Chemistry Research Report: Industrial Production of Sulfuric Acid
VerifiedAdded on 2023/06/07
|10
|1583
|373
Report
AI Summary
This chemistry research report comprehensively examines the industrial production of sulfuric acid, focusing on the contact process. The report begins by defining sulfuric acid and highlighting its applications in various industries. It then delves into the chemical equations involved in the three stages of the contact process: the production of sulfur dioxide, the conversion of sulfur dioxide to sulfur trioxide, and the conversion of sulfur trioxide to concentrated sulfuric acid. The report details the characteristics of the raw materials, including sulfur, oxygen, and water, and discusses their industrial and general uses. A flow diagram illustrates the chemical formation process, followed by a discussion of reaction rates, equilibrium considerations, and the role of catalysts, particularly vanadium pentoxide. The report also covers the uses of the chemicals formed, both in domestic and industrial contexts, including applications as a core substance in the chemical industry, in the manufacturing of phosphoric acid, and as a catalyst in various processes. The report concludes by summarizing the contact process, emphasizing safety precautions and storage considerations. References to various sources are provided to support the information presented.

Running Head: CHEMISTRY 0
Chemistry Research
Production of Sulfuric Acid
(Student Details:)
9/2/2018
Chemistry Research
Production of Sulfuric Acid
(Student Details:)
9/2/2018
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry Research 1
Contents
Chemistry Research Report........................................................................................................2
Introduction................................................................................................................................2
Chemical Information: Chemical Equations..............................................................................2
Production of Sulfuric Acid...................................................................................................2
Contact Process......................................................................................................................2
Steps involved in Contact process for manufacturing of sulphuric acid:...............................2
Characteristics of original reactants involved............................................................................3
Industrial & General utilization of raw products...................................................................4
Chemical Formation Process......................................................................................................4
Flow diagram of process........................................................................................................4
Discussion of equilibrium and catalysts.................................................................................5
Rate of Reaction considerations.........................................................................................5
Equilibrium considerations................................................................................................5
Uses of the chemicals formed (final products)......................................................................5
Conclusion..................................................................................................................................6
References..................................................................................................................................7
Contents
Chemistry Research Report........................................................................................................2
Introduction................................................................................................................................2
Chemical Information: Chemical Equations..............................................................................2
Production of Sulfuric Acid...................................................................................................2
Contact Process......................................................................................................................2
Steps involved in Contact process for manufacturing of sulphuric acid:...............................2
Characteristics of original reactants involved............................................................................3
Industrial & General utilization of raw products...................................................................4
Chemical Formation Process......................................................................................................4
Flow diagram of process........................................................................................................4
Discussion of equilibrium and catalysts.................................................................................5
Rate of Reaction considerations.........................................................................................5
Equilibrium considerations................................................................................................5
Uses of the chemicals formed (final products)......................................................................5
Conclusion..................................................................................................................................6
References..................................................................................................................................7

Chemistry Research 2
Chemistry Research Report
Introduction
This Chemical Research is going to start with answering the query that what is sulphuric acid.
Sulphuric acid is fundamentally an oxidizing, dehydrating agent and a dense mineral acid.
Sulphuric acid (H2SO4) is utilized in the manufacturing industries of paints, detergents,
fertilizers, dyes, plastics, fabrics, and many other useful products. In this task, we will find
out the way to produce this acid within industries (World of Chemicals, 2018).
Chemical Information: Chemical Equations
Stage I
Source: (Patana, 2018)
Stage II
Source: (Patana, 2018)
Stage III
Source: (Patana, 2018)
Chemistry Research Report
Introduction
This Chemical Research is going to start with answering the query that what is sulphuric acid.
Sulphuric acid is fundamentally an oxidizing, dehydrating agent and a dense mineral acid.
Sulphuric acid (H2SO4) is utilized in the manufacturing industries of paints, detergents,
fertilizers, dyes, plastics, fabrics, and many other useful products. In this task, we will find
out the way to produce this acid within industries (World of Chemicals, 2018).
