Ammonia Synthesis from Nitrogen and Hydrogen in Chemical Engineering Contents
VerifiedAdded on 2020/04/15
|11
|1192
|417
AI Summary
Solution 1 As given in question NH3 = 20 mole%, N2 = 18 mole%, H2 = 54 mole%, Inert = 8 mole% Solution 1a) The given Pressure P = 1 ATM, recovery of ammonia = 0.9 = We know that, PNH3 = yNH3P = 0.2 x 1 = 0.2 atm Since, PNH3 = yNH3P = 0.2 x 1 = 0.2 atm Since partial pressure for ammonia is given = 0.2
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.
Problem Solution
1 | P a g e
Chemical Engineering
1 | P a g e
Chemical Engineering
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Problem Solution
Contents
Solution 1.........................................................................................................................................3
Solution 2.........................................................................................................................................3
Solution 3.........................................................................................................................................5
Solution 4.........................................................................................................................................7
Solution 4a)..................................................................................................................................7
Solution 4b).................................................................................................................................7
Solution 4c)..................................................................................................................................8
Solution 4d).................................................................................................................................9
2 | P a g e
Contents
Solution 1.........................................................................................................................................3
Solution 2.........................................................................................................................................3
Solution 3.........................................................................................................................................5
Solution 4.........................................................................................................................................7
Solution 4a)..................................................................................................................................7
Solution 4b).................................................................................................................................7
Solution 4c)..................................................................................................................................8
Solution 4d).................................................................................................................................9
2 | P a g e

Problem Solution
Solution 1
In the synthesis of ammonia from nitrogen and hydrogen, the product gas leaving the ammonia
synthesis is fed to a condenser where essentially pure ammonia is condensed to liquid
Vapour Condenser liquid pure NH3
Based on the feed composition given on the flow diagram, answer the following questions
a) If condenser operates at 1 atm. Pressure (absolute) at what temperature the condenser
must be operated to recover 90% of the ammonia in feed as liquid?
b) If condenser operates at -60o C what would be the pressure to recover 90% of the
ammonia in feed as liquid?
c) If the feed stream is at 1 atm. Pressure what is the dew point temperature of the feed
stream?
Solution 1
As given in question
NH3 = 20 mole%, N2 = 18 mole%, H2 = 54 mole%, Inert = 8 mole%
Solution 1a)
The given Pressure P = 1 ATM, recovery of ammonia = 0.9 =
We know that,
PNH3 = yNH3P = 0.2 x 1 = 0.2 atm
Since, PNH3 = yNH3P = 0.2 x 1 = 0.2 atm
Since partial pressure for ammonia is given = 0.2 atm,
Given for 90% recovery = 0.2*0.9 = 0.18 atm
Now I must use
Antoine equation is derived as log10
p sat
= A− B
T +C
From table of Antoine equation constant
A = 7.55466, B = 1002.711, C = 247.885
Putting this value in above equation
3 | P a g e
Solution 1
In the synthesis of ammonia from nitrogen and hydrogen, the product gas leaving the ammonia
synthesis is fed to a condenser where essentially pure ammonia is condensed to liquid
Vapour Condenser liquid pure NH3
Based on the feed composition given on the flow diagram, answer the following questions
a) If condenser operates at 1 atm. Pressure (absolute) at what temperature the condenser
must be operated to recover 90% of the ammonia in feed as liquid?
b) If condenser operates at -60o C what would be the pressure to recover 90% of the
ammonia in feed as liquid?
c) If the feed stream is at 1 atm. Pressure what is the dew point temperature of the feed
stream?
