Ask a question from expert
Enzyme Kinetics of Yeast Alcohol Dehydrogenase
8 Pages2187 Words316 Views
Added on 2021-08-11
Enzyme Kinetics of Yeast Alcohol Dehydrogenase
Added on 2021-08-11
BookmarkShareRelated Documents
Structure, Function and Analysis of
Proteins (LIFE2087)
Title: Experiment on enzyme kinetics of Yeast
Alcohol Dehydrogenase and its substrate
properties
Name: Priangka Ambheghai Bathianathan
Student ID: 20210653

Page 2 of 8
EXPERIMENT ON ENZYME KINETICS OF YEAST ALCOHOL
DEHYDROGENASE AND ITS SUBSTRATE PROPERTIES
ABSTRACT
Yeast Alcohol Dehydrogenase (YAD) acts as a catalyst in the oxidation of alcohol. The aim of
this experiment is to study the enzyme kinetics of YAD and how different substrates affect
enzyme activity. Methodology uses standard assay conditions for steady state reaction and
spectrophotometer to measure reaction progress. It was found that out of the 9 substrates
used, Propan-1-ol, Butan-1-ol, Propan-2-ol, 2-Propen-1-ol and 1-Cyclopropyl-methanol act as
substrates whereas Methanol, 2-Chloroethanol, and 1,2-Ethanediol act as non-substrate with
2-chloroethanol also being a competitive inhibitor to the enzyme.
INTRODUCTION
Yeast Alcohol Dehydrogenase (YAD) is a tetramer that works as a catalyst to facilitate
conversion of alcohol to aldehydes or ketones with reduction of Nicotinamide adenine
dinucleotide (NAD+) to NADH.
The main reaction of alcohol dehydrogenase is whereby an alcohol group is oxidized
by the removal of a proton from the hydroxyl group with the help of zinc ions. This is done via
the transfer of a hydride ion from the adjacent carbon atom to NAD+. This NAD+ has an
absorbance of 340nm in a spectrophotometer which is used to study the steady state kinetics
of yeast alcohol dehydrogenase (YAD) in this experiment. The reaction is shown below:
R-CH2OH + NAD+ <----> R-CHO + NADH + H+
This experiment aims to study YAD enzyme and its substrate by 4 parts. First, the initial
rate of enzyme reaction is measured under steady state conditions. Second, the enzyme
activity is measured with different alcohols. Thirdly, the alcohols are studied to determine if
they are substrates of the enzyme or not. Fourthly, the non substrates are further analyzed
to determine if they also act as an inhibitor to the YAD enzyme.
METHOD
This experiment is divided into 4 parts. The first part, Part A is to determine initial rate of YAD
enzyme with ethanol as a substrate under standard assay conditions. The standard assay
condition uses 2.2ml H20, 10mmol/L Pyrophosphate buffer at pH 8.5, 1mmol/L NAD, ethanol
at final concentration of 200mmol/L and YAD at 5.7x10-4mg/ml. The final volume for the assay
is fixed at 3ml for all parts. Absorbance reading is taken, and the initial rate can be calculated.

