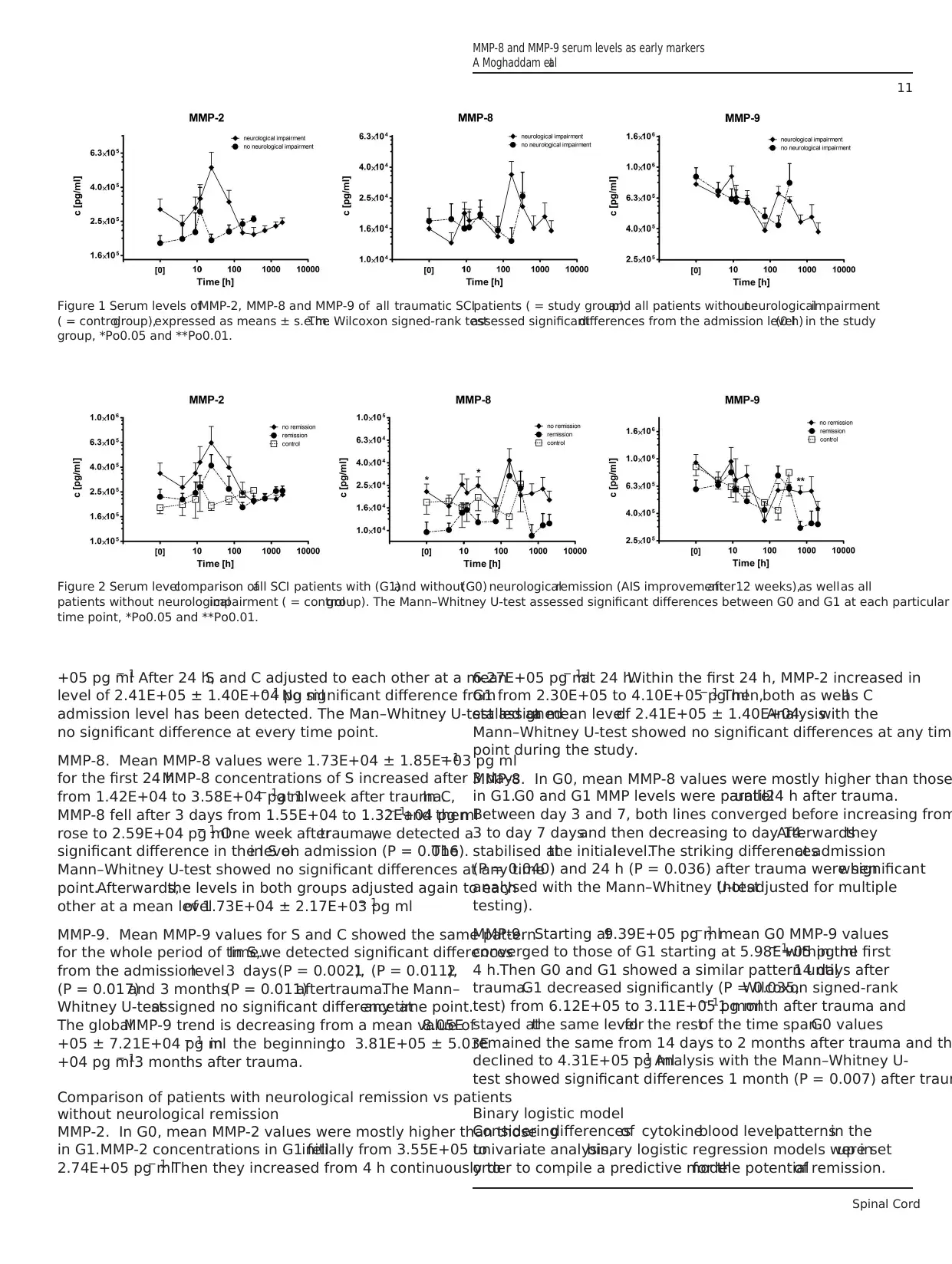
MMP-2. In G0, mean MMP-2 values were mostly higher than those
in G1.MMP-2 concentrations in G1 fellinitially from 3.55E+05 to
2.74E+05 pg ml− 1. Then they increased from 4 h continuously to
6.27E+05 pg ml− 1at 24 h.Within the first 24 h, MMP-2 increased in
G1 from 2.30E+05 to 4.10E+05 pg ml− 1. Then,both as wellas C
stalled ata mean levelof 2.41E+05 ± 1.40E+04.Analysiswith the
Mann–Whitney U-test showed no significant differences at any tim
point during the study.
MMP-8. In G0, mean MMP-8 values were mostly higher than those
in G1.G0 and G1 MMP levels were paralleluntil24 h after trauma.
Between day 3 and 7, both lines converged before increasing from
3 to day 7 daysand then decreasing to day 14.Afterwardsthey
stabilised atthe initiallevel.The striking differencesat admission
(P = 0.040) and 24 h (P = 0.036) after trauma were significantwhen
analysed with the Mann–Whitney U-test(notadjusted for multiple
testing).
MMP-9. Starting at9.39E+05 pg ml− 1, mean G0 MMP-9 values
converged to those of G1 starting at 5.98E+05 pg ml− 1within the first
4 h.Then G0 and G1 showed a similar pattern until14 days after
trauma.G1 decreased significantly (P = 0.035,Wilcoxon signed-rank
test) from 6.12E+05 to 3.11E+05 pg ml− 11 month after trauma and
stayed atthe same levelfor the restof the time span.G0 values
remained the same from 14 days to 2 months after trauma and th
declined to 4.31E+05 pg ml− 1. Analysis with the Mann–Whitney U-
test showed significant differences 1 month (P = 0.007) after traum
Binary logistic model
Consideringdifferencesof cytokineblood levelpatternsin the
univariate analysis,binary logistic regression models were setup in
order to compile a predictive modelfor the potentialof remission.
Figure 1 Serum levels ofMMP-2, MMP-8 and MMP-9 of all traumatic SCIpatients ( = study group)and all patients withoutneurologicalimpairment
( = controlgroup),expressed as means ± s.e.m.The Wilcoxon signed-rank testassessed significantdifferences from the admission level(0 h) in the study
group, *Po0.05 and **Po0.01.
Figure 2 Serum levelcomparison ofall SCI patients with (G1)and without(G0) neurologicalremission (AIS improvementafter12 weeks),as wellas all
patients without neurologicalimpairment ( = controlgroup). The Mann–Whitney U-test assessed significant differences between G0 and G1 at each particular
time point, *Po0.05 and **Po0.01.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
11
Spinal Cord
level of 2.41E+05 ± 1.40E+04 pg ml− 1. No significant difference from
admission level has been detected. The Man–Whitney U-test assigned
no significant difference at every time point.
MMP-8. Mean MMP-8 values were 1.73E+04 ± 1.85E+03 pg ml− 1
for the first 24 h.MMP-8 concentrations of S increased after 3 days
from 1.42E+04 to 3.58E+04 pg ml− 1at 1 week after trauma.In C,
MMP-8 fell after 3 days from 1.55E+04 to 1.32E+04 pg ml− 1and then
rose to 2.59E+04 pg ml− 1. One week aftertrauma,we detected a
significant difference in the levelin S on admission (P = 0.016).The
Mann–Whitney U-test showed no significant differences at any time
point.Afterwards,the levels in both groups adjusted again to each
other at a mean levelof 1.73E+04 ± 2.17E+03 pg ml− 1.
MMP-9. Mean MMP-9 values for S and C showed the same pattern
for the whole period of time.In S,we detected significant differences
from the admissionlevel3 days(P = 0.002),1 (P = 0.011),2
(P = 0.017)and 3 months(P = 0.011)aftertrauma.The Mann–
Whitney U-testassigned no significant difference atany time point.
The globalMMP-9 trend is decreasing from a mean value of8.05E
+05 ± 7.21E+04 pg ml− 1 in the beginningto 3.81E+05 ± 5.03E
+04 pg ml− 13 months after trauma.
