Structure, Bonding and Organic Chemistry
VerifiedAdded on 2020/12/09
|19
|2033
|166
Homework Assignment
AI Summary
This chemistry homework assignment explores various aspects of structure, bonding, and organic chemistry. It includes questions on metallic, covalent, and ionic bonding, VSEPR theory, isomerism, and reactions with different reagents. The assignment provides detailed explanations and diagrams to aid in understanding these concepts.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

STRUCTURE, BONDING AND ORGANIC
CHEMISTRY
CHEMISTRY
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Table of Contents
STRUCTURE, BONDING AND ORGANIC CHEMISTRY.........................................................1
REFERENCES..............................................................................................................................18
STRUCTURE, BONDING AND ORGANIC CHEMISTRY.........................................................1
REFERENCES..............................................................................................................................18

Answer all the following questions
1. Calcium (Ca), chlorine (Cl2) and calcium chloride (MgF2) have different types of
bonding and different properties.
(a) Calcium has metallic bonding.
(i) Draw a diagram to show what is meant by metallic bonding. Label the
diagram.
Use your diagram to explain how calcium conducts electricity.
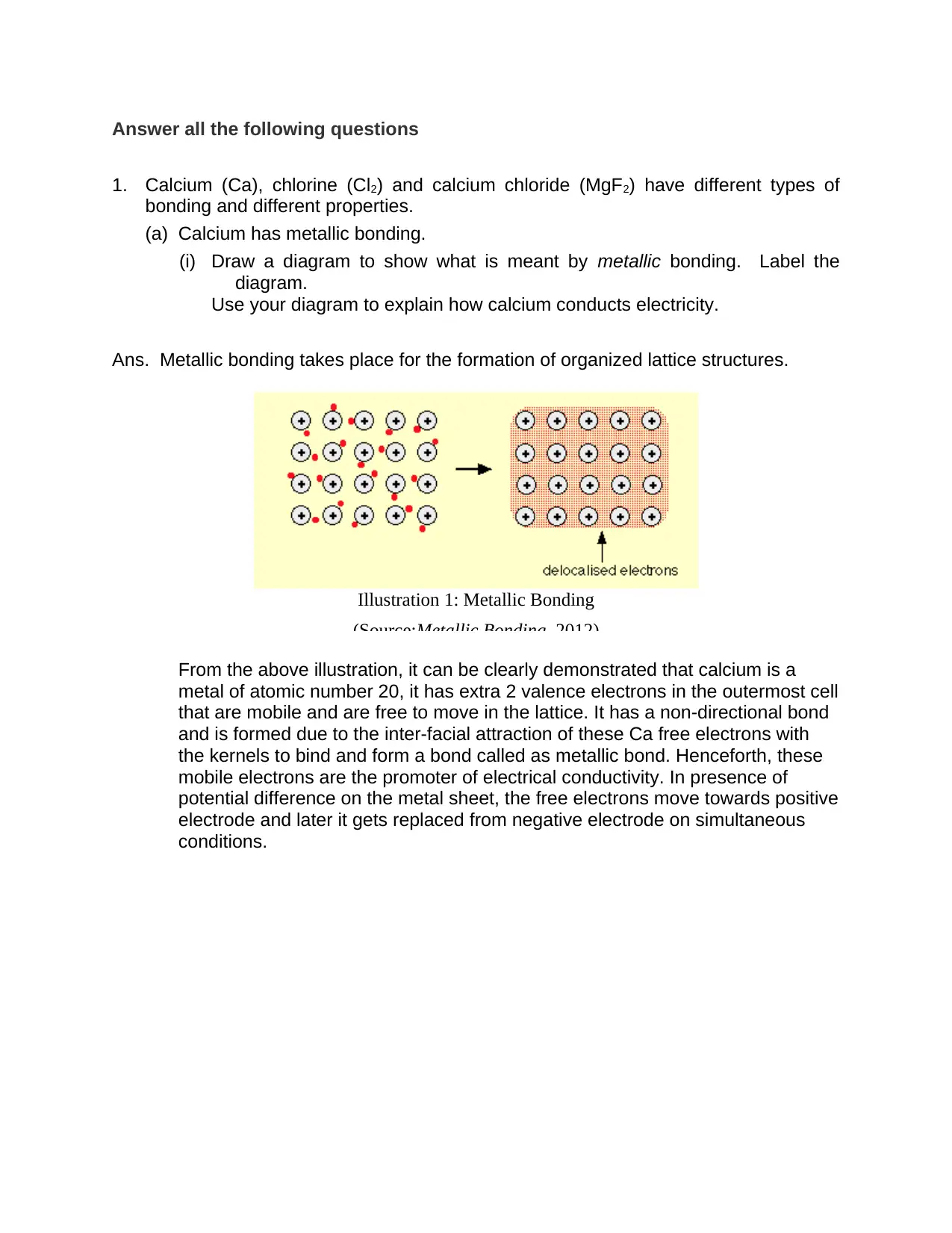
Ans. Metallic bonding takes place for the formation of organized lattice structures.
From the above illustration, it can be clearly demonstrated that calcium is a
metal of atomic number 20, it has extra 2 valence electrons in the outermost cell
that are mobile and are free to move in the lattice. It has a non-directional bond
and is formed due to the inter-facial attraction of these Ca free electrons with
the kernels to bind and form a bond called as metallic bond. Henceforth, these
mobile electrons are the promoter of electrical conductivity. In presence of
potential difference on the metal sheet, the free electrons move towards positive
electrode and later it gets replaced from negative electrode on simultaneous
conditions.
Illustration 1: Metallic Bonding
(Source:Metallic Bonding, 2012)
1. Calcium (Ca), chlorine (Cl2) and calcium chloride (MgF2) have different types of
bonding and different properties.
(a) Calcium has metallic bonding.
(i) Draw a diagram to show what is meant by metallic bonding. Label the
diagram.
Use your diagram to explain how calcium conducts electricity.
Ans. Metallic bonding takes place for the formation of organized lattice structures.
From the above illustration, it can be clearly demonstrated that calcium is a
metal of atomic number 20, it has extra 2 valence electrons in the outermost cell
that are mobile and are free to move in the lattice. It has a non-directional bond
and is formed due to the inter-facial attraction of these Ca free electrons with
the kernels to bind and form a bond called as metallic bond. Henceforth, these
mobile electrons are the promoter of electrical conductivity. In presence of
potential difference on the metal sheet, the free electrons move towards positive
electrode and later it gets replaced from negative electrode on simultaneous
conditions.
