Assignment 1: Sustainable Energy Fundamentals, Thermodynamics Problems
VerifiedAdded on 2023/01/17
|10
|1294
|65
Homework Assignment
AI Summary
This document provides detailed solutions to a Sustainable Energy Fundamentals assignment, focusing on numerical problems related to thermodynamics and energy principles. The solutions cover a range of topics including the compression of nitrogen in a piston-cylinder device, calculation of heat transfer, and analysis of entropy changes during phase transitions of water. Furthermore, the assignment includes the calculation of Rankine cycle efficiency, determination of ammonia vapor discharge from a rigid tank, and consideration of Carnot cycle. The solutions include step-by-step calculations, explanations of the underlying thermodynamic concepts, and discussions of relevant parameters such as entropy, enthalpy, and specific volume. The assignment also includes relevant tables and references to support the solutions.

Sustainable Energy Fundamentals 1
NUMERICAL PROBLEMS BASED ON THERMODYNAMICS AND ENERGY
FUNDAMENTALS
By Name
Course
Instructor
Institution
Location
Date
NUMERICAL PROBLEMS BASED ON THERMODYNAMICS AND ENERGY
FUNDAMENTALS
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sustainable Energy Fundamentals 2
1- A piston cylinder device contains nitrogen with initial volume, temperature, and pressure of
0.1 m3, 25 °C, and 150 kPa, respectively. The nitrogen is then compressed up to 1 MPa when its
temperature reaches 150 °C. If the compression energy (in the form of work) added to the system
is 20 kJ, calculate the amount of heat transfer during the process and comment on its direction
(find nitrogen specific heats attached if required.
Initial volume, V1 = 0.1m3
Temperature, T1 = 25oC = 198K
Pressure, P1 = 150kPa
After nitrogen compression
Pressure, P2 = 1MPa = 106 Pa
Temperature, T2 = 150oC = 423K
Compression energy = 20kJ
Amount of heat transfer = ?
By assuming an ideal behaviou;
P1 V 1
T 1
= P2 V 2
T 2
150 x 103 x 0.1
290 =106 x V 2
423
V2 = 0.02129 m3
Given that Cp = 29.12 + 0.0027(T - 300)
Cv = R – Cp
= 8.314 – 29.12 – 0.0027(T - 300)
= -20.806 – 0.0027(T - 300) J/molK
μ = P1 V 1
RT1
=¿ 150 x 103
298 x 8.314 = 6.05431 moles
Therefore, ∫ dU=∫
T1
T2
μCvdr
dU = 6.0543 ∫
T 1
T 2
{−20.806−0.0027 T +0.81 }dr
dU= 6.0543{-19.996(423-298)-0.0027(4232-2982)/2}
dU= -15.8693 kJ
Since, δw = -20kJ
1- A piston cylinder device contains nitrogen with initial volume, temperature, and pressure of
0.1 m3, 25 °C, and 150 kPa, respectively. The nitrogen is then compressed up to 1 MPa when its
temperature reaches 150 °C. If the compression energy (in the form of work) added to the system
is 20 kJ, calculate the amount of heat transfer during the process and comment on its direction
(find nitrogen specific heats attached if required.
Initial volume, V1 = 0.1m3
Temperature, T1 = 25oC = 198K
Pressure, P1 = 150kPa
After nitrogen compression
Pressure, P2 = 1MPa = 106 Pa
Temperature, T2 = 150oC = 423K
Compression energy = 20kJ
Amount of heat transfer = ?