Solution 1
As given in question
NH3 = 20 mole%, N2 = 18 mole%, H2 = 54 mole%, Inert = 8 mole%
Solution 1a)
The given Pressure P = 1 ATM, recovery of ammonia = 0.9 =
We know that,
PNH3 = yNH3P = 0.2 x 1 = 0.2 atm
Since, PNH3 = yNH3P = 0.2 x 1 = 0.2 atm
Since partial pressure for ammonia is given = 0.2 atm,
Given for 90% recovery = 0.2*0.9 = 0.18 atm
Now I must use
Antoine equation is derived as log10
p sat
= A− B
T +C
From table of Antoine equation constant
A = 7.55466, B = 1002.711, C = 247.885
Putting this value in above equation
3 | P a g e
Problem Solution
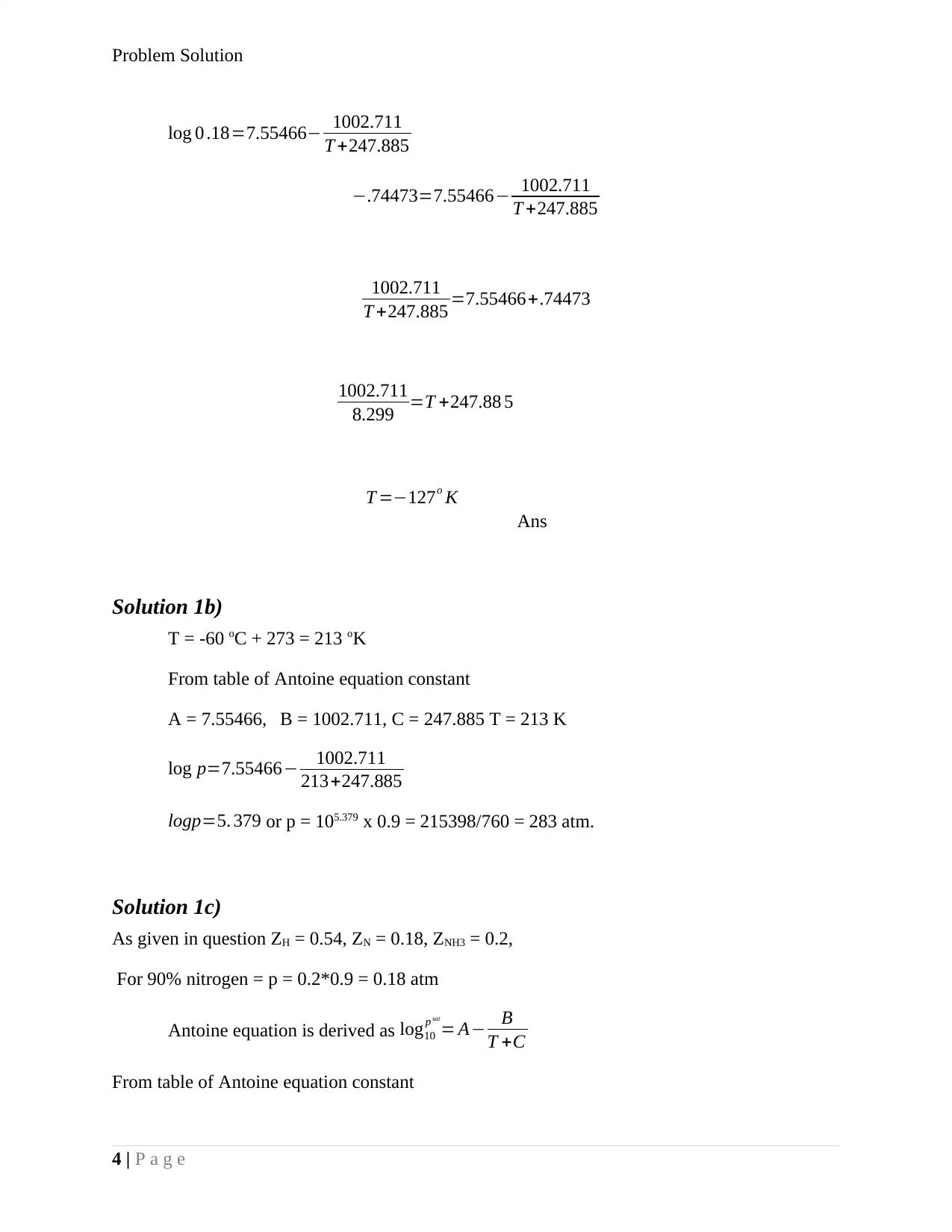
log 0 .18=7.55466− 1002.711
T +247.885
−.74473=7.55466− 1002.711
T +247.885
1002.711
T +247.885 =7.55466+.74473
1002.711
8.299 =T +247.88 5
T =−127o K
Ans
Solution 1b)
T = -60 oC + 273 = 213 oK
From table of Antoine equation constant
A = 7.55466, B = 1002.711, C = 247.885 T = 213 K
log p=7.55466− 1002.711
213+247.885
logp=5. 379 or p = 105.379 x 0.9 = 215398/760 = 283 atm.
