MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury
Develop a Quality Improvement Project proposal to address issues relating to the quality of patient/client care on a ward/unit.
8 Pages8376 Words207 Views
Added on 2022-10-04
About This Document
This study investigates the correlation between matrix metalloprotein serum levels and remission after traumatic spinal cord injury (SCI) and suggests MMP-8 and MMP-9 serum levels as early markers for remission. The study was conducted on 115 patients and the results indicate that further studies with an enlarged collective are warranted. The study was approved by the ethics committee of the University of Heidelberg and the Landesärztekammer Rheinland-Pfalz, Germany.
MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury
Develop a Quality Improvement Project proposal to address issues relating to the quality of patient/client care on a ward/unit.
Added on 2022-10-04
ShareRelated Documents
ORIGINAL ARTICLE
Exploratory study to suggest the possibility of MMP-8 and
MMP-9 serum levels as early markers for remission after
traumatic spinal cord injury
A Moghaddam1, R Heller1 , V Daniel 2, T Swing1 , M Akbar 3, H-J Gerner1 and B Biglari 4
Study design: A prospective observational study reporting the correlation between matrix metalloprotein serum levels and remission
after traumatic spinal cord injury (SCI).
Objectives: To investigate serum cytokine levels as predictive markers.
Setting: Germany, Rhineland-Palatinate (Rheinland-Pfalz).
Methods: Between 2010 and 2015, data sets from 115 patients (33 female, 82 male) after traumatic SCI were recorded at the BG
Trauma Centre Ludwigshafen. We examined the serum levels of Matix metallopraoteinases (MMPs) MMP-2, MMP-8, MMP-9, MMP-10
and MMP-12 over a 12-week period, that is, at admission and 4, 9, 12 h, 1 and 3 days and 1, 2, 4, 8 and 12 weeks after trauma.
Following the same match-pair procedure as in our previous studies, we selected 10 patients with SCI and neurological remission
(Group 1) and 10 patients with an initial American Spinal Injury Association (ASIA) A grade and no neurological remission (Group 0).
Ten patients with an isolated vertebral fracture without neurological deficits served as a control group (Group C). Our analysis was
performed using a Luminex Performance Human High Sensitivity Cytokine Panel. Multivariate logistic regression models were used to
examine the predictive value of MMPs with respect to neurological remission vs no neurological remission.
Results: MMP-8 and MMP-9 provided significantly different values. The favoured predictive model allows to differentiate between
neurological remission and no neurological remission in 97% of cases.
Conclusions: The results indicate that further studies with an enlarged collective are warranted in order to investigate current
monitoring, prognostic and tracking techniques as well as scoring systems.
Spinal Cord (2017) 55, 8–15; doi:10.1038/sc.2016.104; published online 5 July 2016
INTRODUCTION
Spinal cord injury (SCI) impairs patients’ quality of life greatly and
causes immense financial consequences for them and their families.1,2
Current studies report an average annual SCI incidence of 40 cases per
million in the United States.3 Despite substantial research on SCI, no
ground breaking step has been made towards understanding the
mechanisms of SCI and exploring new therapies. New therapies for
reversing neurological deficits are also lacking.2 Current treatments
such as medications or surgical treatment are limited in their success
and results are very poor.4–6 Furthermore, most therapeutic strategies
lack convincing evidence for their beneficial effects.6,7 There exist no
valid markers specifying the potential for remission up to this point.
The pathophysiological process is characterised by a primary and a
secondary phase of injury. Although the first phase is marked by
mechanical trauma, the second phase is more complex due to a variety
of pathophysiological processes.2 The early inflammatory response
involves an initial wave of infiltrating neutrophils, followed by
migration of monocytes and macrophages into the injured segment.