Page 3 of 8
EXPERIMENT ON ENZYME KINETICS OF YEAST ALCOHOL
DEHYDROGENASE AND ITS SUBSTRATE PROPERTIES
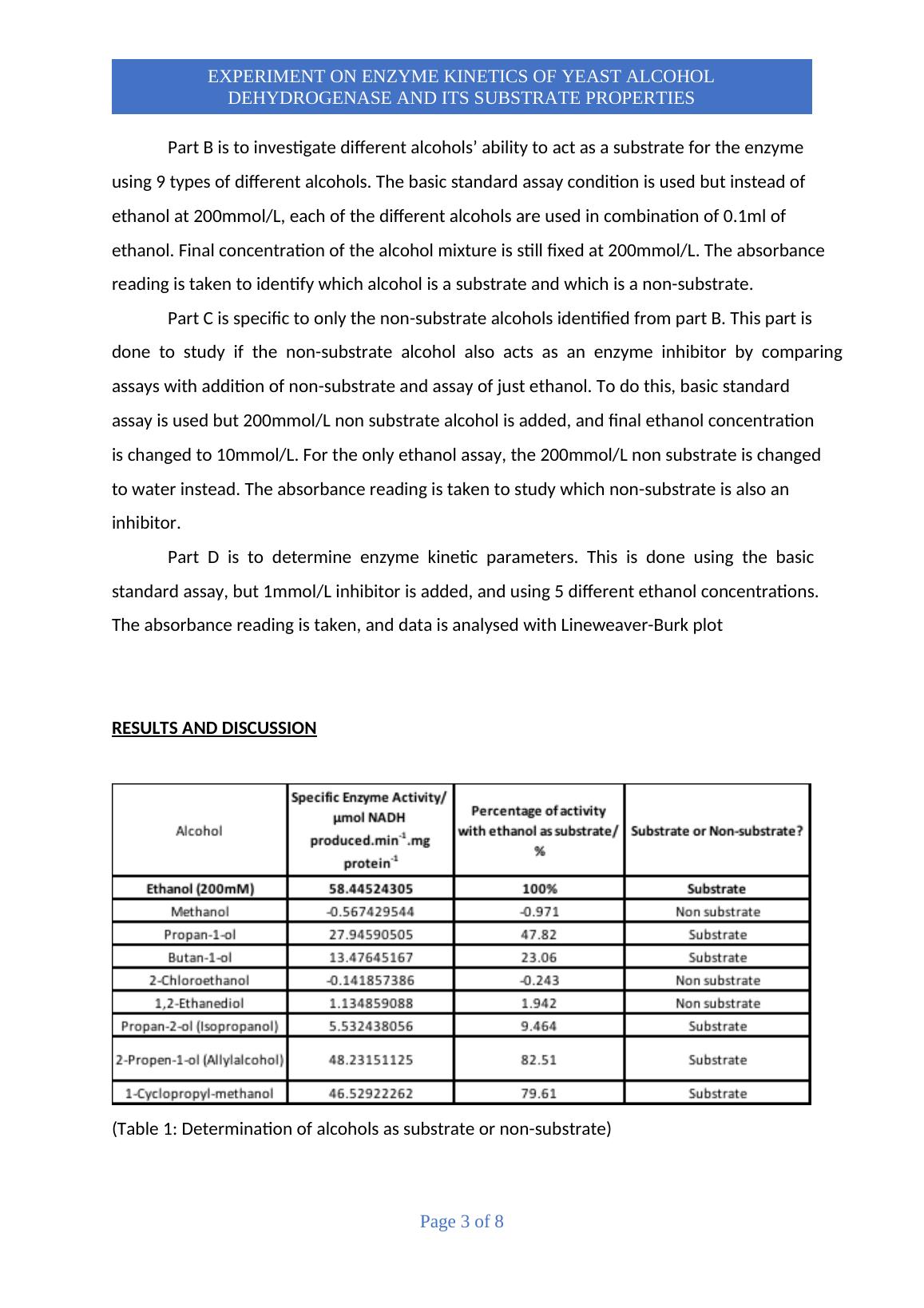
Part B is to investigate different alcohols’ ability to act as a substrate for the enzyme
using 9 types of different alcohols. The basic standard assay condition is used but instead of
ethanol at 200mmol/L, each of the different alcohols are used in combination of 0.1ml of
ethanol. Final concentration of the alcohol mixture is still fixed at 200mmol/L. The absorbance
reading is taken to identify which alcohol is a substrate and which is a non-substrate.
Part C is specific to only the non-substrate alcohols identified from part B. This part is
done to study if the non-substrate alcohol also acts as an enzyme inhibitor by comparing
assays with addition of non-substrate and assay of just ethanol. To do this, basic standard
assay is used but 200mmol/L non substrate alcohol is added, and final ethanol concentration
is changed to 10mmol/L. For the only ethanol assay, the 200mmol/L non substrate is changed
to water instead. The absorbance reading is taken to study which non-substrate is also an
inhibitor.
Part D is to determine enzyme kinetic parameters. This is done using the basic
standard assay, but 1mmol/L inhibitor is added, and using 5 different ethanol concentrations.
The absorbance reading is taken, and data is analysed with Lineweaver-Burk plot
RESULTS AND DISCUSSION
(Table 1: Determination of alcohols as substrate or non-substrate)
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Department of Biomedical Scienceslg...
|13
|3976
|191
Kinetics of Dehydrogenase-Catalyzed Reduction of Pyruvate to Lactatelg...
|5
|616
|423