Comparison of patients with neurological remission vs patients
without neurological remission
MMP-2. In G0, mean MMP-2 values were mostly higher than those
in G1.MMP-2 concentrations in G1 fellinitially from 3.55E+05 to
2.74E+05 pg ml− 1. Then they increased from 4 h continuously to
6.27E+05 pg ml− 1at 24 h.Within the first 24 h, MMP-2 increased in
G1 from 2.30E+05 to 4.10E+05 pg ml− 1. Then,both as wellas C
stalled ata mean levelof 2.41E+05 ± 1.40E+04.Analysiswith the
Mann–Whitney U-test showed no significant differences at any tim
point during the study.
MMP-8. In G0, mean MMP-8 values were mostly higher than those
in G1.G0 and G1 MMP levels were paralleluntil24 h after trauma.
Between day 3 and 7, both lines converged before increasing from
3 to day 7 daysand then decreasing to day 14.Afterwardsthey
stabilised atthe initiallevel.The striking differencesat admission
(P = 0.040) and 24 h (P = 0.036) after trauma were significantwhen
analysed with the Mann–Whitney U-test(notadjusted for multiple
testing).
MMP-9. Starting at9.39E+05 pg ml− 1, mean G0 MMP-9 values
converged to those of G1 starting at 5.98E+05 pg ml− 1within the first
4 h.Then G0 and G1 showed a similar pattern until14 days after
trauma.G1 decreased significantly (P = 0.035,Wilcoxon signed-rank
test) from 6.12E+05 to 3.11E+05 pg ml− 11 month after trauma and
stayed atthe same levelfor the restof the time span.G0 values
remained the same from 14 days to 2 months after trauma and th
declined to 4.31E+05 pg ml− 1. Analysis with the Mann–Whitney U-
test showed significant differences 1 month (P = 0.007) after traum
Binary logistic model
Consideringdifferencesof cytokineblood levelpatternsin the
univariate analysis,binary logistic regression models were setup in
order to compile a predictive modelfor the potentialof remission.
Figure 1 Serum levels ofMMP-2, MMP-8 and MMP-9 of all traumatic SCIpatients ( = study group)and all patients withoutneurologicalimpairment
( = controlgroup),expressed as means ± s.e.m.The Wilcoxon signed-rank testassessed significantdifferences from the admission level(0 h) in the study
group, *Po0.05 and **Po0.01.
Figure 2 Serum levelcomparison ofall SCI patients with (G1)and without(G0) neurologicalremission (AIS improvementafter12 weeks),as wellas all
patients without neurologicalimpairment ( = controlgroup). The Mann–Whitney U-test assessed significant differences between G0 and G1 at each particular
time point, *Po0.05 and **Po0.01.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
11
Spinal Cord
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
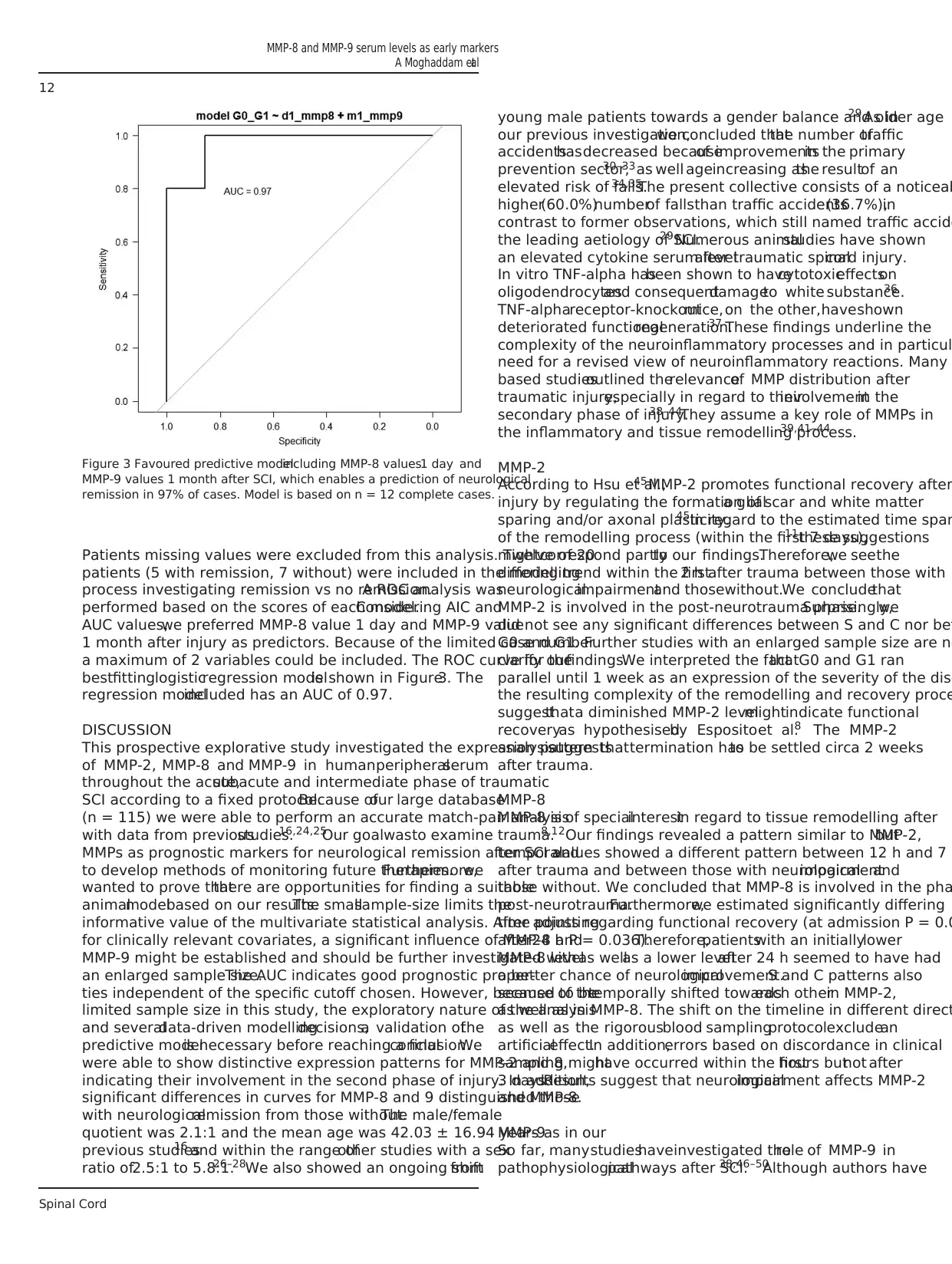
Patients missing values were excluded from this analysis. Twelve of 20
patients (5 with remission, 7 without) were included in the modelling
process investigating remission vs no remission.A ROC analysis was
performed based on the scores of each model.Considering AIC and
AUC values,we preferred MMP-8 value 1 day and MMP-9 value
1 month after injury as predictors. Because of the limited case number
a maximum of 2 variables could be included. The ROC curve for the
bestfittinglogisticregression modelis shown in Figure3. The
regression modelincluded has an AUC of 0.97.