Solution 1c)
As given in question ZH = 0.54, ZN = 0.18, ZNH3 = 0.2,
For 90% nitrogen = p = 0.2*0.9 = 0.18 atm
Antoine equation is derived as log10
p sat
= A− B
T +C
From table of Antoine equation constant
4 | P a g e
log 0 .18=7.55466− 1002.711
T +247.885
−.74473=7.55466− 1002.711
T +247.885
1002.711
T +247.885 =7.55466+.74473
1002.711
8.299 =T +247.88 5
T =−127o K
Ans
Solution 1b)
T = -60 oC + 273 = 213 oK
From table of Antoine equation constant
A = 7.55466, B = 1002.711, C = 247.885 T = 213 K
log p=7.55466− 1002.711
213+247.885
logp=5. 379 or p = 105.379 x 0.9 = 215398/760 = 283 atm.
Solution 1c)
As given in question ZH = 0.54, ZN = 0.18, ZNH3 = 0.2,
For 90% nitrogen = p = 0.2*0.9 = 0.18 atm
Antoine equation is derived as log10
p sat
= A− B
T +C
From table of Antoine equation constant
4 | P a g e
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Problem Solution
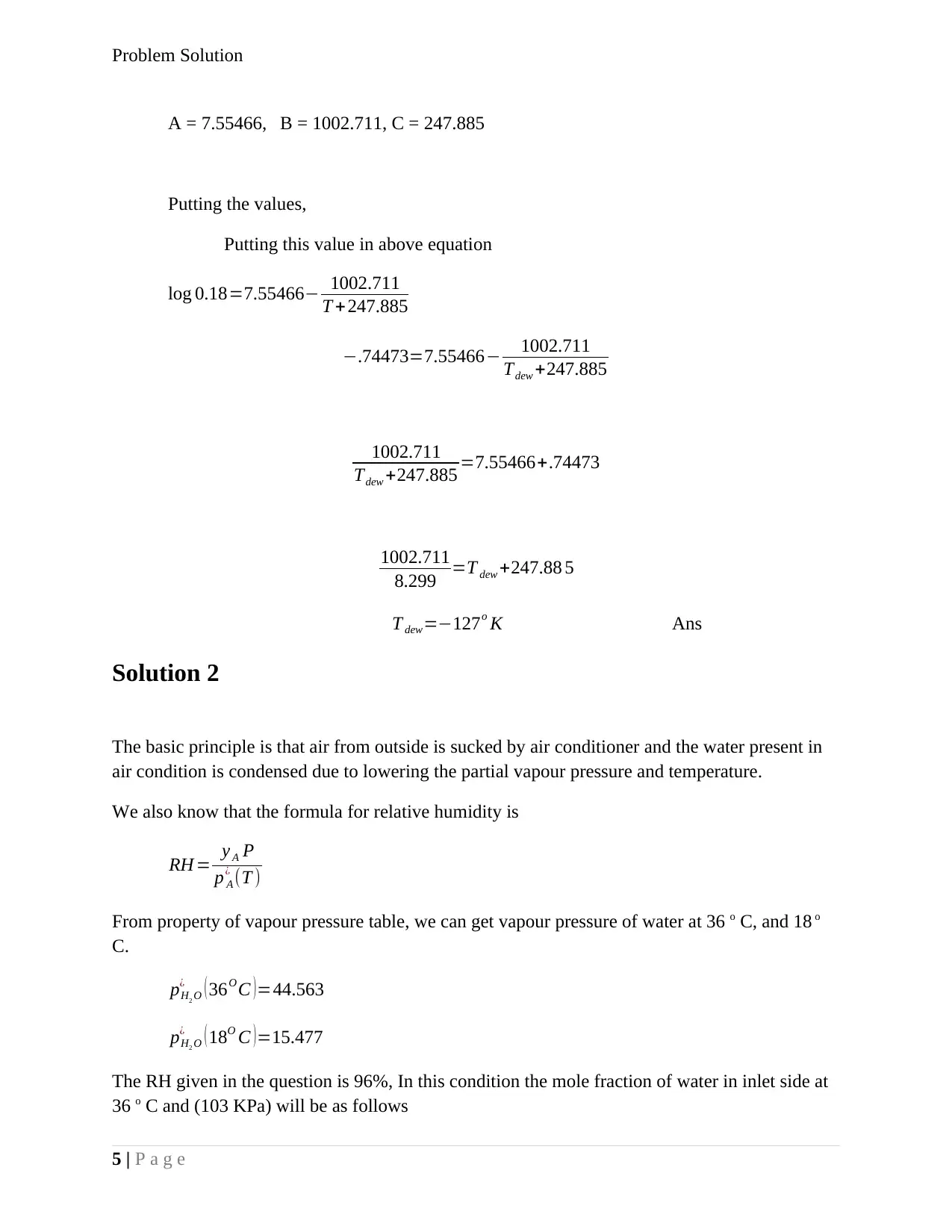
A = 7.55466, B = 1002.711, C = 247.885
Putting the values,
Putting this value in above equation
log 0.18=7.55466− 1002.711
T + 247.885
−.74473=7.55466− 1002.711
Tdew +247.885
1002.711
Tdew +247.885 =7.55466+.74473
1002.711
8.299 =T dew +247.88 5
T dew=−127o K Ans
Solution 2
The basic principle is that air from outside is sucked by air conditioner and the water present in
air condition is condensed due to lowering the partial vapour pressure and temperature.