Illustration 1: Metallic Bonding
(Source:Metallic Bonding, 2012)
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
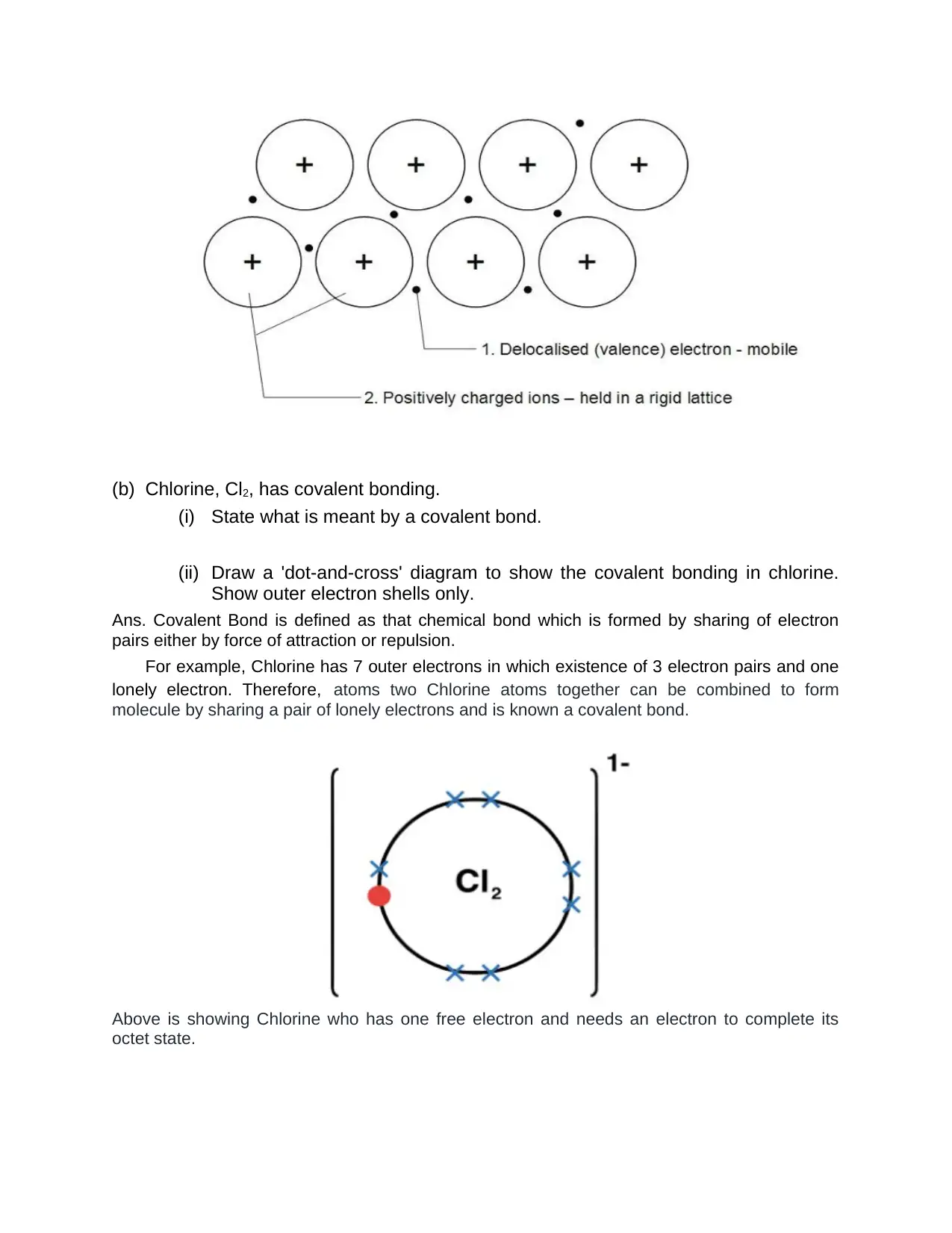
(b) Chlorine, Cl2, has covalent bonding.
(i) State what is meant by a covalent bond.
(ii) Draw a 'dot-and-cross' diagram to show the covalent bonding in chlorine.
Show outer electron shells only.
Ans. Covalent Bond is defined as that chemical bond which is formed by sharing of electron
pairs either by force of attraction or repulsion.
For example, Chlorine has 7 outer electrons in which existence of 3 electron pairs and one
lonely electron. Therefore, atoms two Chlorine atoms together can be combined to form
molecule by sharing a pair of lonely electrons and is known a covalent bond.
Above is showing Chlorine who has one free electron and needs an electron to complete its
octet state.
(i) State what is meant by a covalent bond.
(ii) Draw a 'dot-and-cross' diagram to show the covalent bonding in chlorine.
Show outer electron shells only.
Ans. Covalent Bond is defined as that chemical bond which is formed by sharing of electron
pairs either by force of attraction or repulsion.
For example, Chlorine has 7 outer electrons in which existence of 3 electron pairs and one
lonely electron. Therefore, atoms two Chlorine atoms together can be combined to form
molecule by sharing a pair of lonely electrons and is known a covalent bond.
Above is showing Chlorine who has one free electron and needs an electron to complete its
octet state.
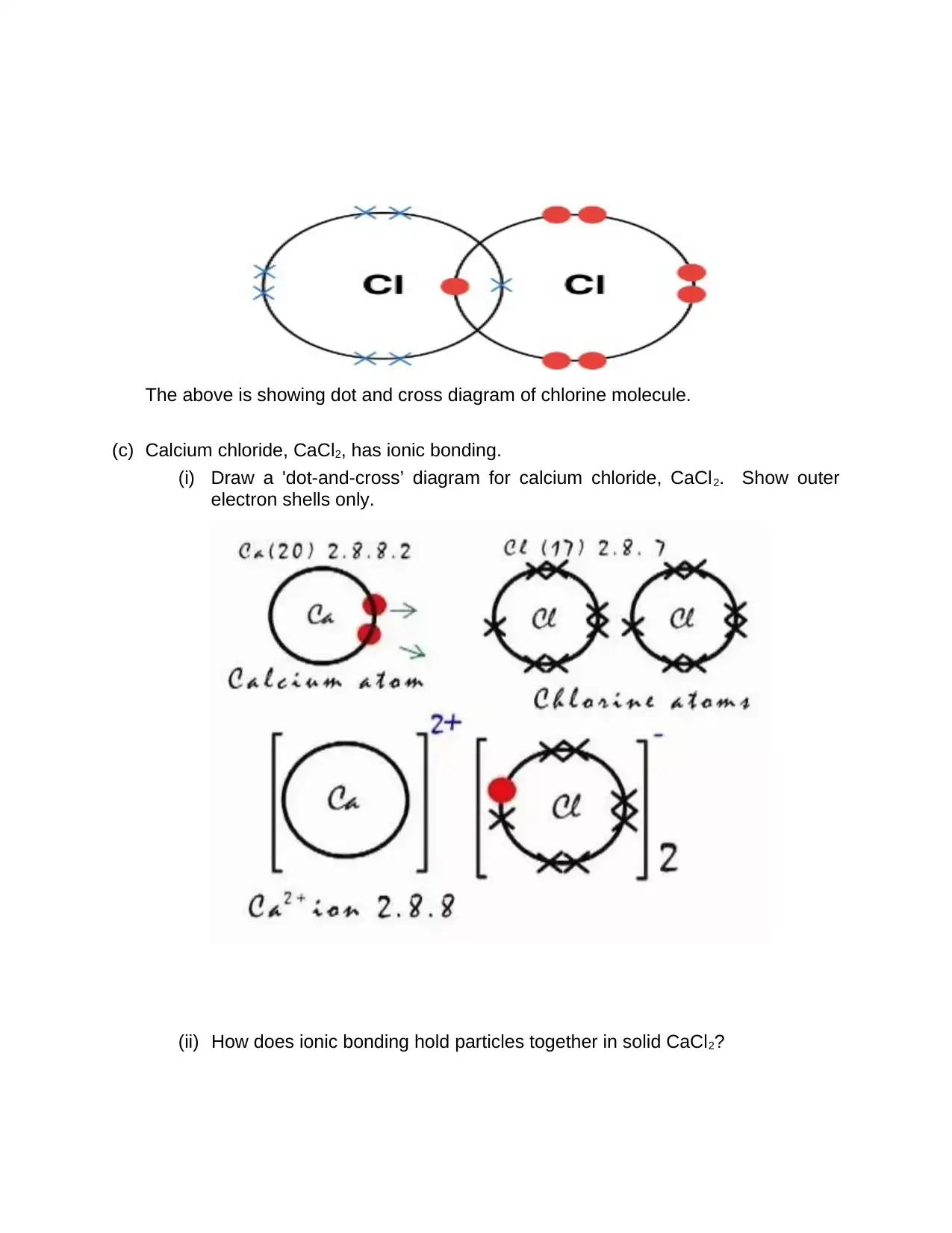
The above is showing dot and cross diagram of chlorine molecule.