DISCUSSION
This prospective explorative study investigated the expression pattern
of MMP-2, MMP-8 and MMP-9 in humanperipheralserum
throughout the acute,subacute and intermediate phase of traumatic
SCI according to a fixed protocol.Because ofour large database
(n = 115) we were able to perform an accurate match-pair analysis
with data from previousstudies.16,24,25Our goalwasto examine
MMPs as prognostic markers for neurological remission after SCI and
to develop methods of monitoring future therapies.Furthermore,we
wanted to prove thatthere are opportunities for finding a suitable
animalmodelbased on our results.The smallsample-size limits the
informative value of the multivariate statistical analysis. After adjusting
for clinically relevant covariates, a significant influence of MMP-8 and
MMP-9 might be established and should be further investigated with
an enlarged sample size.The AUC indicates good prognostic proper-
ties independent of the specific cutoff chosen. However, because of the
limited sample size in this study, the exploratory nature of the analysis
and severaldata-driven modellingdecisions,a validation ofthe
predictive modelis necessary before reaching a finalconclusion.We
were able to show distinctive expression patterns for MMP-2 and 8,
indicating their involvement in the second phase of injury. In addition,
significant differences in curves for MMP-8 and 9 distinguished those
with neurologicalremission from those without.The male/female
quotient was 2.1:1 and the mean age was 42.03 ± 16.94 years as in our
previous studies16 and within the range ofother studies with a sex
ratio of2.5:1 to 5.8:1.26–28We also showed an ongoing shiftfrom
young male patients towards a gender balance and older age29 As in
our previous investigation,we concluded thatthe number oftraffic
accidentshasdecreased becauseof improvementsin the primary
prevention sector,30–33as well ageincreasing asthe resultof an
elevated risk of falls.34,35The present collective consists of a noticeab
higher(60.0%)numberof fallsthan traffic accidents(36.7%),in
contrast to former observations, which still named traffic accide
the leading aetiology of SCI.29 Numerous animalstudies have shown
an elevated cytokine serum levelafter traumatic spinalcord injury.
In vitro TNF-alpha hasbeen shown to havecytotoxiceffectson
oligodendrocytesand consequentdamageto white substance.36
TNF-alphareceptor-knockoutmice,on the other,haveshown
deteriorated functionalregeneration.37 These findings underline the
complexity of the neuroinflammatory processes and in particul
need for a revised view of neuroinflammatory reactions. Many
based studiesoutlined therelevanceof MMP distribution after
traumatic injury,especially in regard to theirinvolvementin the
secondary phase of injury.38–44They assume a key role of MMPs in
the inflammatory and tissue remodelling process.39,41–44
MMP-2
According to Hsu et al.,45MMP-2 promotes functional recovery after
injury by regulating the formation ofa glialscar and white matter
sparing and/or axonal plasticity.45In regard to the estimated time span
of the remodelling process (within the first 7 days),11these suggestions
mightcorrespond partlyto our findings.Therefore,we seethe
differing trend within the first2 h after trauma between those with
neurologicalimpairmentand thosewithout.We concludethat
MMP-2 is involved in the post-neurotrauma phase.Surprisingly,we
did not see any significant differences between S and C nor bet
G0 and G1. Further studies with an enlarged sample size are ne
clarify ourfindings.We interpreted the factthatG0 and G1 ran
parallel until 1 week as an expression of the severity of the dise
the resulting complexity of the remodelling and recovery proce
suggestthata diminished MMP-2 levelmightindicate functional
recoveryas hypothesisedby Espositoet al.8 The MMP-2
analysissuggeststhattermination hasto be settled circa 2 weeks
after trauma.
MMP-8
MMP-8 is of specialinterestin regard to tissue remodelling after
trauma.8,12Our findings revealed a pattern similar to MMP-2,but
temporalvalues showed a different pattern between 12 h and 7
after trauma and between those with neurologicalimpairmentand
those without. We concluded that MMP-8 is involved in the pha
post-neurotrauma.Furthermore,we estimated significantly differing
time points regarding functional recovery (at admission P = 0.0
after24 h P = 0.036).Therefore,patientswith an initiallylower
MMP-8 levelas wellas a lower levelafter 24 h seemed to have had
a better chance of neurologicalimprovement.S and C patterns also
seemed to betemporally shifted towardseach otherin MMP-2,
as well as in MMP-8. The shift on the timeline in different direct
as well as the rigorousblood samplingprotocolexcludean
artificialeffect.In addition,errors based on discordance in clinical
sampling mighthave occurred within the firsthours butnot after
3 days.Results suggest that neurologicalimpairment affects MMP-2
and MMP-8.
MMP-9
So far, manystudieshaveinvestigated therole of MMP-9 in
pathophysiologicalpathways after SCI.38,46–50Although authors have
Figure 3 Favoured predictive modelincluding MMP-8 values1 day and
MMP-9 values 1 month after SCI, which enables a prediction of neurological
remission in 97% of cases. Model is based on n = 12 complete cases.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
12
Spinal Cord
patients (5 with remission, 7 without) were included in the modelling
process investigating remission vs no remission.A ROC analysis was
performed based on the scores of each model.Considering AIC and
AUC values,we preferred MMP-8 value 1 day and MMP-9 value
1 month after injury as predictors. Because of the limited case number
a maximum of 2 variables could be included. The ROC curve for the
bestfittinglogisticregression modelis shown in Figure3. The
regression modelincluded has an AUC of 0.97.
DISCUSSION
This prospective explorative study investigated the expression pattern
of MMP-2, MMP-8 and MMP-9 in humanperipheralserum
throughout the acute,subacute and intermediate phase of traumatic
SCI according to a fixed protocol.Because ofour large database
(n = 115) we were able to perform an accurate match-pair analysis
with data from previousstudies.16,24,25Our goalwasto examine
MMPs as prognostic markers for neurological remission after SCI and
to develop methods of monitoring future therapies.Furthermore,we
wanted to prove thatthere are opportunities for finding a suitable
animalmodelbased on our results.The smallsample-size limits the
informative value of the multivariate statistical analysis. After adjusting
for clinically relevant covariates, a significant influence of MMP-8 and
MMP-9 might be established and should be further investigated with
an enlarged sample size.The AUC indicates good prognostic proper-
ties independent of the specific cutoff chosen. However, because of the
limited sample size in this study, the exploratory nature of the analysis
and severaldata-driven modellingdecisions,a validation ofthe
predictive modelis necessary before reaching a finalconclusion.We
were able to show distinctive expression patterns for MMP-2 and 8,
indicating their involvement in the second phase of injury. In addition,
significant differences in curves for MMP-8 and 9 distinguished those
with neurologicalremission from those without.The male/female
quotient was 2.1:1 and the mean age was 42.03 ± 16.94 years as in our
previous studies16 and within the range ofother studies with a sex
ratio of2.5:1 to 5.8:1.26–28We also showed an ongoing shiftfrom
young male patients towards a gender balance and older age29 As in
our previous investigation,we concluded thatthe number oftraffic
accidentshasdecreased becauseof improvementsin the primary
prevention sector,30–33as well ageincreasing asthe resultof an
elevated risk of falls.34,35The present collective consists of a noticeab
higher(60.0%)numberof fallsthan traffic accidents(36.7%),in
contrast to former observations, which still named traffic accide
the leading aetiology of SCI.29 Numerous animalstudies have shown
an elevated cytokine serum levelafter traumatic spinalcord injury.
In vitro TNF-alpha hasbeen shown to havecytotoxiceffectson
oligodendrocytesand consequentdamageto white substance.36
TNF-alphareceptor-knockoutmice,on the other,haveshown
deteriorated functionalregeneration.37 These findings underline the
complexity of the neuroinflammatory processes and in particul
need for a revised view of neuroinflammatory reactions. Many
based studiesoutlined therelevanceof MMP distribution after
traumatic injury,especially in regard to theirinvolvementin the
secondary phase of injury.38–44They assume a key role of MMPs in
the inflammatory and tissue remodelling process.39,41–44
MMP-2
According to Hsu et al.,45MMP-2 promotes functional recovery after
injury by regulating the formation ofa glialscar and white matter
sparing and/or axonal plasticity.45In regard to the estimated time span
of the remodelling process (within the first 7 days),11these suggestions
mightcorrespond partlyto our findings.Therefore,we seethe
differing trend within the first2 h after trauma between those with
neurologicalimpairmentand thosewithout.We concludethat
MMP-2 is involved in the post-neurotrauma phase.Surprisingly,we
did not see any significant differences between S and C nor bet
G0 and G1. Further studies with an enlarged sample size are ne
clarify ourfindings.We interpreted the factthatG0 and G1 ran
parallel until 1 week as an expression of the severity of the dise
the resulting complexity of the remodelling and recovery proce
suggestthata diminished MMP-2 levelmightindicate functional
recoveryas hypothesisedby Espositoet al.8 The MMP-2
analysissuggeststhattermination hasto be settled circa 2 weeks
after trauma.