By assuming an ideal behaviou;
P1 V 1
T 1
= P2 V 2
T 2
150 x 103 x 0.1
290 =106 x V 2
423
V2 = 0.02129 m3
Given that Cp = 29.12 + 0.0027(T - 300)
Cv = R – Cp
= 8.314 – 29.12 – 0.0027(T - 300)
= -20.806 – 0.0027(T - 300) J/molK
μ = P1 V 1
RT1
=¿ 150 x 103
298 x 8.314 = 6.05431 moles
Therefore, ∫ dU=∫
T1
T2
μCvdr
dU = 6.0543 ∫
T 1
T 2
{−20.806−0.0027 T +0.81 }dr
dU= 6.0543{-19.996(423-298)-0.0027(4232-2982)/2}
dU= -15.8693 kJ
Since, δw = -20kJ

Sustainable Energy Fundamentals 3
Hence, δQ = δw + dU
= -15.8693 – 20
= -35.8693 kJ
Direction of Heat Transfer
Therefore, the heat is lost during the process
Hence, δQ = δw + dU
= -15.8693 – 20
= -35.8693 kJ
Direction of Heat Transfer
Therefore, the heat is lost during the process
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Sustainable Energy Fundamentals 4
2- 1 kg of saturated water vapour at 100 °C in a system becomes saturated liquid water by losing
heat to its surrounding ambient (at 25 °C), while the pressure of the system remains constant.
Answer the following questions by considering that the surrounding ambient receives this heat
reversibly (the process within the system may not be reversible):
a) Calculate the total entropy change of the system and the surrounding ambient. (Mark 2.5)
b) Considering the answer to part (A) and discuss if this process is possible or not
(Mark: 1)
c) By running a Carnot Cycle between the system (at 100 °C) and ambient (at 25
°C), how much heat is rejected to the ambient surrounding the system?
(Mark: 2.5)
a) Given that;
M = 1kg
Tw = 100oC
To = 25oC
According to the saturated steam table at T = 100oC
Enthalpy of saturated water, hf = 419.17 kJ/kg
Enthalpy of saturated vapor, hg = 2675 kJ/kg
Q = m(hg - hf)
ΔQ= -1(2675.6 – 419.17)
= -2256.43 kJ
Entropy change of water, dSw = ∆ Q
T w
=−2256.43
100+273
dSw = -6.04 kJ/K
As surrounding ambient receives this heat reversibly, hence dSs = 0
Total entropy change, dSu = dSw + dSs
= -6.04 + 0
= -6.04 kJ/K
2- 1 kg of saturated water vapour at 100 °C in a system becomes saturated liquid water by losing
heat to its surrounding ambient (at 25 °C), while the pressure of the system remains constant.