Each of these inflammatory cells expresses MMPs including MMP-2
(gelatinase A), MMP-8 (neutrophil collagenase), MMP-9 (gelatinase B),
MMP-11 (stromelysin-3) and MMP-12 (metalloelastase).8 Further-
more, MMP-10 is suspected to have a critical role in controlling tissue
remodelling in macrophages and moderating scar tissue formation
during wound repair.9
Currently, there are still few reports focused on diagnostic
biomarkers in SCI.10,11 In view of devastating consequences of SCI
and the poor therapeutic solutions, there is an urgent need for greater
efforts to innovate reliable biomarkers for remission after SCI.11
Because of their relevance in processes after traumatic injuries such
as traumatic spinal cord injuries, as well as differing catalytic
mechanisms, we decided to investigate MMP-2, MMP-8, MMP-9,
MMP-10 and MMP-12.8,9,12 Our goal was to discover a prognostic
biomarker for remission potential after SCI by investigating matrix-
metalloproteines in peripheral serum and therewith determine neu-
rological outcome. Animal studies investigating possible markers are
limited in terms of transferability.13 The human model in this study
investigated the following: first, if markers can be found in serum to
predict rehabilitation post SCI; second, if there is an effective method
1
HTRG Heidelberg Trauma Research Group, Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Heidelberg University Hospital, Heidelberg, Germany;
2
Transplantation Immunology, Institute of Immunology, University of Heidelberg, Heidelberg, Germany; 3
Spine Center, Center for Orthopedics, Trauma Surgery and Spinal Cord
Injury, Heidelberg University Hospital, Heidelberg, Germany and 4
Department of Paraplegiology, Berufsgenossenschaftliche Unfallklinik Ludwigshafen, Ludwigshafen, Germany
Correspondence: Dr Professor Arash Moghaddam, HTRG Heidelberg Trauma Research Group, Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Heidelberg
University Hospital, Schlierbacher Landstrabe 200a, Heidelberg 69118, Germany.
E-mail: arash.moghaddam@med.uni-heidelberg.de or email@arash.de
Received 7 April 2016; revised 2 May 2016; accepted 9 May 2016; published online 5 July 2016
Spinal Cord (2017) 55, 8–15
& 2017 International Spinal Cord Society All rights reserved 1362-4393/17
www.nature.com/sc
Exploratory study to suggest the possibility of MMP-8 and
MMP-9 serum levels as early markers for remission after
traumatic spinal cord injury
A Moghaddam1, R Heller1 , V Daniel 2, T Swing1 , M Akbar 3, H-J Gerner1 and B Biglari 4
Study design: A prospective observational study reporting the correlation between matrix metalloprotein serum levels and remission
after traumatic spinal cord injury (SCI).
Objectives: To investigate serum cytokine levels as predictive markers.
Setting: Germany, Rhineland-Palatinate (Rheinland-Pfalz).
Methods: Between 2010 and 2015, data sets from 115 patients (33 female, 82 male) after traumatic SCI were recorded at the BG
Trauma Centre Ludwigshafen. We examined the serum levels of Matix metallopraoteinases (MMPs) MMP-2, MMP-8, MMP-9, MMP-10
and MMP-12 over a 12-week period, that is, at admission and 4, 9, 12 h, 1 and 3 days and 1, 2, 4, 8 and 12 weeks after trauma.
Following the same match-pair procedure as in our previous studies, we selected 10 patients with SCI and neurological remission
(Group 1) and 10 patients with an initial American Spinal Injury Association (ASIA) A grade and no neurological remission (Group 0).
Ten patients with an isolated vertebral fracture without neurological deficits served as a control group (Group C). Our analysis was
performed using a Luminex Performance Human High Sensitivity Cytokine Panel. Multivariate logistic regression models were used to
examine the predictive value of MMPs with respect to neurological remission vs no neurological remission.
Results: MMP-8 and MMP-9 provided significantly different values. The favoured predictive model allows to differentiate between
neurological remission and no neurological remission in 97% of cases.
Conclusions: The results indicate that further studies with an enlarged collective are warranted in order to investigate current
monitoring, prognostic and tracking techniques as well as scoring systems.
Spinal Cord (2017) 55, 8–15; doi:10.1038/sc.2016.104; published online 5 July 2016
INTRODUCTION
Spinal cord injury (SCI) impairs patients’ quality of life greatly and
causes immense financial consequences for them and their families.1,2
Current studies report an average annual SCI incidence of 40 cases per
million in the United States.3 Despite substantial research on SCI, no
ground breaking step has been made towards understanding the
mechanisms of SCI and exploring new therapies. New therapies for
reversing neurological deficits are also lacking.2 Current treatments
such as medications or surgical treatment are limited in their success
and results are very poor.4–6 Furthermore, most therapeutic strategies
lack convincing evidence for their beneficial effects.6,7 There exist no
valid markers specifying the potential for remission up to this point.
The pathophysiological process is characterised by a primary and a
secondary phase of injury. Although the first phase is marked by
mechanical trauma, the second phase is more complex due to a variety
of pathophysiological processes.2 The early inflammatory response
involves an initial wave of infiltrating neutrophils, followed by
migration of monocytes and macrophages into the injured segment.