MMP-8
MMP-8 is of specialinterestin regard to tissue remodelling after
trauma.8,12Our findings revealed a pattern similar to MMP-2,but
temporalvalues showed a different pattern between 12 h and 7
after trauma and between those with neurologicalimpairmentand
those without. We concluded that MMP-8 is involved in the pha
post-neurotrauma.Furthermore,we estimated significantly differing
time points regarding functional recovery (at admission P = 0.0
after24 h P = 0.036).Therefore,patientswith an initiallylower
MMP-8 levelas wellas a lower levelafter 24 h seemed to have had
a better chance of neurologicalimprovement.S and C patterns also
seemed to betemporally shifted towardseach otherin MMP-2,
as well as in MMP-8. The shift on the timeline in different direct
as well as the rigorousblood samplingprotocolexcludean
artificialeffect.In addition,errors based on discordance in clinical
sampling mighthave occurred within the firsthours butnot after
3 days.Results suggest that neurologicalimpairment affects MMP-2
and MMP-8.
MMP-9
So far, manystudieshaveinvestigated therole of MMP-9 in
pathophysiologicalpathways after SCI.38,46–50Although authors have
Figure 3 Favoured predictive modelincluding MMP-8 values1 day and
MMP-9 values 1 month after SCI, which enables a prediction of neurological
remission in 97% of cases. Model is based on n = 12 complete cases.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
12
Spinal Cord

provided explicit evidence that MMP-9 is integral to the formation of an
inhibitory glialscarand cytoskeleton-mediated astrocyte migration51
and indicated that MMP-9 is a key factor involved in the disruption
of the endothelialblood-barrier ofthe spinalcord and subsequent
secondary damage after photo thrombotic SCI in rats,39,52we could
not find any clearevidence ofneurologicalimpairmentbesidesa
globaltrend to decline.Regardingfunctionalimprovement,our
findings suggestthatlower MMP-9 levels provide a higher capacity
to recoverfrom neurologicalimpairment(1 month afterinjury
P = 0.007). In mice, MMP-9 was shown to promote motor relearning
after thoracic spinal cord injury.38In contrast, Piao et al.41found that
melatonin improved functionaloutcomevia inhibition ofmatrix
metalloproteinases-9 after photo thrombotic SCI in rats, suggesting an
oppositeeffect.Furthermore,oxidativestressafterSCI induces
MMP-9 expression and endothelialinjury.49 These findings correlate
with Yang etal.,48 showing thathyperbaric oxygen (HBO) reduces
SCI-induced spinalcord oedema,stabilising the blood–spinalcord
barrierand promotingrecoveryof neuronalfunction bydown-
regulating serum levels of IL-6, MMP-2 and MMP-9 and upregulating
VEGF.48 Apparently the MMP-9 pattern reflects partially the upregu-
lation of MMP-2 within the first 72 h and MMP-8 in the time span
from 72 h to 1 month after trauma.In contrast to MMP-8,group C
shows a delayed upregulation ofMMP-9. We know MMP-9 to be
involved in various biochemical processes after traumatic injuries. The
present characteristic shows the systemic level and is therefore difficult
to interpret.To gain more specific information for MMP-9 regarding
neurotrauma,one would need to analysecerebrospinalfluid. In
variousapproaches,MMP-9 also hasbeen directlyor indirectly
targeted to influence neurologicaloutcome after SCI.For example,
Sulforaphane provides a neuroprotective effect by decreasing mRNA
levels of MMP-9.53Increasing NG2+ cell proliferation in the damaged
area by targeting MMP-9 resulted in a functional recovery of the spinal
cord.40
Limitations
The transferability or conclusions from animal to human models have
been shown to hold several deficits;13however, the animal model does
have the advantage that is not as limited in the size of the collective.
Our explorative prospective study involved a small collective (n = 30),
and therefore results may notrepresentlocalprocesses clearly.We
decided to include different locations and thus differing severities of
SCI in G0 and G1.Therefore,in the current study,allpatients in G1
have an initialAISA grade A and G0 varies from A to C.This might
contribute to differences within the groups and thus disguise patterns
in the data.Furtherstudieswith an enlarged sample size should
address an inter-group (G1 vs G0) comparison with each AISA grade,
as the prognosis is often vastly different. Because of the large standard
errors to the mean and the smallnumber of patients,the significant
differences as described for MMP-8 and MMP-9 must be considered
critically. Furthermore, studies must figure out the veracity of the data
by increasing the sample size.The temporalchanges in metallopro-
teins after 24 h (MMP-2), 1 week (MMP-8) and 72 h (MMP-9) might
have a key role in the pathophysiology of SCI and could prove to be
good predictive markers.Because of the smallsample size we cannot
conclude whether they randomly increased atthese time points.In
orderto provethe involvementof MMPs in neuroinflammatory
processes, future studies are needed that correlate MMP levels to other
establishedinflammationmarkerssuch as interferon-γ,tumour
necrosis factor-α,interleukin(IL)-1β,IL-6, IL-8, IL-10,and vascular
endothelialgrowth factor.Furthermore,because ofmissing epitope
mapping we can only provide valid evidence for the assay used in
the study.
MMP-10
MMP-10 is known to regulate the lysis ofcollagen in alternatively
activated resident macrophages. Furthermore, MMP-10, which is n
collagenase,promoted an increase in the serum levelof MMP-8 and
MMP-13,two metallocollagenases released by macrophages.9 Unfor-
tunately,we gainedno furtherinformation regardingMMP-10
because of the low serum level.
MMP-12
Wells et al.42 obtained data revealing that MMP-12 is destructive and
contributes to the developmentof secondary injury in SCI in mice.
This metalloproteinase contributes to the secondary injury process
and in its absence mice recovered better from the insult.42Because of
low serum levels we gained no further information about MMP-12.
Predictive modelling
Although a predictive modelstratifying injury severity and predicting
neurologicaloutcome mightbe extremely usefulfor facilitating the
clinical validation of novel treatments in acute human SCI,14findings
based on the present modelling process must be considered critica
P-values are unadjusted for multiple testing and should be interpre
descriptively.Becauseof the smallsamplesizeof completecases
(n = 12) the predictive value ofregression modelsas wellas ROC
analysis should be interpreted in an explorative way.Considering the
adjusted odds ratios (nearby 1) and their sharp corresponding cis,
have to interpret them in relation to the high serum levels (106 and
more).Nevertheless,by including the MMP-8 value after day 1 and
the MMP-9 valueafter1 month aspredictorsand by including
remission/no remission as a criterion,we gained a predictive model
with an AUC = 0.97. Besides this statistically impressive result, fur
studies are needed with a larger collective. Apart from that, this m
mightbe combined with a costbenefitanalysis thatdetermined by
revealing the ratio of sensitivity/(1 − specificity) based on the resp
clinicalquestion.Thus,an exactcutoffvaluefor sensitivityand
specificity might be estimated.On the basis of this information both
can be calculated for the model. Therefore, the present study prov
key informationto developinga reliableSCI scorebasedon
biometric data.