Chemical Information: Chemical Equations
Stage I
Source: (Patana, 2018)
Stage II
Source: (Patana, 2018)
Stage III
Source: (Patana, 2018)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemistry Research 3
Production of Sulfuric Acid
Sulphuric-acid is generally produced through contact process worldwide. Production of the
acid using contact-process is based on the ‘catalytic-oxidation’ of SO2 that is sulphur-dioxide
into SO3 (sulphur-trioxide) (The Essential chemical industry, 2016).
Contact Process
Steps involved in Contact process for manufacturing of sulphuric-acid:
1. Production of sulphur-dioxide: Either burning sulphur (S) in excessive air obtains
Sulphur dioxide or by heating sulphide ores-pyrite in an excess air will do it.
2. Converting sulphur-dioxide into sulphur-trioxide: the formation process of the sulphur
trioxide is a revocable as well as exothermic reaction.
3. Converting sulphur-trioxide formed into concentrated Sulfuric-acid: Sulphur-trioxide is
initially liquefied within concentrated-sulfuric acid
H2SO4 (l) + SO3 (g) → H2S2O7 (l)
(Oleum)
The product obtained in above step is fuming-sulfuric acid or oleum, which is then made,
react safely with H2O to produce concentrated- H2SO4.
H2S2O7 (l) + H2O → 2 H2SO4 (l)
(Sulfuric acid)
Characteristics of original reactants involved
There are following raw materials used during contact process of acid formation:
Sulphur: Chalcogen and non-metal (ChemiCool, 2018)
Property Value
Atomic weight: 32.06
Colour: yellow
State: solid
Boiling point: 444.7oC, 717.9 K
Melting point: 115.2oC, 388.4 K
Production of Sulfuric Acid
Sulphuric-acid is generally produced through contact process worldwide. Production of the
acid using contact-process is based on the ‘catalytic-oxidation’ of SO2 that is sulphur-dioxide
into SO3 (sulphur-trioxide) (The Essential chemical industry, 2016).
Contact Process
Steps involved in Contact process for manufacturing of sulphuric-acid:
1. Production of sulphur-dioxide: Either burning sulphur (S) in excessive air obtains
Sulphur dioxide or by heating sulphide ores-pyrite in an excess air will do it.
2. Converting sulphur-dioxide into sulphur-trioxide: the formation process of the sulphur
trioxide is a revocable as well as exothermic reaction.
3. Converting sulphur-trioxide formed into concentrated Sulfuric-acid: Sulphur-trioxide is
initially liquefied within concentrated-sulfuric acid
H2SO4 (l) + SO3 (g) → H2S2O7 (l)
(Oleum)
The product obtained in above step is fuming-sulfuric acid or oleum, which is then made,
react safely with H2O to produce concentrated- H2SO4.
H2S2O7 (l) + H2O → 2 H2SO4 (l)
(Sulfuric acid)
Characteristics of original reactants involved
There are following raw materials used during contact process of acid formation:
Sulphur: Chalcogen and non-metal (ChemiCool, 2018)
Property Value
Atomic weight: 32.06
Colour: yellow
State: solid
Boiling point: 444.7oC, 717.9 K
Melting point: 115.2oC, 388.4 K
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Chemistry Research 4
Oxygen: Chalcogen gas and non-metal (ChemiCool, 2018)
Property Value
Atomic weight: 15.9994
Colour: Colourless
State: Gas
Boiling point: -182.9oC, 90.2 K
Melting point: -218.3oC, 54.8 K
Water: tasteless, odourless liquid at ambient temperature/pressure
Property Value
Chemical Formula H2O
Colour: Colourless
State: Liquid (Naturally)
Boiling point: 99.98 °C ( 373.13 K)
Melting point: 0.00 °C ( 273.15 K)
Industrial & General utilization of raw products
Sulphur: Uses of Sulphur are as follows
Being an active reactant in the manufacturing of sulfuric-acid (H2SO4)
For automotive use, sulphur is used as an industrialized chemical in large amounts in
lead-acid batteries.