Each of these inflammatory cells expresses MMPs including MMP-2
(gelatinase A), MMP-8 (neutrophil collagenase), MMP-9 (gelatinase B),
MMP-11 (stromelysin-3) and MMP-12 (metalloelastase).8 Further-
more, MMP-10 is suspected to have a critical role in controlling tissue
remodelling in macrophages and moderating scar tissue formation
during wound repair.9
Currently, there are still few reports focused on diagnostic
biomarkers in SCI.10,11 In view of devastating consequences of SCI
and the poor therapeutic solutions, there is an urgent need for greater
efforts to innovate reliable biomarkers for remission after SCI.11
Because of their relevance in processes after traumatic injuries such
as traumatic spinal cord injuries, as well as differing catalytic
mechanisms, we decided to investigate MMP-2, MMP-8, MMP-9,
MMP-10 and MMP-12.8,9,12 Our goal was to discover a prognostic
biomarker for remission potential after SCI by investigating matrix-
metalloproteines in peripheral serum and therewith determine neu-
rological outcome. Animal studies investigating possible markers are
limited in terms of transferability.13 The human model in this study
investigated the following: first, if markers can be found in serum to
predict rehabilitation post SCI; second, if there is an effective method
1
HTRG Heidelberg Trauma Research Group, Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Heidelberg University Hospital, Heidelberg, Germany;
2
Transplantation Immunology, Institute of Immunology, University of Heidelberg, Heidelberg, Germany; 3
Spine Center, Center for Orthopedics, Trauma Surgery and Spinal Cord
Injury, Heidelberg University Hospital, Heidelberg, Germany and 4
Department of Paraplegiology, Berufsgenossenschaftliche Unfallklinik Ludwigshafen, Ludwigshafen, Germany
Correspondence: Dr Professor Arash Moghaddam, HTRG Heidelberg Trauma Research Group, Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Heidelberg
University Hospital, Schlierbacher Landstrabe 200a, Heidelberg 69118, Germany.
E-mail: arash.moghaddam@med.uni-heidelberg.de or email@arash.de
Received 7 April 2016; revised 2 May 2016; accepted 9 May 2016; published online 5 July 2016
Spinal Cord (2017) 55, 8–15
& 2017 International Spinal Cord Society All rights reserved 1362-4393/17
www.nature.com/sc

of monitoring neurological remission for future treatment strategies
for SCI; third, if results can be used to generate an improved animal
model for investigating SCI.14
The answers to these questions will help us find a way to monitor
improvement, as well as help us develop a useful objective score.
MATERIALS AND METHODS
Between 2010 and 2015, data sets of 115 patients (33 females and 82 males)
were recorded after traumatic SCI in the Berufsgenossenschaftliche Unfallklinik
Ludwigshafen (BG Trauma Centre). Following the same match-pair procedure
as in our previous studies,15 we selected 10 patients with SCI and neurological
remission (Group 1 = G1) and 10 patients with an initial American Spinal
Injury Association (ASIA) A grade and no neurological remission (Group
0 = G0). Furthermore, we chose 10 patients with an isolated vertebral fracture
without neurological deficits to serve as a control group (Group C = C). Blood
was drawn at the same time points in the studies for both groups. Four vials of
serum (each 7.5 ml) were obtained with a standard procedure at different time
points, that is, 4, 9, 12 h, 1 and 3 days and 1, 2, 4, 8 and 12 weeks after SCI.
After 20 min of coagulation, blood was centrifuged at 3000 r.p.m., aliquoted
and stored at − 80 °C until analysis. Serum samples were obtained at the same
time points in both groups. The method of sample collection is the same as in
our previous cytokine analysis.16–18 The ASIA impairment scale (AIS) grades
were conducted in awake and responsive patients at the time of admission and
12 weeks according to the International Standards for Neurological Classifica-
tion of SCI (ISNCSCI; Table 1).19,20 Because BG Ludwigshafen is a primary
trauma centre with its own helicopter patients included in the study were
admitted within 2 h after trauma.16 Surgical stabilisation and decompression
was carried out 3.52 ± 1.35 h after trauma. Quantification was carried out in
accordance with the GLP (good laboratory practice) provisions. A clinical
examination of the patients took place parallel to blood sampling. Therefore,
the recovery process was determined by the parameters of the clinical course
(ASIA score). The quantitative measurement of MMP-2, MMP-8, MMP-9,
MMP-10 and MMP-12 from patient serum was conducted using the Luminex
Performance Human High Sensitivity Cytokine Panel according to the
manufacturer’s instructions (Catalogue Number FCST07-05, Kit Lot Number
1415263). The kits were provided by R&D Systems (Minneapolis, MN, USA).