(c) Calcium chloride, CaCl2, has ionic bonding.
(i) Draw a 'dot-and-cross’ diagram for calcium chloride, CaCl2. Show outer
electron shells only.
(ii) How does ionic bonding hold particles together in solid CaCl2?
(c) Calcium chloride, CaCl2, has ionic bonding.
(i) Draw a 'dot-and-cross’ diagram for calcium chloride, CaCl2. Show outer
electron shells only.
(ii) How does ionic bonding hold particles together in solid CaCl2?

(ii) How does ionic bonding hold particles together in solid CaCl2?
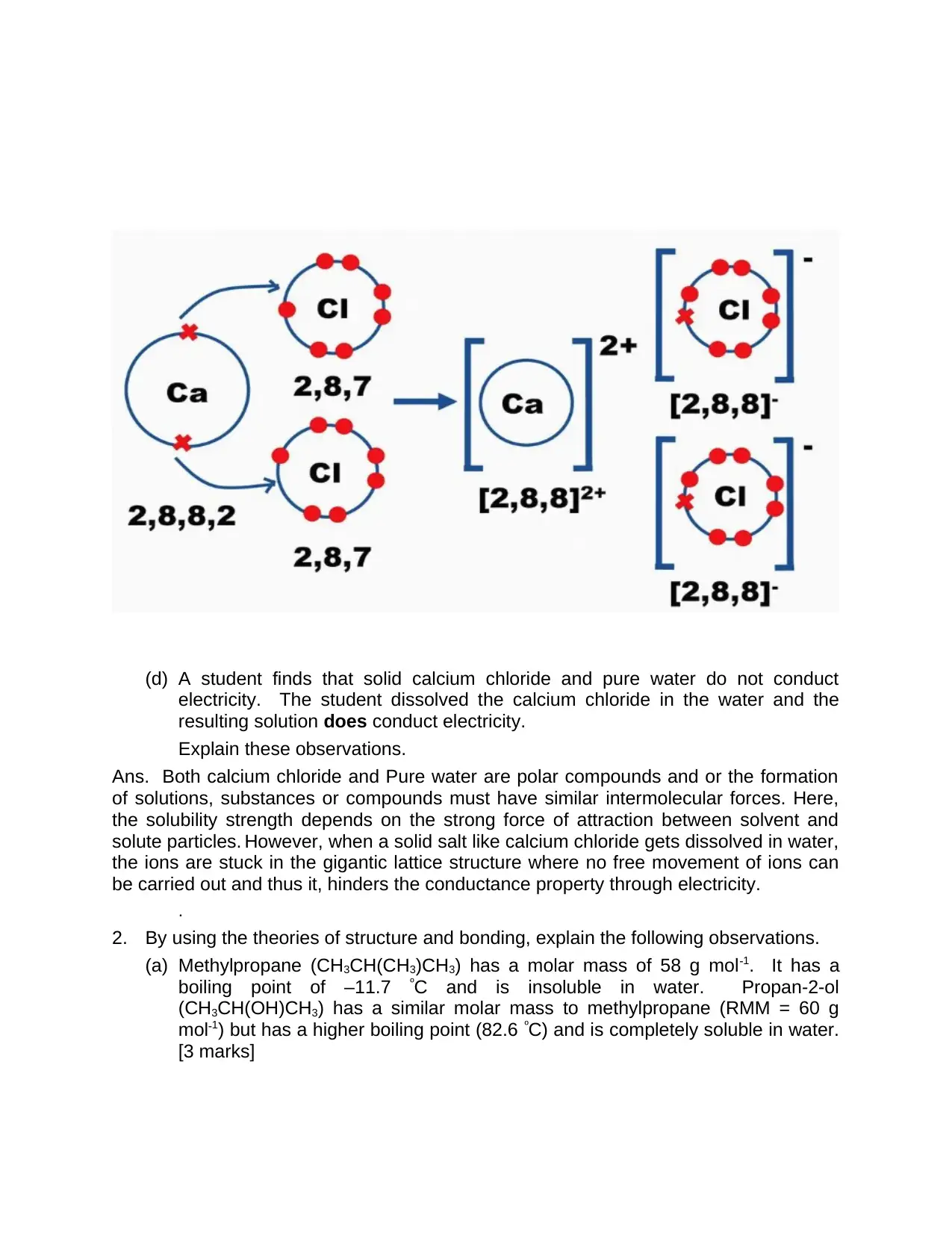
Ans. From the above, it is understandable that by losing two electrons of calcium atom
by donating one electron to one chlorine atom and other electron to second chlorine
atom. That is, total valence electrons of calcium is 2.
The number of valence electrons in chlorine is 7. Along with this, the resultant outcome
is three ions with complete outer shells. Here, formation of solid crystal structural lattice
occurs due to the orientation of Calcium ions with proximity to Chloride ions as shown in
the below CaCl2 crystal formation.
Ans. From the above, it is understandable that by losing two electrons of calcium atom
by donating one electron to one chlorine atom and other electron to second chlorine
atom. That is, total valence electrons of calcium is 2.
The number of valence electrons in chlorine is 7. Along with this, the resultant outcome
is three ions with complete outer shells. Here, formation of solid crystal structural lattice
occurs due to the orientation of Calcium ions with proximity to Chloride ions as shown in
the below CaCl2 crystal formation.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
(d) A student finds that solid calcium chloride and pure water do not conduct
electricity. The student dissolved the calcium chloride in the water and the
resulting solution does conduct electricity.
Explain these observations.
Ans. Both calcium chloride and Pure water are polar compounds and or the formation
of solutions, substances or compounds must have similar intermolecular forces. Here,
the solubility strength depends on the strong force of attraction between solvent and
solute particles. However, when a solid salt like calcium chloride gets dissolved in water,
the ions are stuck in the gigantic lattice structure where no free movement of ions can
be carried out and thus it, hinders the conductance property through electricity.
.