Answer the following questions by considering that the surrounding ambient receives this heat
reversibly (the process within the system may not be reversible):
a) Calculate the total entropy change of the system and the surrounding ambient. (Mark 2.5)
b) Considering the answer to part (A) and discuss if this process is possible or not
(Mark: 1)
c) By running a Carnot Cycle between the system (at 100 °C) and ambient (at 25
°C), how much heat is rejected to the ambient surrounding the system?
(Mark: 2.5)
a) Given that;
M = 1kg
Tw = 100oC
To = 25oC
According to the saturated steam table at T = 100oC
Enthalpy of saturated water, hf = 419.17 kJ/kg
Enthalpy of saturated vapor, hg = 2675 kJ/kg
Q = m(hg - hf)
ΔQ= -1(2675.6 – 419.17)
= -2256.43 kJ
Entropy change of water, dSw = ∆ Q
T w
=−2256.43
100+273
dSw = -6.04 kJ/K
As surrounding ambient receives this heat reversibly, hence dSs = 0
Total entropy change, dSu = dSw + dSs
= -6.04 + 0
= -6.04 kJ/K
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sustainable Energy Fundamentals 5
b) Not possible, this process is not possible since the total change in entropy cannot be
negative fo universe
c) By considering Carnot engine efficiency,
η= 1 - T L
T H
= 1 - 25+273
100+273
= 1 - 298
373
η= 0.202
Since, η = 1 - ∆ Qr
∆ Q
= 1 – 0.202
∆ Qr =0.798 ∆ Q
Rejected heat, ∆ Qr =0.798 x 2256.43
∆ Qr =1800.6311 kJ
Therefore, the rejected heat is 1800.6311 kJ
b) Not possible, this process is not possible since the total change in entropy cannot be
negative fo universe
c) By considering Carnot engine efficiency,
η= 1 - T L
T H
= 1 - 25+273
100+273
= 1 - 298
373
η= 0.202
Since, η = 1 - ∆ Qr
∆ Q
= 1 – 0.202
∆ Qr =0.798 ∆ Q
Rejected heat, ∆ Qr =0.798 x 2256.43
∆ Qr =1800.6311 kJ
Therefore, the rejected heat is 1800.6311 kJ
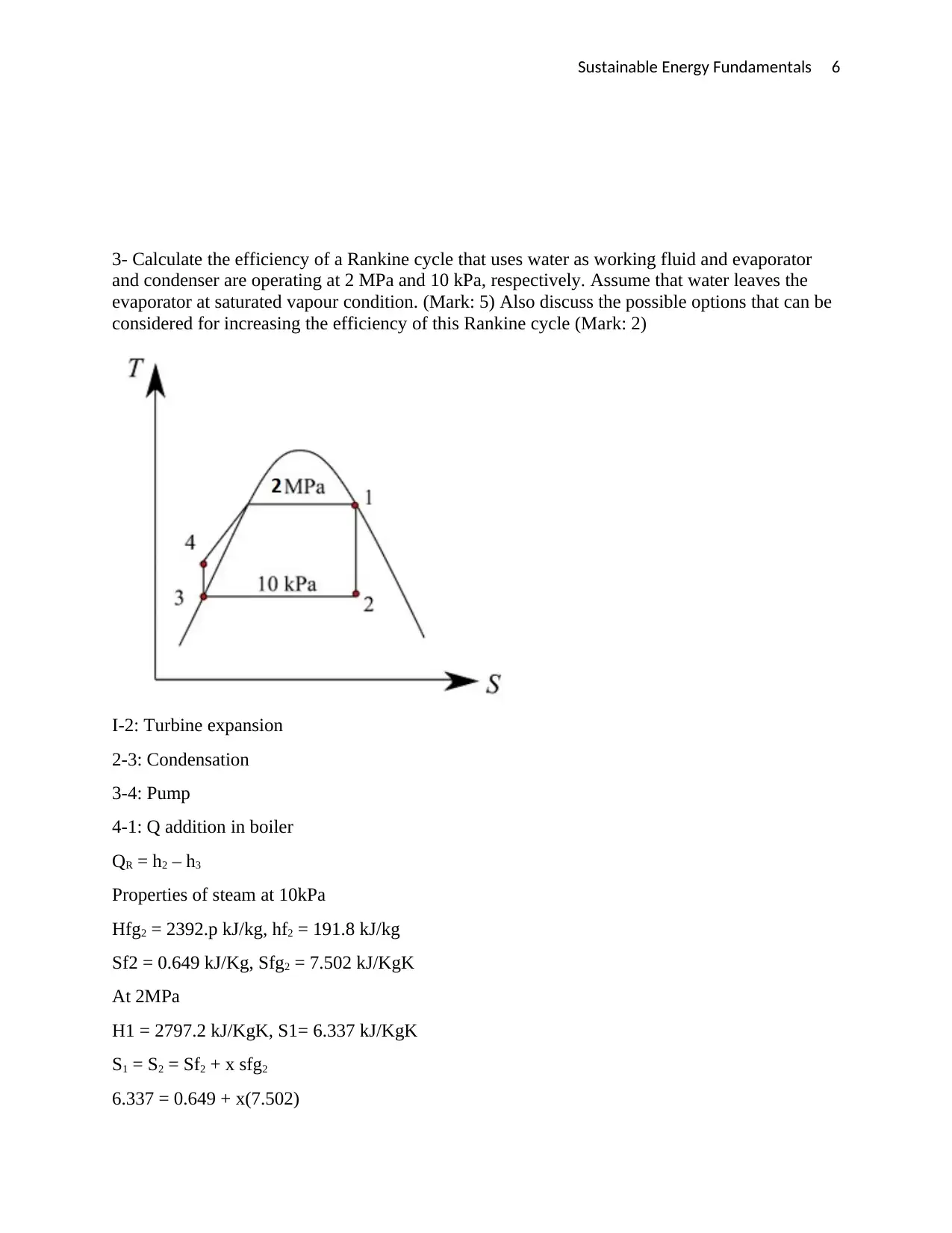
Sustainable Energy Fundamentals 6
3- Calculate the efficiency of a Rankine cycle that uses water as working fluid and evaporator
and condenser are operating at 2 MPa and 10 kPa, respectively. Assume that water leaves the
evaporator at saturated vapour condition. (Mark: 5) Also discuss the possible options that can be
considered for increasing the efficiency of this Rankine cycle (Mark: 2)
I-2: Turbine expansion
2-3: Condensation