The lab technician performing the test was blinded to all patients and clinical
information and all lab work was carried out in the Heidelberg University
Hospital.16 Storage took place until analysis at − 80 °C. Our prospective
observational study has been approved by the ethics committee of the
University of Heidelberg (S-514/2011) and the Landesärztekammer
Rheinland-Pfalz (837.188.12 / 8289-F), Germany. All study participants signed
and dated consent forms willingly and could voluntarily choose to leave the
study at any time and for any reason. Exclusion criteria were the following:
non-traumatic SCI, traumatic brain injury, severe abdominal trauma, traumatic
amputation of extremities and coma and all patients with an additional major
trauma apart from the SCI. Participants were not given methylprednisolone
sodium succinate during study participation.
Matching
We compared three groups of patients as in our previous studies:15
Patients with traumatic SCI without neurological remission
(Group 0 = G0)
Patients with traumatic SCI with neurological remission
(Group 1 = G1)
Fracture patients without neurological impairment (Control
Group = C)
Patients with traumatic SCI who showed no neurological remission within
3 months were assigned to G0. Those with traumatic SCI who showed
neurological remission within 3 months were assigned to G1. We matched the
two groups with a third group of patients with vertebral fractures that presented
no neurological impairment. Patients were enroled in Group C and served as a
control group. Patients were matched on the basis of four criteria: age, sex,
aetiology and AO classification. If more than one match was found for a non-
remission, then patients with the most similar clinical profile were chosen and
vice versa. According to matching criteria, three groups (each group n = 10)
could be formed of the above-mentioned total of 115 study patients (Table 1).
Statistical analysis
All statistical calculations were performed either with SPSS (SPSS Statistics
for Windows 2012, version 21.0, IBM Corp, Armonk, NY, USA) or R version
3.2.321 using the package ‘pROC’22 for receiver operator characteristics (ROC)
analysis. Figures were created by using GraphPad Prism version 5.00 for
Windows, GraphPad Software, CA, USA, www.graphpad.com. Explorative
correlation analyses were conducted between all variables. In order to detect
location shifts between groups, the non-parametric Mann–Whitney U-test for
independent samples was used. To determine location shifts within one group
at different time points, the Wilcoxon signed-rank test for dependent samples
was used. The Χ2-test was used to assess statistically significant differences in
sex, aetiology of accident, AO Classification, AIS, the type of paralysis and GCS.
Comparison of more than two independent samples was conducted using the
Kruskal–Wallis test. Logistic regression models were used to assess the
predictive power of variables for improvement in AIS while adjusting for
potentially clinically relevant covariates. Model selection for logistic regression
was based on AIC,23 and clinical relevance of covariates was taken into account.
When appropriate, we investigated clinically justifiable interactions and
moderation effects. The primary measure for the predictive performance of
any logistic regression model was area under the curve (AUC) of the ROC
curve. All P-values quoted are to be interpreted in a descriptive way as they
were not adjusted for multiple testing, and this is an exploratory post hoc
analysis.
RESULTS
This study was designed to be a prospective, explorative study with
matched pairs and no randomisation.18 Criteria for matching included
the patient’s sex, age, aetiology and AO classification (Table 2). Patient
demographics were documented, and analysis of the entire collective
and comparison of groups was performed as in our previous study.16
Patients demographics
In our match-pair analysis, the collective consisted of 30 patients (9
females and 21 males). On average, subjects were 42.03 ± 17.23 years
of age. Twenty patients were affected by traumatic SCI and serve as the
study group (Group S = S). Ten patients had a traumatic injury
without neurological impairment and serve as the control group
(Group C = C). In the study group, there were 10 patients with AIS
improvement (remission) and 10 AIS A grades with no improvement
(no remission). There were no lesions of the spinal cord. The clinical
characteristics of the entire collective are given in Table 2.
Table 1 ASIA impairment scale (AIS) grade and functional
impairment (clinical state) due to SCI
AIS grade Clinical state
A Complete—no motor or sensory function is preserved in the sacral
segments S4–S5
B Incomplete—sensory but not motor function is preserved below the NLI
and includes the sacral segments S4–S5
C Incomplete—motor function is preserved below the NLI, and more than
half of the key muscles below the NLI have a muscle grade o3
D Incomplete—motor function is preserved below the NLI, and at least half
of the key muscles below the NLI have a muscle grade of 3 or more
E Normal—motor and sensory function is normal
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam et al
9
Spinal Cord
for SCI; third, if results can be used to generate an improved animal
model for investigating SCI.14
The answers to these questions will help us find a way to monitor
improvement, as well as help us develop a useful objective score.