2. By using the theories of structure and bonding, explain the following observations.
(a) Methylpropane (CH3CH(CH3)CH3) has a molar mass of 58 g mol1. It has a
boiling point of –11.7 ºC and is insoluble in water. Propan-2-ol
(CH3CH(OH)CH3) has a similar molar mass to methylpropane (RMM = 60 g
mol1) but has a higher boiling point (82.6 ºC) and is completely soluble in water.
[3 marks]
electricity. The student dissolved the calcium chloride in the water and the
resulting solution does conduct electricity.
Explain these observations.
Ans. Both calcium chloride and Pure water are polar compounds and or the formation
of solutions, substances or compounds must have similar intermolecular forces. Here,
the solubility strength depends on the strong force of attraction between solvent and
solute particles. However, when a solid salt like calcium chloride gets dissolved in water,
the ions are stuck in the gigantic lattice structure where no free movement of ions can
be carried out and thus it, hinders the conductance property through electricity.
.
2. By using the theories of structure and bonding, explain the following observations.
(a) Methylpropane (CH3CH(CH3)CH3) has a molar mass of 58 g mol1. It has a
boiling point of –11.7 ºC and is insoluble in water. Propan-2-ol
(CH3CH(OH)CH3) has a similar molar mass to methylpropane (RMM = 60 g
mol1) but has a higher boiling point (82.6 ºC) and is completely soluble in water.
[3 marks]

Maximum = (2 Marks)
(b) Potassium chloride (KCl) and calcium oxide (CaO) are both ionic and both have the
same crystal structure. The melting point of sodium chloride is 770 ºC whereas
the melting point of calcium oxide is very much higher (2572 ºC).
Ans. Although both consists of ionic bonds where thermal energy is required in order to
break the bonds, that leads to change of state from solid to liquid. Both the
compounds have gigantic ionic lattice structures with strong electrostatic
attractions. In regard to the NaCl, it requires substantial amount of heat energy
needed to melt it and break the strong attraction between oppositely charged
ions. However, CaO lattice structure is twice larger than NaCl, thus it needs
more thermal energy to break and overcome the electrostatic forces.
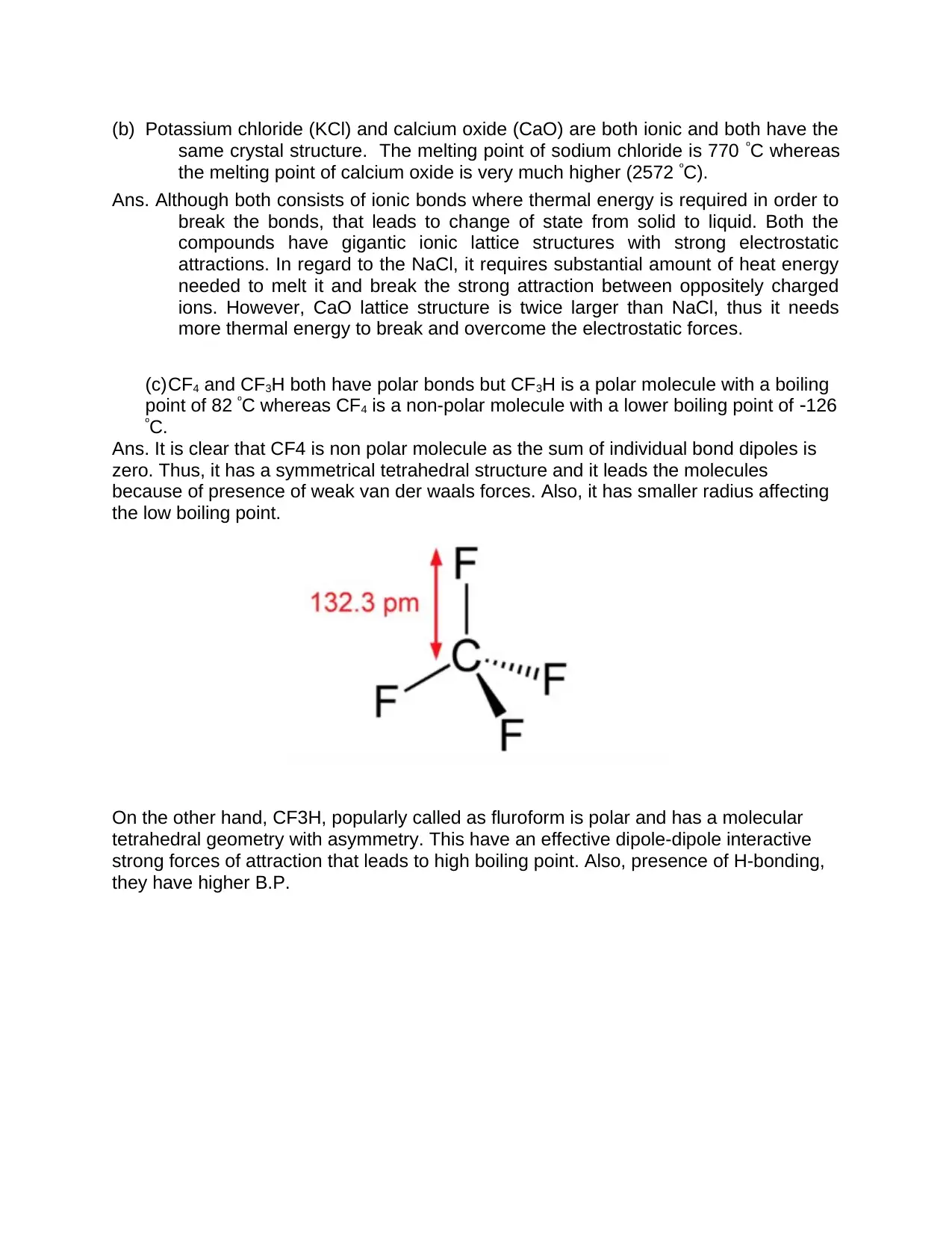
(c)CF4 and CF3H both have polar bonds but CF3H is a polar molecule with a boiling
point of 82 ºC whereas CF4 is a non-polar molecule with a lower boiling point of 126
ºC.
Ans. It is clear that CF4 is non polar molecule as the sum of individual bond dipoles is
zero. Thus, it has a symmetrical tetrahedral structure and it leads the molecules
because of presence of weak van der waals forces. Also, it has smaller radius affecting
the low boiling point.
On the other hand, CF3H, popularly called as fluroform is polar and has a molecular
tetrahedral geometry with asymmetry. This have an effective dipole-dipole interactive
strong forces of attraction that leads to high boiling point. Also, presence of H-bonding,
they have higher B.P.
same crystal structure. The melting point of sodium chloride is 770 ºC whereas
the melting point of calcium oxide is very much higher (2572 ºC).
Ans. Although both consists of ionic bonds where thermal energy is required in order to
break the bonds, that leads to change of state from solid to liquid. Both the
compounds have gigantic ionic lattice structures with strong electrostatic
attractions. In regard to the NaCl, it requires substantial amount of heat energy
needed to melt it and break the strong attraction between oppositely charged
ions. However, CaO lattice structure is twice larger than NaCl, thus it needs
more thermal energy to break and overcome the electrostatic forces.
(c)CF4 and CF3H both have polar bonds but CF3H is a polar molecule with a boiling
point of 82 ºC whereas CF4 is a non-polar molecule with a lower boiling point of 126
ºC.