During vulcanization of natural-rubber Sulphur is used
In detergents as well as in the manufacturing of phosphate-fertilizers
Oxygen: Uses of Sulphur are as follows
During steel production to remove Carbon impurities from steel oxygen is used
During oxyacetylene-welding
In Rocket fuel being an oxidant
During methanol and ethylene-oxide production
Animals and plants depend on oxygen for breathing
To help inhalation in patients in respiratory ailments
Oxygen: Chalcogen gas and non-metal (ChemiCool, 2018)
Property Value
Atomic weight: 15.9994
Colour: Colourless
State: Gas
Boiling point: -182.9oC, 90.2 K
Melting point: -218.3oC, 54.8 K
Water: tasteless, odourless liquid at ambient temperature/pressure
Property Value
Chemical Formula H2O
Colour: Colourless
State: Liquid (Naturally)
Boiling point: 99.98 °C ( 373.13 K)
Melting point: 0.00 °C ( 273.15 K)
Industrial & General utilization of raw products
Sulphur: Uses of Sulphur are as follows
Being an active reactant in the manufacturing of sulfuric-acid (H2SO4)
For automotive use, sulphur is used as an industrialized chemical in large amounts in
lead-acid batteries.
During vulcanization of natural-rubber Sulphur is used
In detergents as well as in the manufacturing of phosphate-fertilizers
Oxygen: Uses of Sulphur are as follows