MATERIALS AND METHODS
Between 2010 and 2015, data sets of 115 patients (33 females and 82 males)
were recorded after traumatic SCI in the Berufsgenossenschaftliche Unfallklinik
Ludwigshafen (BG Trauma Centre). Following the same match-pair procedure
as in our previous studies,15 we selected 10 patients with SCI and neurological
remission (Group 1 = G1) and 10 patients with an initial American Spinal
Injury Association (ASIA) A grade and no neurological remission (Group
0 = G0). Furthermore, we chose 10 patients with an isolated vertebral fracture
without neurological deficits to serve as a control group (Group C = C). Blood
was drawn at the same time points in the studies for both groups. Four vials of
serum (each 7.5 ml) were obtained with a standard procedure at different time
points, that is, 4, 9, 12 h, 1 and 3 days and 1, 2, 4, 8 and 12 weeks after SCI.
After 20 min of coagulation, blood was centrifuged at 3000 r.p.m., aliquoted
and stored at − 80 °C until analysis. Serum samples were obtained at the same
time points in both groups. The method of sample collection is the same as in
our previous cytokine analysis.16–18 The ASIA impairment scale (AIS) grades
were conducted in awake and responsive patients at the time of admission and
12 weeks according to the International Standards for Neurological Classifica-
tion of SCI (ISNCSCI; Table 1).19,20 Because BG Ludwigshafen is a primary
trauma centre with its own helicopter patients included in the study were
admitted within 2 h after trauma.16 Surgical stabilisation and decompression
was carried out 3.52 ± 1.35 h after trauma. Quantification was carried out in
accordance with the GLP (good laboratory practice) provisions. A clinical
examination of the patients took place parallel to blood sampling. Therefore,
the recovery process was determined by the parameters of the clinical course
(ASIA score). The quantitative measurement of MMP-2, MMP-8, MMP-9,
MMP-10 and MMP-12 from patient serum was conducted using the Luminex
Performance Human High Sensitivity Cytokine Panel according to the
manufacturer’s instructions (Catalogue Number FCST07-05, Kit Lot Number
1415263). The kits were provided by R&D Systems (Minneapolis, MN, USA).
The lab technician performing the test was blinded to all patients and clinical
information and all lab work was carried out in the Heidelberg University
Hospital.16 Storage took place until analysis at − 80 °C. Our prospective
observational study has been approved by the ethics committee of the
University of Heidelberg (S-514/2011) and the Landesärztekammer
Rheinland-Pfalz (837.188.12 / 8289-F), Germany. All study participants signed
and dated consent forms willingly and could voluntarily choose to leave the
study at any time and for any reason. Exclusion criteria were the following:
non-traumatic SCI, traumatic brain injury, severe abdominal trauma, traumatic
amputation of extremities and coma and all patients with an additional major
trauma apart from the SCI. Participants were not given methylprednisolone
sodium succinate during study participation.
Matching
We compared three groups of patients as in our previous studies:15
Patients with traumatic SCI without neurological remission
(Group 0 = G0)
Patients with traumatic SCI with neurological remission
(Group 1 = G1)
Fracture patients without neurological impairment (Control
Group = C)
Patients with traumatic SCI who showed no neurological remission within
3 months were assigned to G0. Those with traumatic SCI who showed
neurological remission within 3 months were assigned to G1. We matched the
two groups with a third group of patients with vertebral fractures that presented
no neurological impairment. Patients were enroled in Group C and served as a
control group. Patients were matched on the basis of four criteria: age, sex,
aetiology and AO classification. If more than one match was found for a non-
remission, then patients with the most similar clinical profile were chosen and
vice versa. According to matching criteria, three groups (each group n = 10)
could be formed of the above-mentioned total of 115 study patients (Table 1).
Statistical analysis
All statistical calculations were performed either with SPSS (SPSS Statistics
for Windows 2012, version 21.0, IBM Corp, Armonk, NY, USA) or R version
3.2.321 using the package ‘pROC’22 for receiver operator characteristics (ROC)
analysis. Figures were created by using GraphPad Prism version 5.00 for
Windows, GraphPad Software, CA, USA, www.graphpad.com. Explorative
correlation analyses were conducted between all variables. In order to detect
location shifts between groups, the non-parametric Mann–Whitney U-test for
independent samples was used. To determine location shifts within one group
at different time points, the Wilcoxon signed-rank test for dependent samples
was used. The Χ2-test was used to assess statistically significant differences in
sex, aetiology of accident, AO Classification, AIS, the type of paralysis and GCS.