Ans. It is clear that CF4 is non polar molecule as the sum of individual bond dipoles is
zero. Thus, it has a symmetrical tetrahedral structure and it leads the molecules
because of presence of weak van der waals forces. Also, it has smaller radius affecting
the low boiling point.
On the other hand, CF3H, popularly called as fluroform is polar and has a molecular
tetrahedral geometry with asymmetry. This have an effective dipole-dipole interactive
strong forces of attraction that leads to high boiling point. Also, presence of H-bonding,
they have higher B.P.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
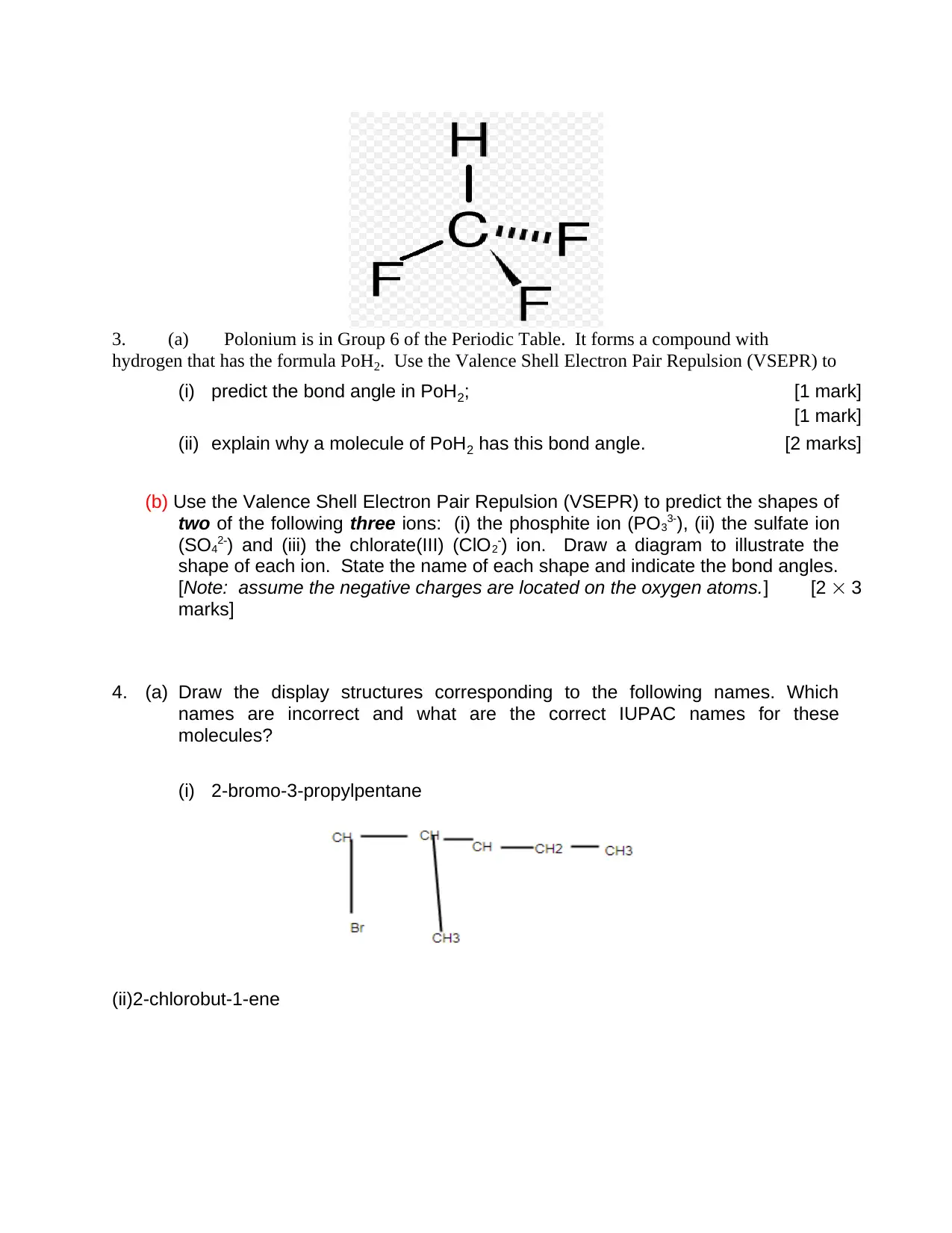
3. (a) Polonium is in Group 6 of the Periodic Table. It forms a compound with
hydrogen that has the formula PoH2. Use the Valence Shell Electron Pair Repulsion (VSEPR) to
(i) predict the bond angle in PoH2; [1 mark]
[1 mark]
(ii) explain why a molecule of PoH2 has this bond angle. [2 marks]
(b) Use the Valence Shell Electron Pair Repulsion (VSEPR) to predict the shapes of
two of the following three ions: (i) the phosphite ion (PO33-), (ii) the sulfate ion
(SO42) and (iii) the chlorate(III) (ClO2) ion. Draw a diagram to illustrate the
shape of each ion. State the name of each shape and indicate the bond angles.
[Note: assume the negative charges are located on the oxygen atoms.] [2 3
marks]
4. (a) Draw the display structures corresponding to the following names. Which
names are incorrect and what are the correct IUPAC names for these
molecules?