We also know that the formula for relative humidity is
RH = y A P
p A
¿ (T )
From property of vapour pressure table, we can get vapour pressure of water at 36 o C, and 18 o
C.
pH2 O
¿ ( 36O C )=44.563
pH2 O
¿ ( 18O C )=15.477
The RH given in the question is 96%, In this condition the mole fraction of water in inlet side at
36 o C and (103 KPa) will be as follows
5 | P a g e
A = 7.55466, B = 1002.711, C = 247.885
Putting the values,
Putting this value in above equation
log 0.18=7.55466− 1002.711
T + 247.885
−.74473=7.55466− 1002.711
Tdew +247.885
1002.711
Tdew +247.885 =7.55466+.74473
1002.711
8.299 =T dew +247.88 5
T dew=−127o K Ans
Solution 2
The basic principle is that air from outside is sucked by air conditioner and the water present in
air condition is condensed due to lowering the partial vapour pressure and temperature.
We also know that the formula for relative humidity is
RH = y A P
p A
¿ (T )
From property of vapour pressure table, we can get vapour pressure of water at 36 o C, and 18 o
C.
pH2 O
¿ ( 36O C )=44.563
pH2 O
¿ ( 18O C )=15.477
The RH given in the question is 96%, In this condition the mole fraction of water in inlet side at
36 o C and (103 KPa) will be as follows
5 | P a g e

Problem Solution
First, we must convert the inflow into mole / h
y H2 O =RH pH 2 O
¿ ( 36O C )
P
y H2 O =0.96 44.563
103 x 101.325 kPa
760 mm Hg =0.0554 mol H2 O
mol air
Similarly, mole fraction of water in outlet side (18 o C and 103 kPa)
y H2 O =15.477
103 x 101.325 kPa
760 mm Hg =0.0200 mol H2 O
mol air
Now according to ideal gas equation
n= PV
RT
Putting the value in above formula,
¿
103 KPax 10000 l
h x 1 atm
101.325 KPa
0.08206 L . atm/ ( mol . K ) x (273.2+18)=425.002 mol/ h
Balancing the equation,
Nx(inlet side) x y(inlet side) = N(outlet side) x (y outlet side)
Nx(inlet side) x (1 – yH2O) inlet side = Nx(Outlet side) x (1 – yH2O) outlet side.
N(1-0.0554 ) = 425.002 x (1- 0.020)
N (inlet side) = 425.002 x 0.98/0.9446 = 440.93 mole /h
Similarly, for volumetric flow, putting ideal gas law equation again
V = nRT
P = 440.93 x 0.08206 x(273.2+ 36)
103
101.325
=11005.51 l/hr
The volumetric flow rate of AC unit is 11005.51 l/hr
6 | P a g e
First, we must convert the inflow into mole / h
y H2 O =RH pH 2 O
¿ ( 36O C )
P
y H2 O =0.96 44.563
103 x 101.325 kPa
760 mm Hg =0.0554 mol H2 O
mol air
Similarly, mole fraction of water in outlet side (18 o C and 103 kPa)
y H2 O =15.477
103 x 101.325 kPa
760 mm Hg =0.0200 mol H2 O
mol air
Now according to ideal gas equation
n= PV
RT
Putting the value in above formula,
¿
103 KPax 10000 l
h x 1 atm
101.325 KPa
0.08206 L . atm/ ( mol . K ) x (273.2+18)=425.002 mol/ h
Balancing the equation,
Nx(inlet side) x y(inlet side) = N(outlet side) x (y outlet side)
Nx(inlet side) x (1 – yH2O) inlet side = Nx(Outlet side) x (1 – yH2O) outlet side.