Comparison of more than two independent samples was conducted using the
Kruskal–Wallis test. Logistic regression models were used to assess the
predictive power of variables for improvement in AIS while adjusting for
potentially clinically relevant covariates. Model selection for logistic regression
was based on AIC,23 and clinical relevance of covariates was taken into account.
When appropriate, we investigated clinically justifiable interactions and
moderation effects. The primary measure for the predictive performance of
any logistic regression model was area under the curve (AUC) of the ROC
curve. All P-values quoted are to be interpreted in a descriptive way as they
were not adjusted for multiple testing, and this is an exploratory post hoc
analysis.
RESULTS
This study was designed to be a prospective, explorative study with
matched pairs and no randomisation.18 Criteria for matching included
the patient’s sex, age, aetiology and AO classification (Table 2). Patient
demographics were documented, and analysis of the entire collective
and comparison of groups was performed as in our previous study.16
Patients demographics
In our match-pair analysis, the collective consisted of 30 patients (9
females and 21 males). On average, subjects were 42.03 ± 17.23 years
of age. Twenty patients were affected by traumatic SCI and serve as the
study group (Group S = S). Ten patients had a traumatic injury
without neurological impairment and serve as the control group
(Group C = C). In the study group, there were 10 patients with AIS
improvement (remission) and 10 AIS A grades with no improvement
(no remission). There were no lesions of the spinal cord. The clinical
characteristics of the entire collective are given in Table 2.
Table 1 ASIA impairment scale (AIS) grade and functional
impairment (clinical state) due to SCI
AIS grade Clinical state
A Complete—no motor or sensory function is preserved in the sacral
segments S4–S5
B Incomplete—sensory but not motor function is preserved below the NLI
and includes the sacral segments S4–S5
C Incomplete—motor function is preserved below the NLI, and more than
half of the key muscles below the NLI have a muscle grade o3
D Incomplete—motor function is preserved below the NLI, and at least half
of the key muscles below the NLI have a muscle grade of 3 or more
E Normal—motor and sensory function is normal
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam et al
9
Spinal Cord

There was no significant difference in the distribution of age,
gender, aetiology and AO classification between patients with and
without neurological remission (referred to as G1 and G0 in the
following text, respectively). All 20 patients with traumatic SCI (G0
and G1) received surgery (9 ventral 45%; 11 dorsal 55%); 14 were
treated with spondylodesis (70.0%). All 30 patients included in the
collective suffered vertebral fractures. The AIS grades at admission and
discharge as well as the NLI and the type of paralysis were significantly
different in G1 and G0 (Po0.05).
Analysis of the entire patient collective
For exact MMP concentrations (pg ml − 1) and a graphic comparison
of the groups, see Figures 1 and 2. There were no significant
differences in cytokine serum levels in regard to gender, age, paralysis,
AO classification, aetiology or NLI.
Serum values of MMP-10 and MMP-12 remained undetectable.
Comparison of SCI patients vs control group
We investigated how MMP values reflect the biochemical processes
after SCI by comparing patients with (S) with patients without (C)
neurological impairment.