(i) 2-bromo-3-propylpentane
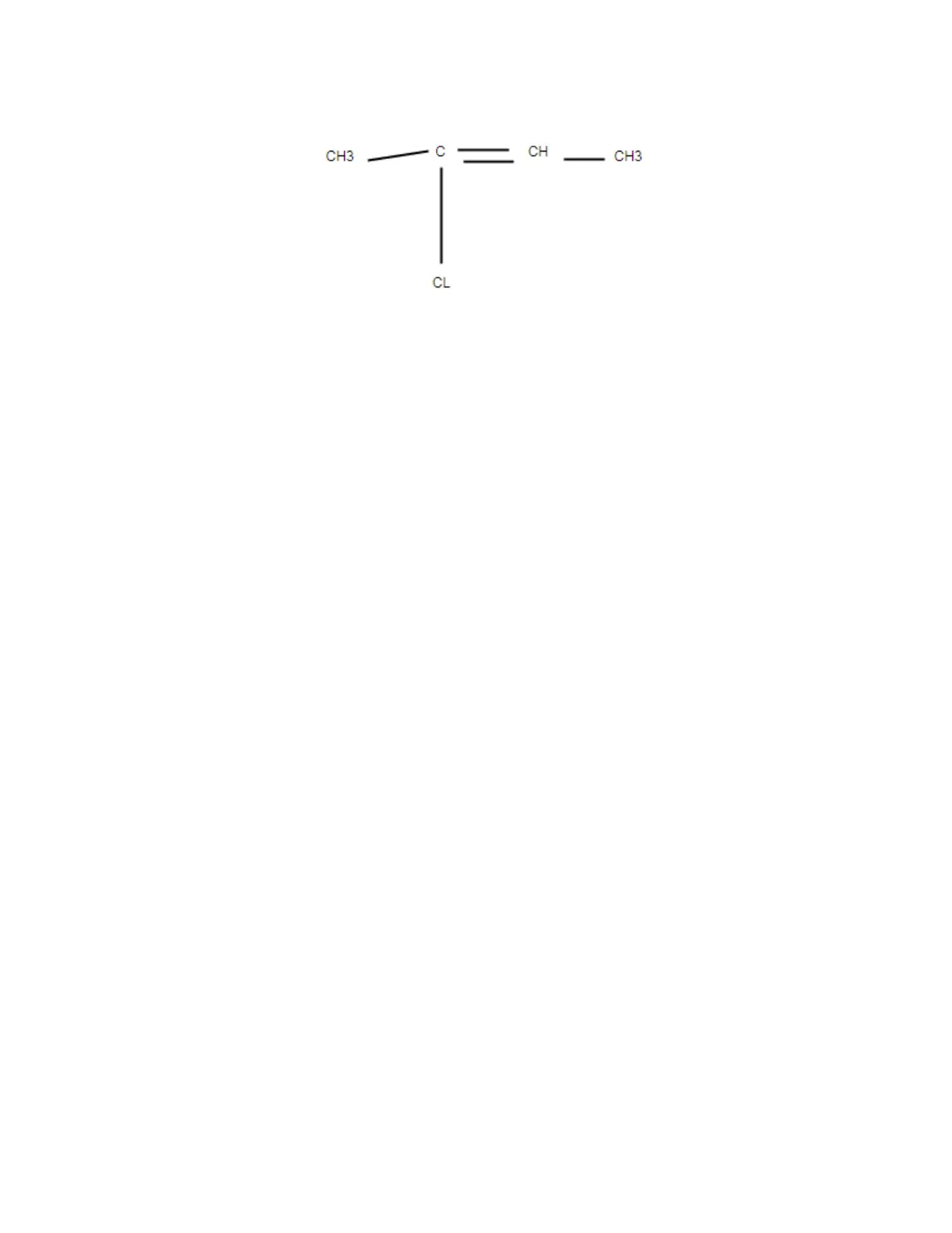
(ii)2-chlorobut-1-ene
hydrogen that has the formula PoH2. Use the Valence Shell Electron Pair Repulsion (VSEPR) to
(i) predict the bond angle in PoH2; [1 mark]
[1 mark]
(ii) explain why a molecule of PoH2 has this bond angle. [2 marks]
(b) Use the Valence Shell Electron Pair Repulsion (VSEPR) to predict the shapes of
two of the following three ions: (i) the phosphite ion (PO33-), (ii) the sulfate ion
(SO42) and (iii) the chlorate(III) (ClO2) ion. Draw a diagram to illustrate the
shape of each ion. State the name of each shape and indicate the bond angles.
[Note: assume the negative charges are located on the oxygen atoms.] [2 3
marks]
4. (a) Draw the display structures corresponding to the following names. Which
names are incorrect and what are the correct IUPAC names for these
molecules?
(i) 2-bromo-3-propylpentane
(ii)2-chlorobut-1-ene
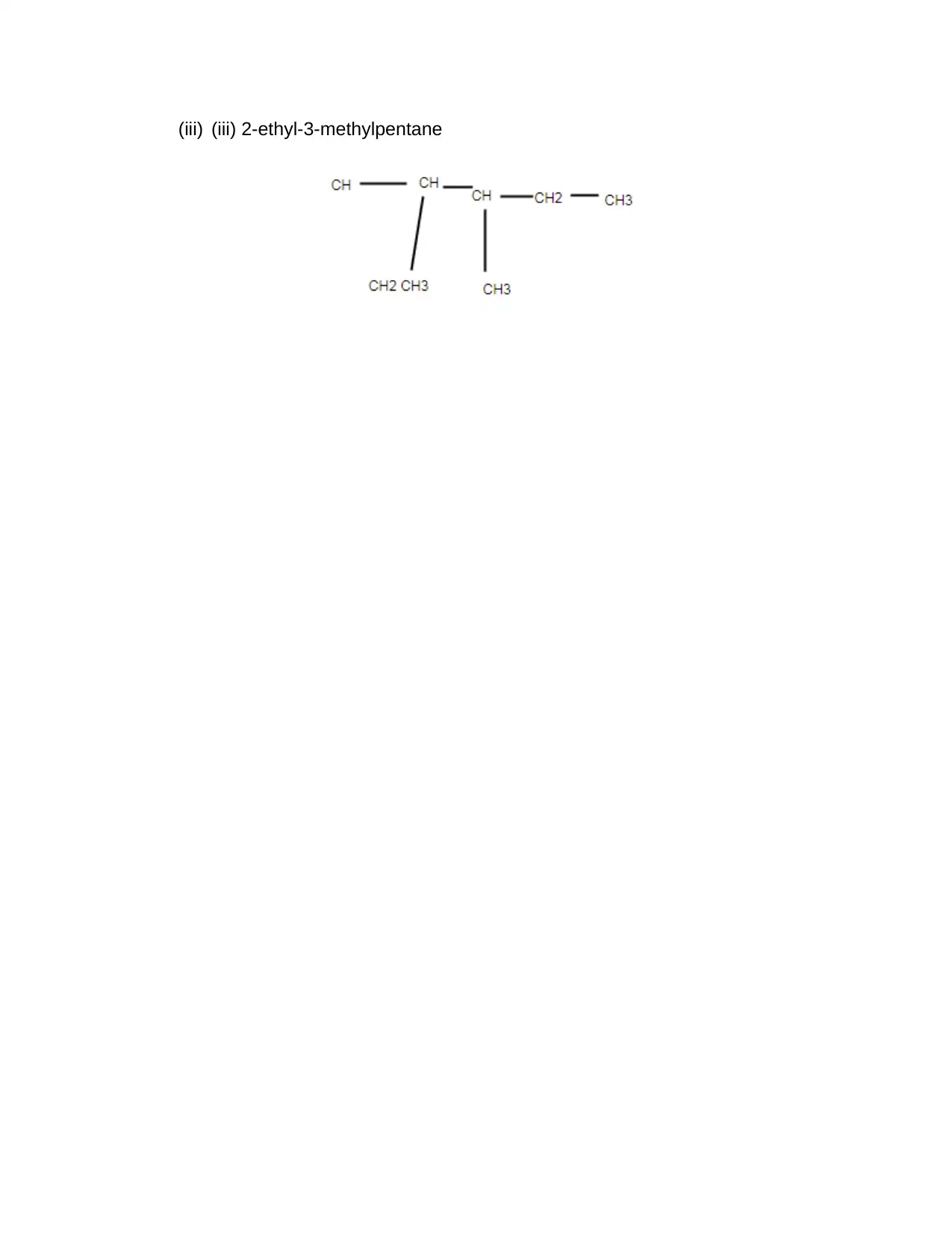
(iii) (iii) 2-ethyl-3-methylpentane
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
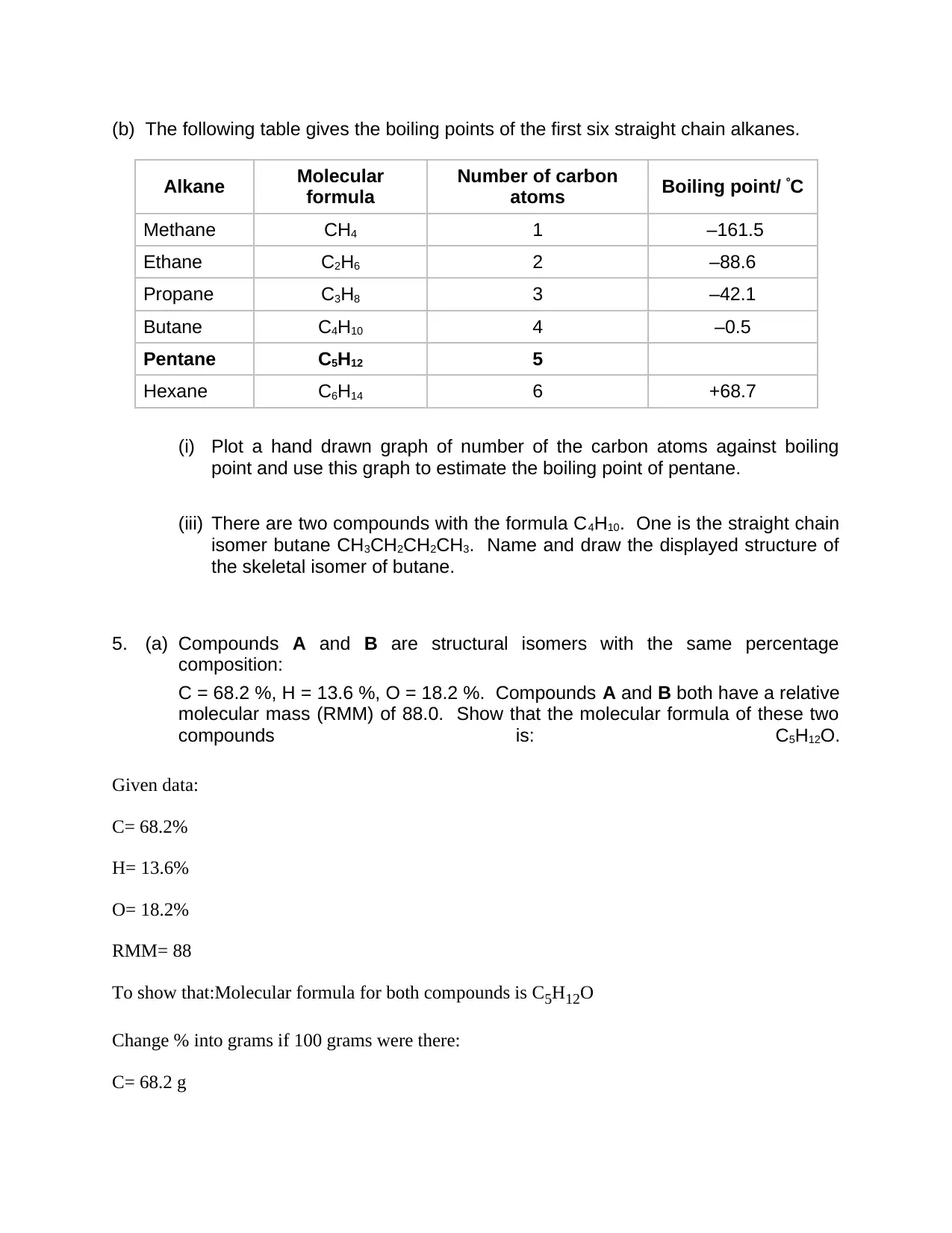
(b) The following table gives the boiling points of the first six straight chain alkanes.
(i) Plot a hand drawn graph of number of the carbon atoms against boiling
point and use this graph to estimate the boiling point of pentane.
(iii) There are two compounds with the formula C4H10. One is the straight chain
isomer butane CH3CH2CH2CH3. Name and draw the displayed structure of
the skeletal isomer of butane.
5. (a) Compounds A and B are structural isomers with the same percentage
composition:
C = 68.2 %, H = 13.6 %, O = 18.2 %. Compounds A and B both have a relative
molecular mass (RMM) of 88.0. Show that the molecular formula of these two
compounds is: C5H12O.
Given data:
C= 68.2%
H= 13.6%
O= 18.2%
RMM= 88
To show that:Molecular formula for both compounds is C5H12O
Change % into grams if 100 grams were there:
C= 68.2 g
Alkane Molecular
formula
Number of carbon
atoms Boiling point/ ºC
Methane CH4 1 –161.5
Ethane C2H6 2 –88.6
Propane C3H8 3 –42.1
Butane C4H10 4 –0.5
Pentane C5H12 5
Hexane C6H14 6 +68.7
(i) Plot a hand drawn graph of number of the carbon atoms against boiling