Perspective
So far, our results stage interesting references for the understandi
biochemical processes following SCI. Further studies with an enlarg
collective should also focus on patients with an initial AISA A gradi
and examine their specific cytokine profile regarding the incidence
neurologicalrecovery.Additively,the inclusion ofapproaches in the
radiologicalassessmentstill remainsvery promisingsourcesof
information and therefore should also be investigated in the future
In particular,in a setting where a satisfactory clinicalexamination is
not possible,they might provide relevant supplementary information
The current study showed peripheralserum analysis to be a suitable
concept for assessing the patient’s potential for neurological remis
after tSCI.In addition,the present data supply promising approaches
for future monitoring concepts for current therapies, as well as ani
models. Moreover, the results provide key information for a predic
scoring system for SCI patients.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
13
Spinal Cord
inhibitory glialscarand cytoskeleton-mediated astrocyte migration51
and indicated that MMP-9 is a key factor involved in the disruption
of the endothelialblood-barrier ofthe spinalcord and subsequent
secondary damage after photo thrombotic SCI in rats,39,52we could
not find any clearevidence ofneurologicalimpairmentbesidesa
globaltrend to decline.Regardingfunctionalimprovement,our
findings suggestthatlower MMP-9 levels provide a higher capacity
to recoverfrom neurologicalimpairment(1 month afterinjury
P = 0.007). In mice, MMP-9 was shown to promote motor relearning
after thoracic spinal cord injury.38In contrast, Piao et al.41found that
melatonin improved functionaloutcomevia inhibition ofmatrix
metalloproteinases-9 after photo thrombotic SCI in rats, suggesting an
oppositeeffect.Furthermore,oxidativestressafterSCI induces
MMP-9 expression and endothelialinjury.49 These findings correlate
with Yang etal.,48 showing thathyperbaric oxygen (HBO) reduces
SCI-induced spinalcord oedema,stabilising the blood–spinalcord
barrierand promotingrecoveryof neuronalfunction bydown-
regulating serum levels of IL-6, MMP-2 and MMP-9 and upregulating
VEGF.48 Apparently the MMP-9 pattern reflects partially the upregu-
lation of MMP-2 within the first 72 h and MMP-8 in the time span
from 72 h to 1 month after trauma.In contrast to MMP-8,group C
shows a delayed upregulation ofMMP-9. We know MMP-9 to be
involved in various biochemical processes after traumatic injuries. The
present characteristic shows the systemic level and is therefore difficult
to interpret.To gain more specific information for MMP-9 regarding
neurotrauma,one would need to analysecerebrospinalfluid. In
variousapproaches,MMP-9 also hasbeen directlyor indirectly
targeted to influence neurologicaloutcome after SCI.For example,
Sulforaphane provides a neuroprotective effect by decreasing mRNA
levels of MMP-9.53Increasing NG2+ cell proliferation in the damaged
area by targeting MMP-9 resulted in a functional recovery of the spinal
cord.40
Limitations
The transferability or conclusions from animal to human models have
been shown to hold several deficits;13however, the animal model does
have the advantage that is not as limited in the size of the collective.
Our explorative prospective study involved a small collective (n = 30),
and therefore results may notrepresentlocalprocesses clearly.We
decided to include different locations and thus differing severities of
SCI in G0 and G1.Therefore,in the current study,allpatients in G1
have an initialAISA grade A and G0 varies from A to C.This might
contribute to differences within the groups and thus disguise patterns
in the data.Furtherstudieswith an enlarged sample size should
address an inter-group (G1 vs G0) comparison with each AISA grade,
as the prognosis is often vastly different. Because of the large standard
errors to the mean and the smallnumber of patients,the significant
differences as described for MMP-8 and MMP-9 must be considered
critically. Furthermore, studies must figure out the veracity of the data
by increasing the sample size.The temporalchanges in metallopro-
teins after 24 h (MMP-2), 1 week (MMP-8) and 72 h (MMP-9) might
have a key role in the pathophysiology of SCI and could prove to be
good predictive markers.Because of the smallsample size we cannot
conclude whether they randomly increased atthese time points.In
orderto provethe involvementof MMPs in neuroinflammatory
processes, future studies are needed that correlate MMP levels to other
establishedinflammationmarkerssuch as interferon-γ,tumour
necrosis factor-α,interleukin(IL)-1β,IL-6, IL-8, IL-10,and vascular
endothelialgrowth factor.Furthermore,because ofmissing epitope
mapping we can only provide valid evidence for the assay used in
the study.
MMP-10
MMP-10 is known to regulate the lysis ofcollagen in alternatively
activated resident macrophages. Furthermore, MMP-10, which is n
collagenase,promoted an increase in the serum levelof MMP-8 and
MMP-13,two metallocollagenases released by macrophages.9 Unfor-
tunately,we gainedno furtherinformation regardingMMP-10
because of the low serum level.
MMP-12
Wells et al.42 obtained data revealing that MMP-12 is destructive and
contributes to the developmentof secondary injury in SCI in mice.
This metalloproteinase contributes to the secondary injury process
and in its absence mice recovered better from the insult.42Because of
low serum levels we gained no further information about MMP-12.
Predictive modelling
Although a predictive modelstratifying injury severity and predicting
neurologicaloutcome mightbe extremely usefulfor facilitating the
clinical validation of novel treatments in acute human SCI,14findings
based on the present modelling process must be considered critica
P-values are unadjusted for multiple testing and should be interpre
descriptively.Becauseof the smallsamplesizeof completecases
(n = 12) the predictive value ofregression modelsas wellas ROC
analysis should be interpreted in an explorative way.Considering the
adjusted odds ratios (nearby 1) and their sharp corresponding cis,
have to interpret them in relation to the high serum levels (106 and
more).Nevertheless,by including the MMP-8 value after day 1 and
the MMP-9 valueafter1 month aspredictorsand by including
remission/no remission as a criterion,we gained a predictive model
with an AUC = 0.97. Besides this statistically impressive result, fur
studies are needed with a larger collective. Apart from that, this m
mightbe combined with a costbenefitanalysis thatdetermined by
revealing the ratio of sensitivity/(1 − specificity) based on the resp
clinicalquestion.Thus,an exactcutoffvaluefor sensitivityand
specificity might be estimated.On the basis of this information both
can be calculated for the model. Therefore, the present study prov
key informationto developinga reliableSCI scorebasedon
biometric data.
Perspective
So far, our results stage interesting references for the understandi
biochemical processes following SCI. Further studies with an enlarg
collective should also focus on patients with an initial AISA A gradi
and examine their specific cytokine profile regarding the incidence
neurologicalrecovery.Additively,the inclusion ofapproaches in the
radiologicalassessmentstill remainsvery promisingsourcesof
information and therefore should also be investigated in the future
In particular,in a setting where a satisfactory clinicalexamination is
not possible,they might provide relevant supplementary information
The current study showed peripheralserum analysis to be a suitable
concept for assessing the patient’s potential for neurological remis
after tSCI.In addition,the present data supply promising approaches
for future monitoring concepts for current therapies, as well as ani
models. Moreover, the results provide key information for a predic
scoring system for SCI patients.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
13
Spinal Cord

CONCLUSIONS
In the presentstudy,serum levelpatterns ofMMP-2 and MMP-8
showed distinctive patterns for patients with neurological impairment.
MMP-8 and MMP-9 patterns showed significant differences regarding
functional recovery. Our binary logistic regression model showed that,
according to neurologicaldamage,measuring peripheralserum levels
can be used to monitor and predictlocomotor recovery after SCI.
Even though the predictive value of the modelin this study must be
considered critically,results provided a basis for further approaches
investigating standardized monitoring as wellas prognostic tracking
techniques and scoring.
DATA ARCHIVING
There were no data to deposit.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We thank Martina Kutsche-Bauer for performing the Luminex assays.
Statistical consulting was provided by the Institute for Medical Biometrics and
Information Technology at the University of Heidelberg,Germany.
1 Boakye M,Leigh BC, Skelly AC.Quality oflife in persons with spinalcord injury:
comparisons with other populations. J Neurosurg Spine 2012; 17: 29–37.
2 Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord
injury pathophysiology and emerging therapies:promise on the horizon.Neurosurg
Focus 2008; 25: E2.
3 Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications.
Spinal Cord 2012; 50: 365–372.