MMP-2. Mean MMP-2 values were higher in S than in C. MMP-2
concentrations of the same first sample fell from 2.95E+05 to 2.43E
+05 pg ml − 1 at 4 h after trauma. Then they rose continuously to 5.19E
+05 pg ml − 1 at 24 h. Within the first 12 h, MMP-2 in C rose from
1.88E+05 to 2.87E+05 pg ml − 1 initially and then decreased to 1.95E
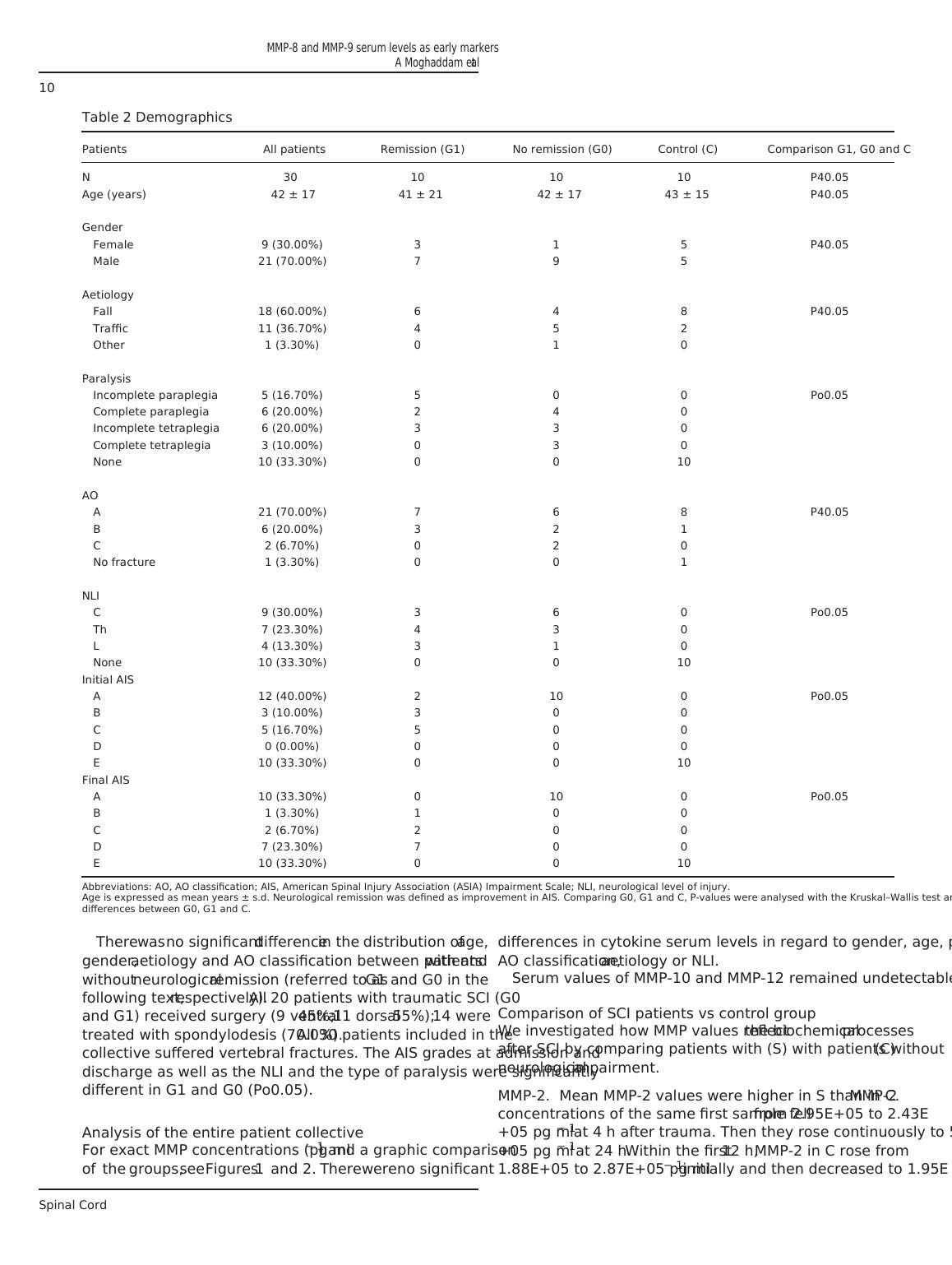
Table 2 Demographics
Patients All patients Remission (G1) No remission (G0) Control (C) Comparison G1, G0 and C
N 30 10 10 10 P40.05
Age (years) 42 ± 17 41 ± 21 42 ± 17 43 ± 15 P40.05
Gender
Female 9 (30.00%) 3 1 5 P40.05
Male 21 (70.00%) 7 9 5
Aetiology
Fall 18 (60.00%) 6 4 8 P40.05
Traffic 11 (36.70%) 4 5 2
Other 1 (3.30%) 0 1 0
Paralysis
Incomplete paraplegia 5 (16.70%) 5 0 0 Po0.05
Complete paraplegia 6 (20.00%) 2 4 0
Incomplete tetraplegia 6 (20.00%) 3 3 0
Complete tetraplegia 3 (10.00%) 0 3 0
None 10 (33.30%) 0 0 10
AO
A 21 (70.00%) 7 6 8 P40.05
B 6 (20.00%) 3 2 1
C 2 (6.70%) 0 2 0
No fracture 1 (3.30%) 0 0 1
NLI
C 9 (30.00%) 3 6 0 Po0.05
Th 7 (23.30%) 4 3 0
L 4 (13.30%) 3 1 0
None 10 (33.30%) 0 0 10
Initial AIS
A 12 (40.00%) 2 10 0 Po0.05
B 3 (10.00%) 3 0 0
C 5 (16.70%) 5 0 0
D 0 (0.00%) 0 0 0
E 10 (33.30%) 0 0 10
Final AIS
A 10 (33.30%) 0 10 0 Po0.05
B 1 (3.30%) 1 0 0
C 2 (6.70%) 2 0 0
D 7 (23.30%) 7 0 0
E 10 (33.30%) 0 0 10
Abbreviations: AO, AO classification; AIS, American Spinal Injury Association (ASIA) Impairment Scale; NLI, neurological level of injury.