point and use this graph to estimate the boiling point of pentane.
(iii) There are two compounds with the formula C4H10. One is the straight chain
isomer butane CH3CH2CH2CH3. Name and draw the displayed structure of
the skeletal isomer of butane.
5. (a) Compounds A and B are structural isomers with the same percentage
composition:
C = 68.2 %, H = 13.6 %, O = 18.2 %. Compounds A and B both have a relative
molecular mass (RMM) of 88.0. Show that the molecular formula of these two
compounds is: C5H12O.
Given data:
C= 68.2%
H= 13.6%
O= 18.2%
RMM= 88
To show that:Molecular formula for both compounds is C5H12O
Change % into grams if 100 grams were there:
C= 68.2 g
Alkane Molecular
formula
Number of carbon
atoms Boiling point/ ºC
Methane CH4 1 –161.5
Ethane C2H6 2 –88.6
Propane C3H8 3 –42.1
Butane C4H10 4 –0.5
Pentane C5H12 5
Hexane C6H14 6 +68.7
H= 13.6 g
O= 18.2 g
Next step is changing grams into moles:
Moles of C= 68.2 / 12 = 5.684
Moles of H= 13.6 / 1 = 13.6
Moles of O= 18.2 / 16= 1.1375
Now, the Simplest moles' ratio:
C / O = 5.6084/1.1375 = 4.99=5
H / O = 13.6 / 1.1374 =11.95 = 12
Thus, the ratios of C: H: O respectively is 5: 12: 1. Hence, proved.
(b) Compounds A and B were separately heated with acidified potassium dichromate
[Cr2O72- (aq) / H+ (aq)] as in Scheme 1 below. Compounds C, D and E were formed.
Compounds D and E could not be oxidized further and none of the compounds are
branched. Compound B exists as a pair of stereo-isomers.
Scheme 1
(i) Identify compounds A to E by either writing their name or their formula.
A-
B-
C-
D-
O= 18.2 g
Next step is changing grams into moles:
Moles of C= 68.2 / 12 = 5.684
Moles of H= 13.6 / 1 = 13.6
Moles of O= 18.2 / 16= 1.1375
Now, the Simplest moles' ratio:
C / O = 5.6084/1.1375 = 4.99=5
H / O = 13.6 / 1.1374 =11.95 = 12
Thus, the ratios of C: H: O respectively is 5: 12: 1. Hence, proved.
(b) Compounds A and B were separately heated with acidified potassium dichromate
[Cr2O72- (aq) / H+ (aq)] as in Scheme 1 below. Compounds C, D and E were formed.
Compounds D and E could not be oxidized further and none of the compounds are
branched. Compound B exists as a pair of stereo-isomers.