4 Ito Y, Sugimoto Y, Tomioka M, KaiN, Tanaka M. Does high dose methylprednisolone
sodium succinate really improve neurological status in patient with acute cervical cord
injury?: a prospective study about neurological recovery and early complications. Spine
(Phila Pa 1976) 2009; 34: 2121–2124.
5 Kwon BK, Okon EB, Plunet W, Baptiste D, Fouad K, Hillyer J et al. A systematic review
of directly applied biologic therapies for acute spinal cord injury. J Neurotrauma 2011;
28: 1589–1610.
6 Evaniew N, Belley-Cote EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF. Methylpred-
nisolone forthe treatmentof patients with acute spinalcord injuries:a systematic
review and meta-analysis. J Neurotrauma 2015; 33: 468–481.
7 Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE et al. Guidelines for
the managementof acute cervicalspine and spinalcord injuries:2013 update.
Neurosurgery 2013; 60: 82–91.
8 Esposito E,Genovese T,CaminitiR, BramantiP, Meli R, Cuzzocrea S.Melatonin
regulates matrix metalloproteinases aftertraumatic experimentalspinalcord injury.
J Pineal Res 2008; 45: 149–156.
9 RohaniMG, McMahan RS,Razumova MV,Hertz AL, Cieslewicz M,Pun SH et al.
MMP-10 regulatescollagenolytic activityof alternatively activated residentmacro-
phages. J Invest Dermatol 2015; 135: 2377–2384.
10 Kwon BK, Casha S, Hurlbert RJ, Yong VW. Inflammatory and structural biomarkers in
acute traumatic spinal cord injury. Clin Chem Lab Med 2011; 49: 425–433.
11 YokoboriS, Zhang Z,Moghieb A,Mondello S,GajavelliS, Dietrich WD et al.Acute
diagnostic biomarkers forspinalcord injury:review ofthe literature and preliminary
research report. World Neurosurg 2015; 83: 867–878.
12 Zhang H,Chang M, Hansen CN,Basso DM, Noble-Haeusslein LJ.Role of matrix
metalloproteinases and therapeutic benefits oftheirinhibition in spinalcord injury.
Neurotherapeutics 2011; 8: 206–220.
13 Kwon BK, Streijger F, Hill CE, Anderson AJ, Bacon M, Beattie MS et al. Large animal
and primate models of spinal cord injury for the testing of novel therapies. Exp Neurol
2015; 269: 154–168.
14 Kwon BK, Stammers AM,BelangerLM, Bernardo A,Chan D, Bishop CM et al.
Cerebrospinalfluid inflammatory cytokines and biomarkers of injury severity in acute
human spinal cord injury. J Neurotrauma 2010; 27: 669–682.
15 Moghaddam A, Breier L, Haubruck P, Bender D, Biglari B, Wentzensen A et al. Non-
unions treatedwith bone morphogenicprotein 7: introducingthe quantitative
measurement of human serum cytokine levels as promising tool in evaluation of adjunct
non-union therapy. J Inflamm (Lond) 2016; 13: 3.
16 Moghaddam A,Child C, BrucknerT, GernerHJ, DanielV, BiglariB. Posttraumatic
inflammation as a key to neuroregeneration after traumatic spinal cord injury. Int J Mol
Sci 2015; 16: 7900–7916.
17 Moghaddam A, Zimmermann G, Hammer K, Bruckner T, Grutzner PA, von Recum J.
Cigarette smoking influences the clinicaland occupationaloutcome of patients with
tibial shaft fractures. Injury 2011; 42: 1435–1442.
18 Moghaddam A, Muller U, Roth HJ, Wentzensen A, Grutzner PA, Zimmermann G. TRAC
5b and CTX as osteologicalmarkers ofdelayed fracture healing.Injury 2011;42:
758–764.
19 Kirshblum SC,Burns SP, Biering-Sorensen F,Donovan W,Graves DE,Jha A et al.
Internationalstandards for neurologicalclassificationof spinal cord injury
(revised 2011). J Spinal Cord Med 2011; 34: 535–546.
20 Marino RJ, Ditunno JF Jr, Donovan WH,Maynard F Jr Neurologic recovery after
traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Ph
Med Rehabil 1999; 80: 1391–1396.
21 R DevelopmentCore Team (2015).R: A Language and Environmentfor Statistical
Computing. Vienna, Austria, R Foundation for Statistical Computing.
22 Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC et al.pROC: an open-
source package for R and S+ to analyze and compare ROC curves. BMC Bioinformati
2011; 12: 77.
23 Akaike H. Information Theory and an Extension of the Maximum Likelihood Principle.
Selected Papersof Hirotugu Akaike.(eds. Parzen E, Tanabe K, KitagawaG)
New York, NY, Springer New York: 199–213 (1998).
24 Biglari B, Buchler A, Swing T, Biehl E, Roth HJ, Bruckner T et al. Increase in soluble
CD95L during subacutephases after human spinal cord injury: a potential
therapeutic target. Spinal Cord 2013; 51: 183–187.
25 BiglariB, BuchlerA, Swing T, Child C, Biehl E, ReitzelT et al. Serum sCD95L
concentration in patients with spinal cord injury. J Int Med Res 2015; 43: 250–256.
26 Osterthun R, Post MW, van Asbeck FW. Characteristics, length of stay and functional
outcome of patients with spinal cord injury in Dutch and Flemish rehabilitation centre
Spinal Cord 2009; 47: 339–344.
27 Milicevic S, Bukumiric Z, Nikolic AK, Babovic R, Jankovic S. Demographic character-
istics and functional outcomes in patients with traumatic and nontraumatic spinal cor
injuries. Vojnosanit Pregl 2012; 69: 1061–1066.
28 Wyndaele M,Wyndaele JJ.Incidence,prevalence and epidemiology ofspinalcord
injury: what learns a worldwide literature survey? Spinal Cord 2006; 44: 523–529.
29 Jackson AB, DijkersM, Devivo MJ, Poczatek RB.A demographic profile ofnew
traumatic spinalcord injuries:change and stability over30 years.Arch Phys Med
Rehabil 2004; 85: 1740–1748.
30 Cummins JS, Koval KJ, Cantu RV, Spratt KF. Do seat belts and air bags reduce mortali
and injury severity aftercar accidents? Am J Orthop (Belle Mead,NJ) 2011; 40:
E26–E29.
31 Smith JL, Ackerman LL.Managementof cervicalspine injuries in young children:
lessons learned. J Neurosurg Pediatr 2009; 4: 64–73.
32 Weninger P, Hertz H. Factors influencing the injury pattern and injury severity after h
speed motor vehicle accident–a retrospective study. Resuscitation 2007; 75: 35–41.
33 Richter M, Thermann H, Wippermann B, Otte D, Schratt HE, Tscherne H. Foot fracture
in restrained frontseat car occupants:a long-term study overtwenty-three years.
J Orthop Trauma 2001; 15: 287–293.
34 Kaminska MS,BrodowskiJ, Karakiewicz B.Fall risk factors in community-dwelling
elderlydepending on theirphysicalfunction,cognitive statusand symptomsof
depression. Int J Environ Res Public Health 2015; 12: 3406–3416.
35 Chen Y, Tang Y, Allen V, DeVivo MJ. Aging and spinalcord injury: externalcauses of
injury and implicationsfor prevention.Top Spinal Cord Inj Rehabil 2015; 21:
218–226.
36 Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of
oligodendrocytes is mediated through nitric oxide. J Immunol 1993; 151: 2132–2141.
37 Kim GM,Xu J, Song SK, Yan P, Ku G, Xu XM et al.Tumor necrosis factor receptor
deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis prot
2 expression,and functionalrecovery aftertraumatic spinalcord injury.J Neurosci
2001; 21: 6617–6625.
38 Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L et al.
Elevated MMP-9 in the lumbarcord early afterthoracic spinalcord injury impedes
motor relearning in mice. J Neurosci 2013; 33: 13101–13111.