During steel production to remove Carbon impurities from steel oxygen is used
During oxyacetylene-welding
In Rocket fuel being an oxidant
During methanol and ethylene-oxide production
Animals and plants depend on oxygen for breathing
To help inhalation in patients in respiratory ailments
Chemistry Research 5
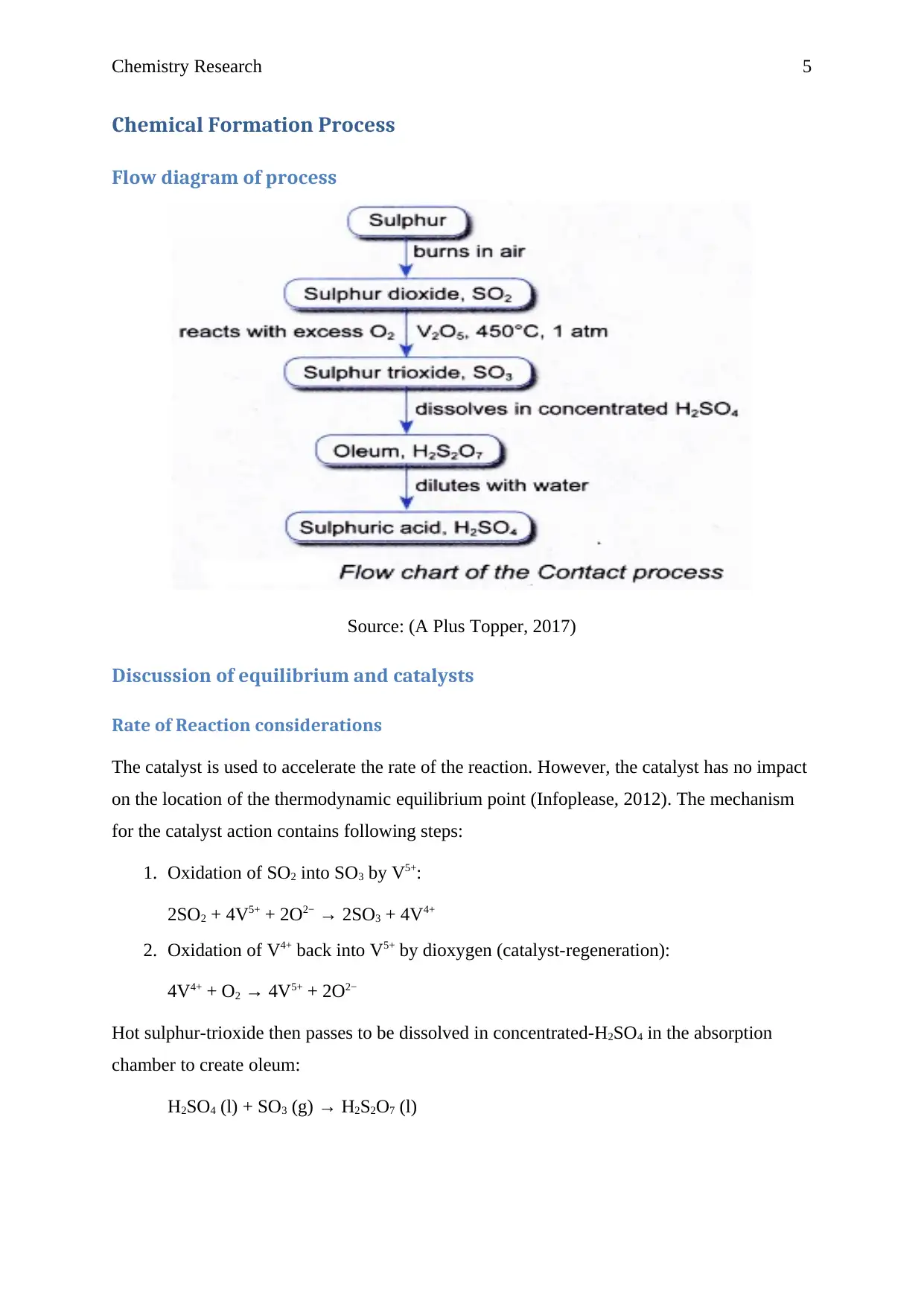
Chemical Formation Process
Flow diagram of process
Source: (A Plus Topper, 2017)
Discussion of equilibrium and catalysts
Rate of Reaction considerations
The catalyst is used to accelerate the rate of the reaction. However, the catalyst has no impact
on the location of the thermodynamic equilibrium point (Infoplease, 2012). The mechanism
for the catalyst action contains following steps:
1. Oxidation of SO2 into SO3 by V5+:
2SO2 + 4V5+ + 2O2− → 2SO3 + 4V4+
2. Oxidation of V4+ back into V5+ by dioxygen (catalyst-regeneration):
4V4+ + O2 → 4V5+ + 2O2−
Hot sulphur-trioxide then passes to be dissolved in concentrated-H2SO4 in the absorption
chamber to create oleum:
H2SO4 (l) + SO3 (g) → H2S2O7 (l)
Chemical Formation Process
Flow diagram of process
Source: (A Plus Topper, 2017)
Discussion of equilibrium and catalysts
Rate of Reaction considerations
The catalyst is used to accelerate the rate of the reaction. However, the catalyst has no impact