3-4: Pump
4-1: Q addition in boiler
QR = h2 – h3
Properties of steam at 10kPa
Hfg2 = 2392.p kJ/kg, hf2 = 191.8 kJ/kg
Sf2 = 0.649 kJ/Kg, Sfg2 = 7.502 kJ/KgK
At 2MPa
H1 = 2797.2 kJ/KgK, S1= 6.337 kJ/KgK
S1 = S2 = Sf2 + x sfg2
6.337 = 0.649 + x(7.502)
3- Calculate the efficiency of a Rankine cycle that uses water as working fluid and evaporator
and condenser are operating at 2 MPa and 10 kPa, respectively. Assume that water leaves the
evaporator at saturated vapour condition. (Mark: 5) Also discuss the possible options that can be
considered for increasing the efficiency of this Rankine cycle (Mark: 2)
I-2: Turbine expansion
2-3: Condensation
3-4: Pump
4-1: Q addition in boiler
QR = h2 – h3
Properties of steam at 10kPa
Hfg2 = 2392.p kJ/kg, hf2 = 191.8 kJ/kg
Sf2 = 0.649 kJ/Kg, Sfg2 = 7.502 kJ/KgK
At 2MPa
H1 = 2797.2 kJ/KgK, S1= 6.337 kJ/KgK
S1 = S2 = Sf2 + x sfg2
6.337 = 0.649 + x(7.502)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Sustainable Energy Fundamentals 7
x = 0.758
H2 = hf2 + x hfg2
= 191.8 + 0.758(2392.9)
H2 = 2006.1 kJ/kg
Hf2 = h3 = 191.8 kJ/Kg
QR = 2006.1 – 191.8 = 1814.3 kJ/kg
η Rankine = W Net
Qadded
=W T−W p
Qadded
= (h¿ ¿1−h2 )−( h4 −h3)
h1−h4
¿
Pump work; h4 – h3 = Vf3 (P4 – P3)
h4 – 191.8 = 0.001(2 x 103 - 10)
h4 = 193.79 kJ/Kg
ηR = ( 2797.2−2006.1 ) −(193.79−191.8)
2797.2−193.79
ηR = 0.30 = 30%
x = 0.758
H2 = hf2 + x hfg2
= 191.8 + 0.758(2392.9)
H2 = 2006.1 kJ/kg
Hf2 = h3 = 191.8 kJ/Kg
QR = 2006.1 – 191.8 = 1814.3 kJ/kg
η Rankine = W Net
Qadded
=W T−W p
Qadded
= (h¿ ¿1−h2 )−( h4 −h3)
h1−h4
¿
Pump work; h4 – h3 = Vf3 (P4 – P3)
h4 – 191.8 = 0.001(2 x 103 - 10)
h4 = 193.79 kJ/Kg
ηR = ( 2797.2−2006.1 ) −(193.79−191.8)
2797.2−193.79
ηR = 0.30 = 30%
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sustainable Energy Fundamentals 8
4- A well-insulated 2-m3 rigid tank contains saturated ammonia at 40 °C. Initially the tank
contains 50% (volume based) of this ammonia in liquid form and the rest (50%) in vapour form.
The valve (on the tank) is opened, discharging ammonia vapour (not liquid) until the temperature
reaches 10 ̊ C. How much is the mass of discharged ammonia vapour? (Mark: 8) (see ammonia’s
properties attached if required).