39 Jang JW, Lee JK, Kim SH. Activation of matrix metalloproteinases-9 after photothrom-
botic spinal cord injury model in rats. J Korean Neurosurg Soc 2011; 50: 288–292.
40 Liu H, ShubayevVI. Matrix metalloproteinase-9 controlsproliferation ofNG2+
progenitorcells immediatelyafter spinal cord injury. Exp Neurol 2011; 231:
236–246.
41 Piao MS, Lee JK, Jang JW, Hur H, Lee SS, Xiao L et al. Melatonin improves functional
outcome via inhibition of matrix metalloproteinases-9 after photothrombotic spinal co
injury in rats. Acta Neurochir (Wien) 2014; 156: 2173–2182.
42 Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S et al. An adverse role for
matrix metalloproteinase 12 afterspinalcord injury in mice.J Neurosci 2003; 23:
10107–10115.
43 Zhang H, Trivedi A, Lee JU, Lohela M, Lee SM, Fandel TM et al. Matrix
metalloproteinase-9 and stromalcell-derived factor-1 actsynergistically to support
migration of blood-borne monocytes into the injured spinal cord. J Neurosci 2011; 31:
15894–15903.
44 Zhou Y, CuiZ, Xia X, Liu C, Zhu X, Cao J et al. Matrix metalloproteinase-1 (MMP-1)
expression in rat spinal cord injury model. Cell Mol Neurobiol 2014; 34: 1151–1163.
45 Hsu JY, McKeon R, GoussevS, Werb Z, Lee JU, Trivedi A et al. Matrix
metalloproteinase-2 facilitates wound healing events that promote functionalrecovery
after spinal cord injury. J Neurosci 2006; 26: 9841–9850.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
14
Spinal Cord
In the presentstudy,serum levelpatterns ofMMP-2 and MMP-8
showed distinctive patterns for patients with neurological impairment.
MMP-8 and MMP-9 patterns showed significant differences regarding
functional recovery. Our binary logistic regression model showed that,
according to neurologicaldamage,measuring peripheralserum levels
can be used to monitor and predictlocomotor recovery after SCI.
Even though the predictive value of the modelin this study must be
considered critically,results provided a basis for further approaches
investigating standardized monitoring as wellas prognostic tracking
techniques and scoring.
DATA ARCHIVING
There were no data to deposit.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We thank Martina Kutsche-Bauer for performing the Luminex assays.
Statistical consulting was provided by the Institute for Medical Biometrics and
Information Technology at the University of Heidelberg,Germany.
1 Boakye M,Leigh BC, Skelly AC.Quality oflife in persons with spinalcord injury:
comparisons with other populations. J Neurosurg Spine 2012; 17: 29–37.
2 Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord
injury pathophysiology and emerging therapies:promise on the horizon.Neurosurg
Focus 2008; 25: E2.
3 Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications.
Spinal Cord 2012; 50: 365–372.
4 Ito Y, Sugimoto Y, Tomioka M, KaiN, Tanaka M. Does high dose methylprednisolone
sodium succinate really improve neurological status in patient with acute cervical cord
injury?: a prospective study about neurological recovery and early complications. Spine
(Phila Pa 1976) 2009; 34: 2121–2124.
5 Kwon BK, Okon EB, Plunet W, Baptiste D, Fouad K, Hillyer J et al. A systematic review
of directly applied biologic therapies for acute spinal cord injury. J Neurotrauma 2011;
28: 1589–1610.
6 Evaniew N, Belley-Cote EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF. Methylpred-
nisolone forthe treatmentof patients with acute spinalcord injuries:a systematic
review and meta-analysis. J Neurotrauma 2015; 33: 468–481.
7 Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE et al. Guidelines for
the managementof acute cervicalspine and spinalcord injuries:2013 update.
Neurosurgery 2013; 60: 82–91.
8 Esposito E,Genovese T,CaminitiR, BramantiP, Meli R, Cuzzocrea S.Melatonin
regulates matrix metalloproteinases aftertraumatic experimentalspinalcord injury.
J Pineal Res 2008; 45: 149–156.
9 RohaniMG, McMahan RS,Razumova MV,Hertz AL, Cieslewicz M,Pun SH et al.
MMP-10 regulatescollagenolytic activityof alternatively activated residentmacro-
phages. J Invest Dermatol 2015; 135: 2377–2384.
10 Kwon BK, Casha S, Hurlbert RJ, Yong VW. Inflammatory and structural biomarkers in
acute traumatic spinal cord injury. Clin Chem Lab Med 2011; 49: 425–433.
11 YokoboriS, Zhang Z,Moghieb A,Mondello S,GajavelliS, Dietrich WD et al.Acute
diagnostic biomarkers forspinalcord injury:review ofthe literature and preliminary
research report. World Neurosurg 2015; 83: 867–878.
12 Zhang H,Chang M, Hansen CN,Basso DM, Noble-Haeusslein LJ.Role of matrix
metalloproteinases and therapeutic benefits oftheirinhibition in spinalcord injury.
Neurotherapeutics 2011; 8: 206–220.
13 Kwon BK, Streijger F, Hill CE, Anderson AJ, Bacon M, Beattie MS et al. Large animal
and primate models of spinal cord injury for the testing of novel therapies. Exp Neurol
2015; 269: 154–168.
14 Kwon BK, Stammers AM,BelangerLM, Bernardo A,Chan D, Bishop CM et al.
Cerebrospinalfluid inflammatory cytokines and biomarkers of injury severity in acute
human spinal cord injury. J Neurotrauma 2010; 27: 669–682.
15 Moghaddam A, Breier L, Haubruck P, Bender D, Biglari B, Wentzensen A et al. Non-
unions treatedwith bone morphogenicprotein 7: introducingthe quantitative
measurement of human serum cytokine levels as promising tool in evaluation of adjunct
non-union therapy. J Inflamm (Lond) 2016; 13: 3.
16 Moghaddam A,Child C, BrucknerT, GernerHJ, DanielV, BiglariB. Posttraumatic
inflammation as a key to neuroregeneration after traumatic spinal cord injury. Int J Mol
Sci 2015; 16: 7900–7916.
17 Moghaddam A, Zimmermann G, Hammer K, Bruckner T, Grutzner PA, von Recum J.
Cigarette smoking influences the clinicaland occupationaloutcome of patients with
tibial shaft fractures. Injury 2011; 42: 1435–1442.
18 Moghaddam A, Muller U, Roth HJ, Wentzensen A, Grutzner PA, Zimmermann G. TRAC
5b and CTX as osteologicalmarkers ofdelayed fracture healing.Injury 2011;42:
758–764.
19 Kirshblum SC,Burns SP, Biering-Sorensen F,Donovan W,Graves DE,Jha A et al.
Internationalstandards for neurologicalclassificationof spinal cord injury
(revised 2011). J Spinal Cord Med 2011; 34: 535–546.
20 Marino RJ, Ditunno JF Jr, Donovan WH,Maynard F Jr Neurologic recovery after
traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Ph
Med Rehabil 1999; 80: 1391–1396.
21 R DevelopmentCore Team (2015).R: A Language and Environmentfor Statistical
Computing. Vienna, Austria, R Foundation for Statistical Computing.
22 Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC et al.pROC: an open-
source package for R and S+ to analyze and compare ROC curves. BMC Bioinformati
2011; 12: 77.
23 Akaike H. Information Theory and an Extension of the Maximum Likelihood Principle.
Selected Papersof Hirotugu Akaike.(eds. Parzen E, Tanabe K, KitagawaG)
New York, NY, Springer New York: 199–213 (1998).
24 Biglari B, Buchler A, Swing T, Biehl E, Roth HJ, Bruckner T et al. Increase in soluble
CD95L during subacutephases after human spinal cord injury: a potential
therapeutic target. Spinal Cord 2013; 51: 183–187.
25 BiglariB, BuchlerA, Swing T, Child C, Biehl E, ReitzelT et al. Serum sCD95L
concentration in patients with spinal cord injury. J Int Med Res 2015; 43: 250–256.