on the location of the thermodynamic equilibrium point (Infoplease, 2012). The mechanism
for the catalyst action contains following steps:
1. Oxidation of SO2 into SO3 by V5+:
2SO2 + 4V5+ + 2O2− → 2SO3 + 4V4+
2. Oxidation of V4+ back into V5+ by dioxygen (catalyst-regeneration):
4V4+ + O2 → 4V5+ + 2O2−
Hot sulphur-trioxide then passes to be dissolved in concentrated-H2SO4 in the absorption
chamber to create oleum:
H2SO4 (l) + SO3 (g) → H2S2O7 (l)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Chemistry Research 6
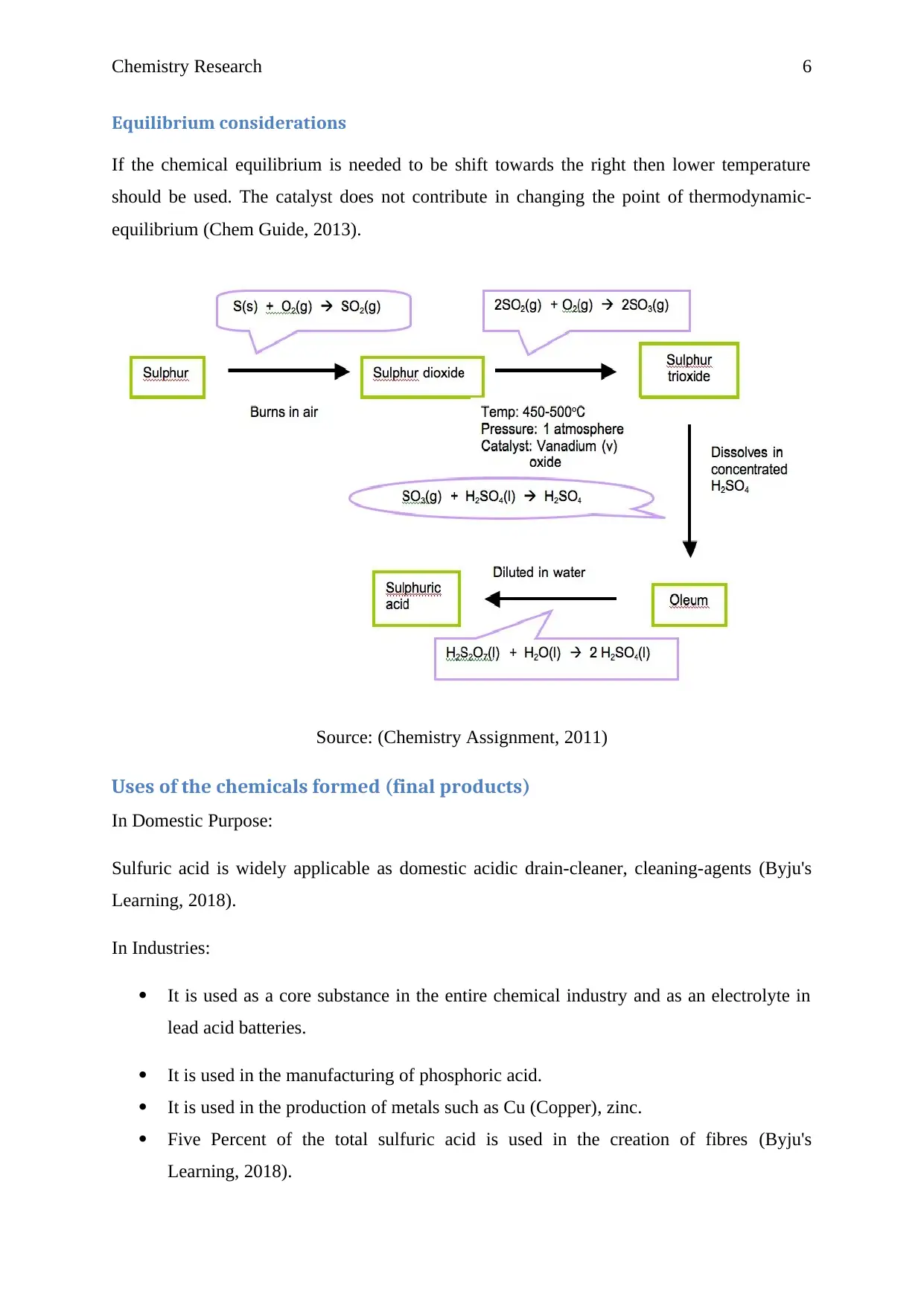
Equilibrium considerations
If the chemical equilibrium is needed to be shift towards the right then lower temperature
should be used. The catalyst does not contribute in changing the point of thermodynamic-
equilibrium (Chem Guide, 2013).
Source: (Chemistry Assignment, 2011)
Uses of the chemicals formed (final products)
In Domestic Purpose:
Sulfuric acid is widely applicable as domestic acidic drain-cleaner, cleaning-agents (Byju's
Learning, 2018).
In Industries:
It is used as a core substance in the entire chemical industry and as an electrolyte in
lead acid batteries.
It is used in the manufacturing of phosphoric acid.
It is used in the production of metals such as Cu (Copper), zinc.
Five Percent of the total sulfuric acid is used in the creation of fibres (Byju's
Learning, 2018).