Scheme 1
(i) Identify compounds A to E by either writing their name or their formula.
A-
B-
C-
D-
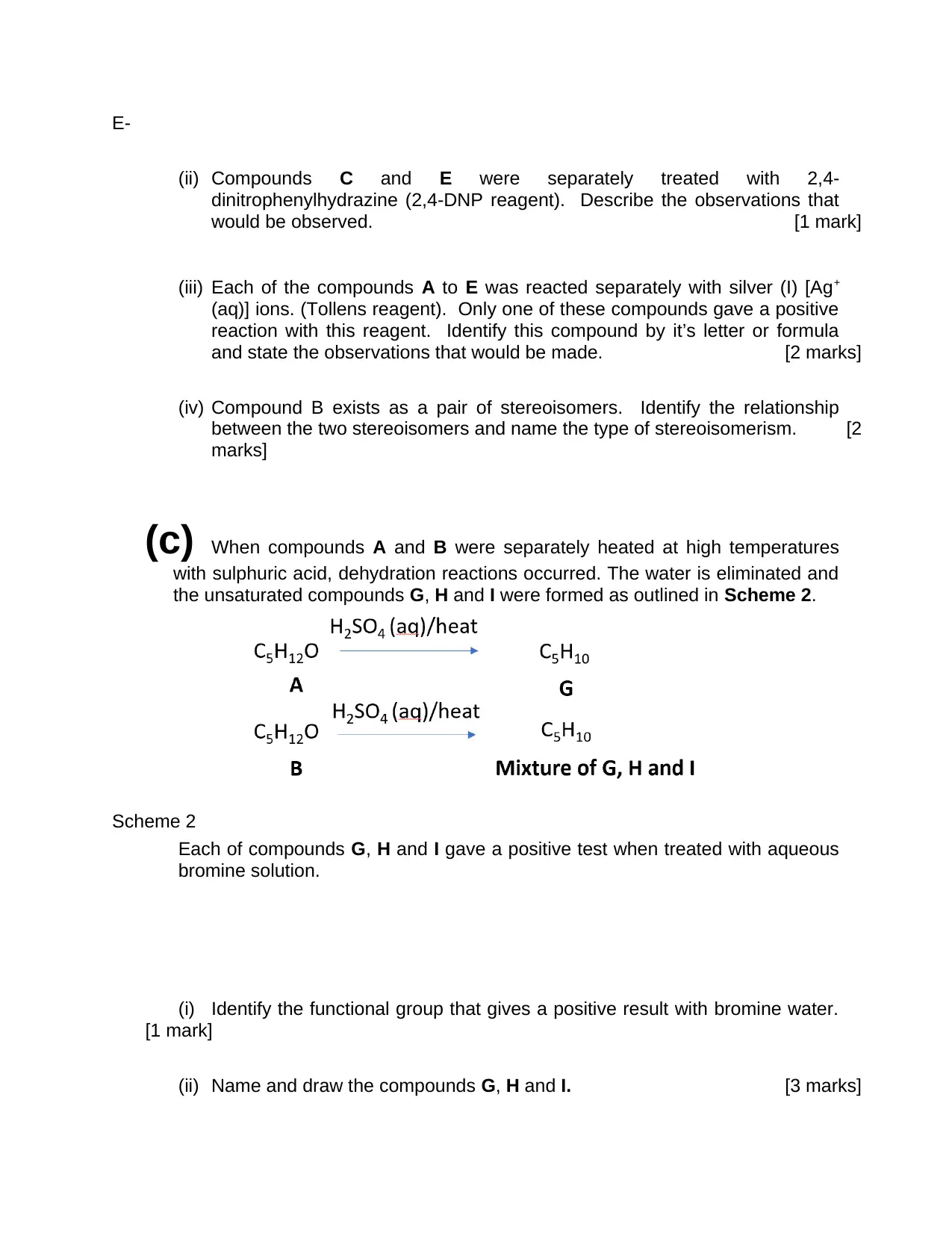
E-
(ii) Compounds C and E were separately treated with 2,4-
dinitrophenylhydrazine (2,4-DNP reagent). Describe the observations that
would be observed. [1 mark]
(iii) Each of the compounds A to E was reacted separately with silver (I) [Ag+
(aq)] ions. (Tollens reagent). Only one of these compounds gave a positive
reaction with this reagent. Identify this compound by it’s letter or formula
and state the observations that would be made. [2 marks]
(iv) Compound B exists as a pair of stereoisomers. Identify the relationship
between the two stereoisomers and name the type of stereoisomerism. [2
marks]
(c) When compounds A and B were separately heated at high temperatures
with sulphuric acid, dehydration reactions occurred. The water is eliminated and
the unsaturated compounds G, H and I were formed as outlined in Scheme 2.
Scheme 2
Each of compounds G, H and I gave a positive test when treated with aqueous
bromine solution.
(i) Identify the functional group that gives a positive result with bromine water.
[1 mark]
(ii) Name and draw the compounds G, H and I. [3 marks]
(ii) Compounds C and E were separately treated with 2,4-
dinitrophenylhydrazine (2,4-DNP reagent). Describe the observations that
would be observed. [1 mark]
(iii) Each of the compounds A to E was reacted separately with silver (I) [Ag+
(aq)] ions. (Tollens reagent). Only one of these compounds gave a positive
reaction with this reagent. Identify this compound by it’s letter or formula
and state the observations that would be made. [2 marks]
(iv) Compound B exists as a pair of stereoisomers. Identify the relationship
between the two stereoisomers and name the type of stereoisomerism. [2
marks]
(c) When compounds A and B were separately heated at high temperatures
with sulphuric acid, dehydration reactions occurred. The water is eliminated and
the unsaturated compounds G, H and I were formed as outlined in Scheme 2.
Scheme 2
Each of compounds G, H and I gave a positive test when treated with aqueous
bromine solution.
(i) Identify the functional group that gives a positive result with bromine water.
[1 mark]
(ii) Name and draw the compounds G, H and I. [3 marks]
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

(iii) Compounds H and I are stereoisomers but the relationship between this
type of stereoisomerisom is different from that discussed in part (b) (v).
What type of stereoisomerism is shown by compounds H and I? [1 mark]
(iv) Discuss why compounds H and I are stereoisomers whereas compound G
does not form steroisomers. You should state in your answer the properties
of the functional group that makes this form of steroisomerism and the
effects that the various atoms or groups bonded either side of the C=C
double bond can have on stereoisomerism.
[3 marks]
type of stereoisomerisom is different from that discussed in part (b) (v).
What type of stereoisomerism is shown by compounds H and I? [1 mark]
(iv) Discuss why compounds H and I are stereoisomers whereas compound G
does not form steroisomers. You should state in your answer the properties
of the functional group that makes this form of steroisomerism and the
effects that the various atoms or groups bonded either side of the C=C
double bond can have on stereoisomerism.
[3 marks]

REFERENCES
Metallic Bonding, 2012. [Online]. Available
through:<https://www.chemguide.co.uk/atoms/bonding/metallic.html>.
Metallic Bonding, 2012. [Online]. Available
through:<https://www.chemguide.co.uk/atoms/bonding/metallic.html>.

1
1 out of 19
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