26 Osterthun R, Post MW, van Asbeck FW. Characteristics, length of stay and functional
outcome of patients with spinal cord injury in Dutch and Flemish rehabilitation centre
Spinal Cord 2009; 47: 339–344.
27 Milicevic S, Bukumiric Z, Nikolic AK, Babovic R, Jankovic S. Demographic character-
istics and functional outcomes in patients with traumatic and nontraumatic spinal cor
injuries. Vojnosanit Pregl 2012; 69: 1061–1066.
28 Wyndaele M,Wyndaele JJ.Incidence,prevalence and epidemiology ofspinalcord
injury: what learns a worldwide literature survey? Spinal Cord 2006; 44: 523–529.
29 Jackson AB, DijkersM, Devivo MJ, Poczatek RB.A demographic profile ofnew
traumatic spinalcord injuries:change and stability over30 years.Arch Phys Med
Rehabil 2004; 85: 1740–1748.
30 Cummins JS, Koval KJ, Cantu RV, Spratt KF. Do seat belts and air bags reduce mortali
and injury severity aftercar accidents? Am J Orthop (Belle Mead,NJ) 2011; 40:
E26–E29.
31 Smith JL, Ackerman LL.Managementof cervicalspine injuries in young children:
lessons learned. J Neurosurg Pediatr 2009; 4: 64–73.
32 Weninger P, Hertz H. Factors influencing the injury pattern and injury severity after h
speed motor vehicle accident–a retrospective study. Resuscitation 2007; 75: 35–41.
33 Richter M, Thermann H, Wippermann B, Otte D, Schratt HE, Tscherne H. Foot fracture
in restrained frontseat car occupants:a long-term study overtwenty-three years.
J Orthop Trauma 2001; 15: 287–293.
34 Kaminska MS,BrodowskiJ, Karakiewicz B.Fall risk factors in community-dwelling
elderlydepending on theirphysicalfunction,cognitive statusand symptomsof
depression. Int J Environ Res Public Health 2015; 12: 3406–3416.
35 Chen Y, Tang Y, Allen V, DeVivo MJ. Aging and spinalcord injury: externalcauses of
injury and implicationsfor prevention.Top Spinal Cord Inj Rehabil 2015; 21:
218–226.
36 Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of
oligodendrocytes is mediated through nitric oxide. J Immunol 1993; 151: 2132–2141.
37 Kim GM,Xu J, Song SK, Yan P, Ku G, Xu XM et al.Tumor necrosis factor receptor
deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis prot
2 expression,and functionalrecovery aftertraumatic spinalcord injury.J Neurosci
2001; 21: 6617–6625.
38 Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L et al.
Elevated MMP-9 in the lumbarcord early afterthoracic spinalcord injury impedes
motor relearning in mice. J Neurosci 2013; 33: 13101–13111.
39 Jang JW, Lee JK, Kim SH. Activation of matrix metalloproteinases-9 after photothrom-
botic spinal cord injury model in rats. J Korean Neurosurg Soc 2011; 50: 288–292.
40 Liu H, ShubayevVI. Matrix metalloproteinase-9 controlsproliferation ofNG2+
progenitorcells immediatelyafter spinal cord injury. Exp Neurol 2011; 231:
236–246.
41 Piao MS, Lee JK, Jang JW, Hur H, Lee SS, Xiao L et al. Melatonin improves functional
outcome via inhibition of matrix metalloproteinases-9 after photothrombotic spinal co
injury in rats. Acta Neurochir (Wien) 2014; 156: 2173–2182.
42 Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S et al. An adverse role for
matrix metalloproteinase 12 afterspinalcord injury in mice.J Neurosci 2003; 23:
10107–10115.
43 Zhang H, Trivedi A, Lee JU, Lohela M, Lee SM, Fandel TM et al. Matrix
metalloproteinase-9 and stromalcell-derived factor-1 actsynergistically to support
migration of blood-borne monocytes into the injured spinal cord. J Neurosci 2011; 31:
15894–15903.
44 Zhou Y, CuiZ, Xia X, Liu C, Zhu X, Cao J et al. Matrix metalloproteinase-1 (MMP-1)
expression in rat spinal cord injury model. Cell Mol Neurobiol 2014; 34: 1151–1163.
45 Hsu JY, McKeon R, GoussevS, Werb Z, Lee JU, Trivedi A et al. Matrix
metalloproteinase-2 facilitates wound healing events that promote functionalrecovery
after spinal cord injury. J Neurosci 2006; 26: 9841–9850.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
14
Spinal Cord
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

46 Lee JY, Choi HY, Na WH, Ju BG, Yune TY. 17beta-estradiol inhibits MMP-9 and SUR1/
TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage
after spinal cord injury in male rats. Endocrinology 2015; 156: 1838–1850.
47 Li XQ, Cao XZ, Wang J, Fang B, Tan WF, Ma H. Sevoflurane preconditioning ameliorates
neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/
reperfusion in rats. Mol Brain 2014; 7: 69.
48 Yang J, Wang G, Gao C, Shao G, Kang N. Effects of hyperbaric oxygen on MMP-2 and
MMP-9 expression and spinal cord edema after spinal cord injury. Life Sci 2013; 93:
1033–1038.
49 Yu F, Kamada H, Niizuma K, Endo H, Chan PH. Induction of mmp-9 expression and
endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma 2008; 25:
184–195.
50 Zhang P, Ma X. Effect of rutin on spinal cord injury through inhibition of the expression
of MIP-2 and activation ofMMP-9, and downregulation ofAkt phosphorylation.Mol
Med Rep 2015; 12: 7554–7560.
51 Hsu JY, Bourguignon LY,Adams CM,PeyrollierK, Zhang H,FandelT et al. Matrix
metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci
2008; 28: 13467–13477.
52 Austin JW, Afshar M, Fehlings MG.The relationship between localized subarachnoid
inflammation and parenchymal pathophysiology after spinal cord injury. J Neurotrauma
2012; 29: 1838–1849.
53 Benedict AL, Mountney A, Hurtado A, Bryan KE, Schnaar RL, Dinkova-Kostova AT et al.
Neuroprotective effects ofsulforaphane aftercontusive spinalcord injury.J Neuro-
trauma 2012; 29: 2576–2586.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
15
Spinal Cord
TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage
after spinal cord injury in male rats. Endocrinology 2015; 156: 1838–1850.
47 Li XQ, Cao XZ, Wang J, Fang B, Tan WF, Ma H. Sevoflurane preconditioning ameliorates
neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/
reperfusion in rats. Mol Brain 2014; 7: 69.
48 Yang J, Wang G, Gao C, Shao G, Kang N. Effects of hyperbaric oxygen on MMP-2 and
MMP-9 expression and spinal cord edema after spinal cord injury. Life Sci 2013; 93:
1033–1038.
49 Yu F, Kamada H, Niizuma K, Endo H, Chan PH. Induction of mmp-9 expression and
endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma 2008; 25:
184–195.
50 Zhang P, Ma X. Effect of rutin on spinal cord injury through inhibition of the expression
of MIP-2 and activation ofMMP-9, and downregulation ofAkt phosphorylation.Mol
Med Rep 2015; 12: 7554–7560.
51 Hsu JY, Bourguignon LY,Adams CM,PeyrollierK, Zhang H,FandelT et al. Matrix
metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci
2008; 28: 13467–13477.
52 Austin JW, Afshar M, Fehlings MG.The relationship between localized subarachnoid
inflammation and parenchymal pathophysiology after spinal cord injury. J Neurotrauma
2012; 29: 1838–1849.
53 Benedict AL, Mountney A, Hurtado A, Bryan KE, Schnaar RL, Dinkova-Kostova AT et al.
Neuroprotective effects ofsulforaphane aftercontusive spinalcord injury.J Neuro-
trauma 2012; 29: 2576–2586.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam etal
15
Spinal Cord
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.