Age is expressed as mean years ± s.d. Neurological remission was defined as improvement in AIS. Comparing G0, G1 and C, P-values were analysed with the Kruskal–Wallis test and show
differences between G0, G1 and C.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam et al
10
Spinal Cord
gender, aetiology and AO classification between patients with and
without neurological remission (referred to as G1 and G0 in the
following text, respectively). All 20 patients with traumatic SCI (G0
and G1) received surgery (9 ventral 45%; 11 dorsal 55%); 14 were
treated with spondylodesis (70.0%). All 30 patients included in the
collective suffered vertebral fractures. The AIS grades at admission and
discharge as well as the NLI and the type of paralysis were significantly
different in G1 and G0 (Po0.05).
Analysis of the entire patient collective
For exact MMP concentrations (pg ml − 1) and a graphic comparison
of the groups, see Figures 1 and 2. There were no significant
differences in cytokine serum levels in regard to gender, age, paralysis,
AO classification, aetiology or NLI.
Serum values of MMP-10 and MMP-12 remained undetectable.
Comparison of SCI patients vs control group
We investigated how MMP values reflect the biochemical processes
after SCI by comparing patients with (S) with patients without (C)
neurological impairment.
MMP-2. Mean MMP-2 values were higher in S than in C. MMP-2
concentrations of the same first sample fell from 2.95E+05 to 2.43E
+05 pg ml − 1 at 4 h after trauma. Then they rose continuously to 5.19E
+05 pg ml − 1 at 24 h. Within the first 12 h, MMP-2 in C rose from
1.88E+05 to 2.87E+05 pg ml − 1 initially and then decreased to 1.95E
Table 2 Demographics
Patients All patients Remission (G1) No remission (G0) Control (C) Comparison G1, G0 and C
N 30 10 10 10 P40.05
Age (years) 42 ± 17 41 ± 21 42 ± 17 43 ± 15 P40.05
Gender
Female 9 (30.00%) 3 1 5 P40.05
Male 21 (70.00%) 7 9 5
Aetiology
Fall 18 (60.00%) 6 4 8 P40.05
Traffic 11 (36.70%) 4 5 2
Other 1 (3.30%) 0 1 0
Paralysis
Incomplete paraplegia 5 (16.70%) 5 0 0 Po0.05
Complete paraplegia 6 (20.00%) 2 4 0
Incomplete tetraplegia 6 (20.00%) 3 3 0
Complete tetraplegia 3 (10.00%) 0 3 0
None 10 (33.30%) 0 0 10
AO
A 21 (70.00%) 7 6 8 P40.05
B 6 (20.00%) 3 2 1
C 2 (6.70%) 0 2 0
No fracture 1 (3.30%) 0 0 1
NLI
C 9 (30.00%) 3 6 0 Po0.05
Th 7 (23.30%) 4 3 0
L 4 (13.30%) 3 1 0
None 10 (33.30%) 0 0 10
Initial AIS
A 12 (40.00%) 2 10 0 Po0.05
B 3 (10.00%) 3 0 0
C 5 (16.70%) 5 0 0
D 0 (0.00%) 0 0 0
E 10 (33.30%) 0 0 10
Final AIS
A 10 (33.30%) 0 10 0 Po0.05
B 1 (3.30%) 1 0 0
C 2 (6.70%) 2 0 0
D 7 (23.30%) 7 0 0
E 10 (33.30%) 0 0 10
Abbreviations: AO, AO classification; AIS, American Spinal Injury Association (ASIA) Impairment Scale; NLI, neurological level of injury.
Age is expressed as mean years ± s.d. Neurological remission was defined as improvement in AIS. Comparing G0, G1 and C, P-values were analysed with the Kruskal–Wallis test and show
differences between G0, G1 and C.
MMP-8 and MMP-9 serum levels as early markers
A Moghaddam et al
10
Spinal Cord
End of preview
Want to access all the pages? Upload your documents or become a member.