Volume of tank = 2m3
Volume of liquid = 50%, Hence volume of liquid = 2 x 0.5 = 1m3 and Volume of vapor = 1m3
Specific volume at saturated conditions of 40oC (from steam tables)
Liquid = 0.001735m3/kg and gas= 0.0788m3/kg
Density, liquid = 1/0.001735 = 576.369kg/m3 and gas= 1/0.0788 = 12.69 kg/m3
Masses: liquid = 576.369 x 1 = 576.369 kg and gas= 12.69 x 1 = 12.69 kg
Total mass of the contents in the tank = 576.369 + 12.69 = 589.06 kg
4- A well-insulated 2-m3 rigid tank contains saturated ammonia at 40 °C. Initially the tank
contains 50% (volume based) of this ammonia in liquid form and the rest (50%) in vapour form.
The valve (on the tank) is opened, discharging ammonia vapour (not liquid) until the temperature
reaches 10 ̊ C. How much is the mass of discharged ammonia vapour? (Mark: 8) (see ammonia’s
properties attached if required).
Volume of tank = 2m3
Volume of liquid = 50%, Hence volume of liquid = 2 x 0.5 = 1m3 and Volume of vapor = 1m3
Specific volume at saturated conditions of 40oC (from steam tables)
Liquid = 0.001735m3/kg and gas= 0.0788m3/kg
Density, liquid = 1/0.001735 = 576.369kg/m3 and gas= 1/0.0788 = 12.69 kg/m3
Masses: liquid = 576.369 x 1 = 576.369 kg and gas= 12.69 x 1 = 12.69 kg
Total mass of the contents in the tank = 576.369 + 12.69 = 589.06 kg

Sustainable Energy Fundamentals 9
BIBLIOGRAPHY
Al-Nimr, A., 2010. Non-equilibrium thermodynamics of heterogeneous systems. s.l.:Energy.
Bourouga, B. & Ding, J., 2013. Transient boiling heat transfer performances of subcooled water during
quenching process. s.l.:International Communications in Heat and Mass Transfer. Vol 48. pp. 15-21.
Dariusz, M., Jan, W. & Żmuda, E., 2013. Organic Rankine Cycle as Bottoming Cycle to a Combined
Brayton and Clausius - Rankine Cycle. s.l.:Key Engineering Materials. Vol 597. pp. 87-98.
Gaggioli, R., 2010. Teaching elementary thermodynamics and energy conversion: Opinions. s.l.:Energy.
Vol 35. pp. 1047-1056.
Siviter, J., Montecucco, A. & Knox, R., 2015. Rankine cycle efficiency gain using thermoelectric heat
pumps. s.l.:Applied Energy. Vol 140. pp. 161-170.
Wei, W. & Fang, C., 2011. Efficiency Analysis on Low Temperature Energy Conversion System Based on
Organic Rankine Cycle. s.l.:Advanced Materials Research. Vol 347. pp. 498-503.
BIBLIOGRAPHY
Al-Nimr, A., 2010. Non-equilibrium thermodynamics of heterogeneous systems. s.l.:Energy.
Bourouga, B. & Ding, J., 2013. Transient boiling heat transfer performances of subcooled water during
quenching process. s.l.:International Communications in Heat and Mass Transfer. Vol 48. pp. 15-21.
Dariusz, M., Jan, W. & Żmuda, E., 2013. Organic Rankine Cycle as Bottoming Cycle to a Combined
Brayton and Clausius - Rankine Cycle. s.l.:Key Engineering Materials. Vol 597. pp. 87-98.
Gaggioli, R., 2010. Teaching elementary thermodynamics and energy conversion: Opinions. s.l.:Energy.
Vol 35. pp. 1047-1056.
Siviter, J., Montecucco, A. & Knox, R., 2015. Rankine cycle efficiency gain using thermoelectric heat
pumps. s.l.:Applied Energy. Vol 140. pp. 161-170.
Wei, W. & Fang, C., 2011. Efficiency Analysis on Low Temperature Energy Conversion System Based on
Organic Rankine Cycle. s.l.:Advanced Materials Research. Vol 347. pp. 498-503.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Sustainable Energy Fundamentals 10
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
