Structures of matter, properties and interactions PDF
Added on 2021-09-28
8 Pages1563 Words226 Views
Structures of matter, properties and
interactions1
STRUCTURES OF MATTER, PROPERTIES AND INTERACTIONS
Name:
Department:
School:
Date:
interactions1
STRUCTURES OF MATTER, PROPERTIES AND INTERACTIONS
Name:
Department:
School:
Date:

Structures of matter, properties and interactions 2
Structures of matter, properties and interactions
Question 1
The attraction amongst oppositely charged molecule is referred to as ionic bonding, and it
is one of the various forms of chemical bonding in chemistry. Ionic bonding is as a result of
electrons (e-) moving from one particle to another. The trends that atoms have eight electrons in
their valence shell are referred to as the octet decree. Sodium in its elemental sort, it has one
valence e- and is firm (Svidzinsky, Scully and Herschbach 2014). It is reactive and does not
need a lot of vigor to dislodge an e- to create the Na+ ions. To form Na2+ from Na+ ions, it will
require a lot of energy that is normally available in chemical reactions. Now, considering a Na
atom in existence of a Cl bit, the two atoms have these Lewis e- dot figures and electronic
configuration (Moruzzi, Janak and Williams 2013).
For a Na particle to have an octet, it needs to lose an e-; for the Cl atom to gain an octet,
it must gain an e-
Structures of matter, properties and interactions
Question 1
The attraction amongst oppositely charged molecule is referred to as ionic bonding, and it
is one of the various forms of chemical bonding in chemistry. Ionic bonding is as a result of
electrons (e-) moving from one particle to another. The trends that atoms have eight electrons in
their valence shell are referred to as the octet decree. Sodium in its elemental sort, it has one
valence e- and is firm (Svidzinsky, Scully and Herschbach 2014). It is reactive and does not
need a lot of vigor to dislodge an e- to create the Na+ ions. To form Na2+ from Na+ ions, it will
require a lot of energy that is normally available in chemical reactions. Now, considering a Na
atom in existence of a Cl bit, the two atoms have these Lewis e- dot figures and electronic
configuration (Moruzzi, Janak and Williams 2013).
For a Na particle to have an octet, it needs to lose an e-; for the Cl atom to gain an octet,
it must gain an e-

Structures of matter, properties and interactions 3
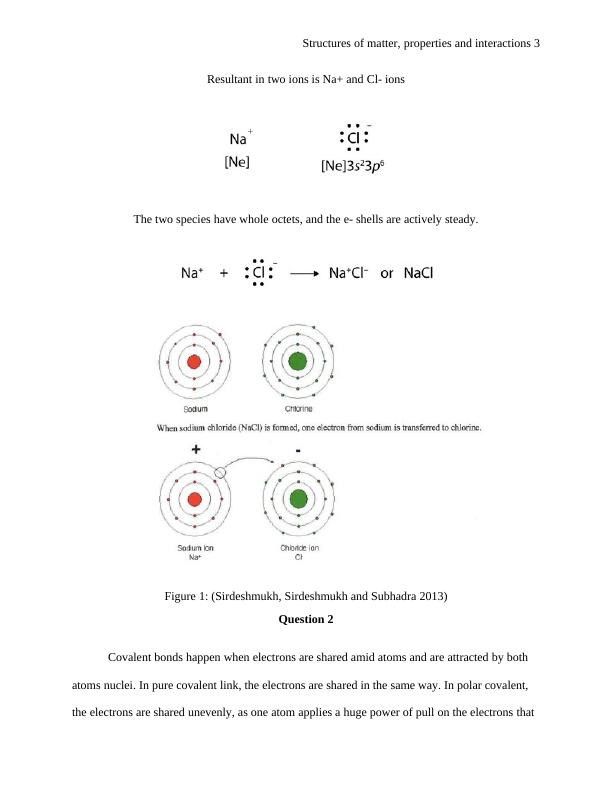
Resultant in two ions is Na+ and Cl- ions
The two species have whole octets, and the e- shells are actively steady.
Figure 1: (Sirdeshmukh, Sirdeshmukh and Subhadra 2013)
Question 2
Covalent bonds happen when electrons are shared amid atoms and are attracted by both
atoms nuclei. In pure covalent link, the electrons are shared in the same way. In polar covalent,
the electrons are shared unevenly, as one atom applies a huge power of pull on the electrons that
Resultant in two ions is Na+ and Cl- ions
The two species have whole octets, and the e- shells are actively steady.
Figure 1: (Sirdeshmukh, Sirdeshmukh and Subhadra 2013)
Question 2
Covalent bonds happen when electrons are shared amid atoms and are attracted by both
atoms nuclei. In pure covalent link, the electrons are shared in the same way. In polar covalent,
the electrons are shared unevenly, as one atom applies a huge power of pull on the electrons that
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Structure, Bonding and Organic Chemistrylg...
|19
|2033
|166