Equilibrium considerations
If the chemical equilibrium is needed to be shift towards the right then lower temperature
should be used. The catalyst does not contribute in changing the point of thermodynamic-
equilibrium (Chem Guide, 2013).
Source: (Chemistry Assignment, 2011)
Uses of the chemicals formed (final products)
In Domestic Purpose:
Sulfuric acid is widely applicable as domestic acidic drain-cleaner, cleaning-agents (Byju's
Learning, 2018).
In Industries:
It is used as a core substance in the entire chemical industry and as an electrolyte in
lead acid batteries.
It is used in the manufacturing of phosphoric acid.
It is used in the production of metals such as Cu (Copper), zinc.
Five Percent of the total sulfuric acid is used in the creation of fibres (Byju's
Learning, 2018).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Chemistry Research 7
As Catalyst:
During Production of nylon
During the Manheim method in the manufacturing of hydrochloric acid( HCl)
During petroleum refining (Byju's Learning, 2018)
Source: (Byju's Learning, 2018)
Conclusion
The Manufacturing of Sulphuric acid by contact process gives a pure, concentrated acid but
necessitates purer reactants and expensive catalysts. Firstly, sulphur is burned through excess
air to produce sulphur-dioxide. Further, additional air is mixed with sulphur-dioxide to
produce sulphur -trioxide during reversible reaction. A temperature of about 450 °C is to be
set to increase the speed of the reaction. Gas passing from layers of vanadium-oxide catalyst,
will also improve reaction rate.
Lastly, sulphur-trioxide is mixed with 98% solution of sulphuric-acid to create concentrated
form of sulfuric acid (99.5 per cent). In this way, acid of all chosen concentration can be
prepared by adding or diluting the reactants of contact process. Production of this acid also
includes safety precautions specially while handling pure sulphuric acid within a laboratory
As Catalyst:
During Production of nylon
During the Manheim method in the manufacturing of hydrochloric acid( HCl)
During petroleum refining (Byju's Learning, 2018)
Source: (Byju's Learning, 2018)
Conclusion
The Manufacturing of Sulphuric acid by contact process gives a pure, concentrated acid but
necessitates purer reactants and expensive catalysts. Firstly, sulphur is burned through excess
air to produce sulphur-dioxide. Further, additional air is mixed with sulphur-dioxide to
produce sulphur -trioxide during reversible reaction. A temperature of about 450 °C is to be
set to increase the speed of the reaction. Gas passing from layers of vanadium-oxide catalyst,
will also improve reaction rate.
Lastly, sulphur-trioxide is mixed with 98% solution of sulphuric-acid to create concentrated
form of sulfuric acid (99.5 per cent). In this way, acid of all chosen concentration can be
prepared by adding or diluting the reactants of contact process. Production of this acid also
includes safety precautions specially while handling pure sulphuric acid within a laboratory

Chemistry Research 8
or industry, or when utilizing products containing pure sulfuric acid. Protective accessories
like chemical safety glasses, respirator, boots, face shield, long rubber gloves and industrial
apron should be worn during its use. Another considerable fact about this usable acid is that it
reacts violently when encountering H2O. Storage and disposal of this acid is also a concern, it
should be stored within a cool and dry area distant from direct sunlight as well as other heat
sources. In order to reduce accumulation of vapours inside acid large quantity storage should
be avoided and product storage containers should be examined regularly. Therefore, whether
it is pure or diluted Sulphuric acid (H2SO4) being an extraordinary industrial as well as
domestic agent, it requires many precautions throughout the process of production to
utilization.
or industry, or when utilizing products containing pure sulfuric acid. Protective accessories
like chemical safety glasses, respirator, boots, face shield, long rubber gloves and industrial
apron should be worn during its use. Another considerable fact about this usable acid is that it
reacts violently when encountering H2O. Storage and disposal of this acid is also a concern, it
should be stored within a cool and dry area distant from direct sunlight as well as other heat
sources. In order to reduce accumulation of vapours inside acid large quantity storage should
be avoided and product storage containers should be examined regularly. Therefore, whether
it is pure or diluted Sulphuric acid (H2SO4) being an extraordinary industrial as well as
domestic agent, it requires many precautions throughout the process of production to
utilization.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemistry Research 9
References
A Plus Topper (2017) How is Sulphuric Acid Made?, 27 Apr, [Online], Available:
https://www.aplustopper.com/sulfuric-acid-made/ [02 Sep 2018].
Byju's Learning (2018) Preparation of Sulphuric Acid by Contact process, 22 Jul, [Online],
Available: https://byjus.com/chemistry/mass-production-of-sulphuric-acid/ [28 Aug 2018].
Chem Guide (2013) THE CONTACT PROCESS, Apr, [Online], Available:
https://www.chemguide.co.uk/physical/equilibria/contact.html [28 Aug 2018].
ChemiCool (2018) Sulfur Element Facts, [Online], Available:
https://www.chemicool.com/elements/sulfur.html [01 Sep 2018].
Chemistry Assignment (2011) Sulphuric Acid and its Uses, 03 Sep, [Online], Available:
http://my-chem-assignment.blogspot.com/2011/09/sulphuric-acid.html [02 Sep 2018].
Infoplease (2012) sulfuric acid: Production of Sulfuric Acid, [Online], Available:
https://www.infoplease.com/encyclopedia/science-and-technology/chemistry/compounds-
and-elements/sulfuric-acid/production-of-sulfuric-acid [28 Aug 2018].
Patana (2018) The Manufacture of Sulfuric Acid, [Online], Available:
https://www.patana.ac.th/parents/curriculum/Chemistry/units/LR1702.html [02 Sep 2018].
The Essential chemical industry (2016) Sulfuric acid, 09 Oct, [Online], Available:
http://www.essentialchemicalindustry.org/chemicals/sulfuric-acid.html [28 Aug 2018].
World of Chemicals (2018) Manufacturing of sulfuric acid by Contact process, [Online],
Available: https://www.worldofchemicals.com/441/chemistry-articles/manufacturing-of-
sulfuric-acid-by-contact-process.html [28 Aug 2018].
References
A Plus Topper (2017) How is Sulphuric Acid Made?, 27 Apr, [Online], Available:
https://www.aplustopper.com/sulfuric-acid-made/ [02 Sep 2018].
Byju's Learning (2018) Preparation of Sulphuric Acid by Contact process, 22 Jul, [Online],
Available: https://byjus.com/chemistry/mass-production-of-sulphuric-acid/ [28 Aug 2018].
Chem Guide (2013) THE CONTACT PROCESS, Apr, [Online], Available:
https://www.chemguide.co.uk/physical/equilibria/contact.html [28 Aug 2018].
ChemiCool (2018) Sulfur Element Facts, [Online], Available:
https://www.chemicool.com/elements/sulfur.html [01 Sep 2018].
Chemistry Assignment (2011) Sulphuric Acid and its Uses, 03 Sep, [Online], Available:
http://my-chem-assignment.blogspot.com/2011/09/sulphuric-acid.html [02 Sep 2018].
Infoplease (2012) sulfuric acid: Production of Sulfuric Acid, [Online], Available:
https://www.infoplease.com/encyclopedia/science-and-technology/chemistry/compounds-
and-elements/sulfuric-acid/production-of-sulfuric-acid [28 Aug 2018].
Patana (2018) The Manufacture of Sulfuric Acid, [Online], Available:
https://www.patana.ac.th/parents/curriculum/Chemistry/units/LR1702.html [02 Sep 2018].
The Essential chemical industry (2016) Sulfuric acid, 09 Oct, [Online], Available:
http://www.essentialchemicalindustry.org/chemicals/sulfuric-acid.html [28 Aug 2018].
World of Chemicals (2018) Manufacturing of sulfuric acid by Contact process, [Online],
Available: https://www.worldofchemicals.com/441/chemistry-articles/manufacturing-of-
sulfuric-acid-by-contact-process.html [28 Aug 2018].
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