N(1-0.0554 ) = 425.002 x (1- 0.020)
N (inlet side) = 425.002 x 0.98/0.9446 = 440.93 mole /h
Similarly, for volumetric flow, putting ideal gas law equation again
V = nRT
P = 440.93 x 0.08206 x(273.2+ 36)
103
101.325
=11005.51 l/hr
The volumetric flow rate of AC unit is 11005.51 l/hr
6 | P a g e
Problem Solution
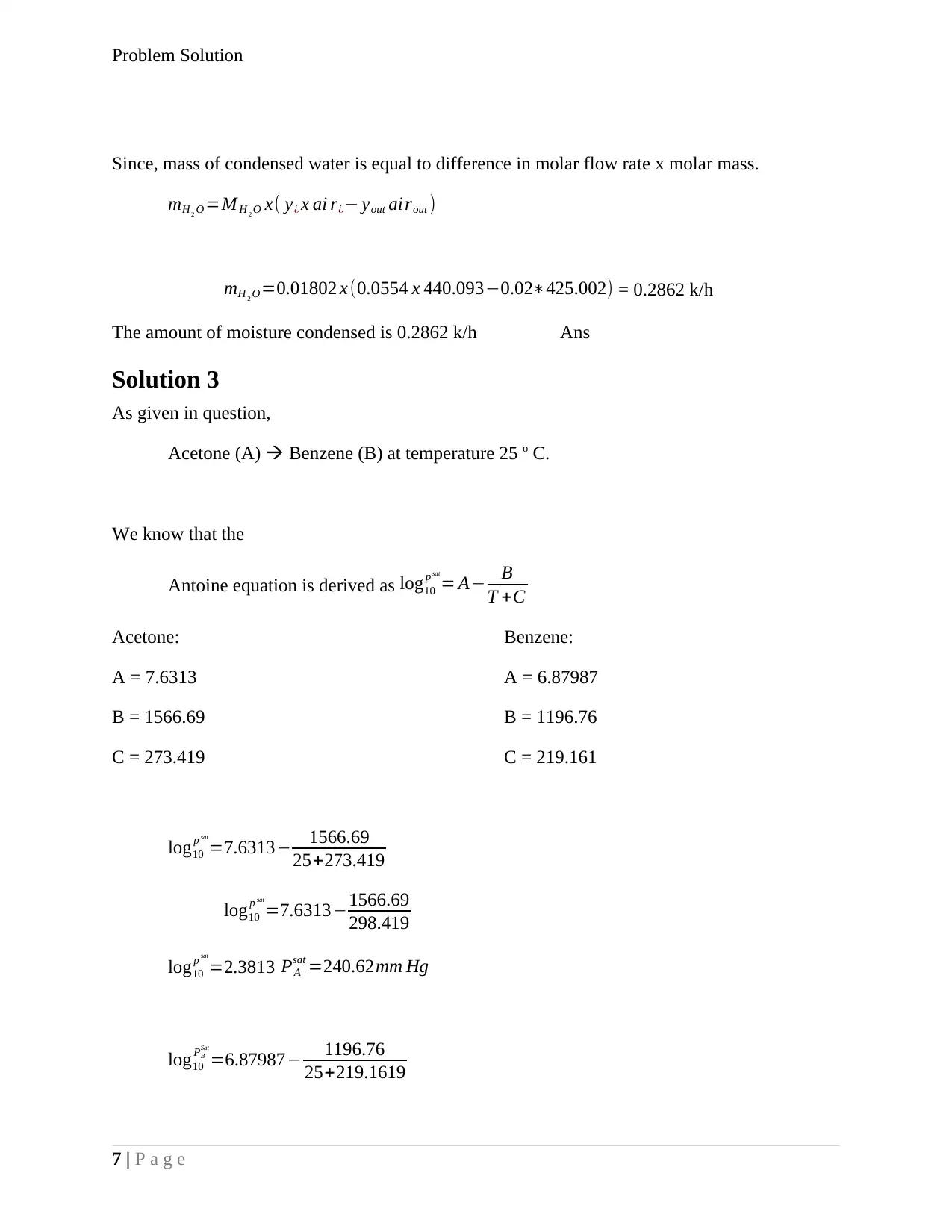
Since, mass of condensed water is equal to difference in molar flow rate x molar mass.
mH 2 O =M H 2 O x( y¿ x ai r¿− yout airout )
mH 2 O =0.01802 x (0.0554 x 440.093−0.02∗425.002) = 0.2862 k/h
The amount of moisture condensed is 0.2862 k/h Ans
Solution 3
As given in question,
Acetone (A) Benzene (B) at temperature 25 o C.
We know that the
Antoine equation is derived as log10
p sat
= A− B
T +C
Acetone: Benzene:
A = 7.6313 A = 6.87987
B = 1566.69 B = 1196.76
C = 273.419 C = 219.161
log10
p sat
=7.6313− 1566.69
25+273.419
log10
p sat
=7.6313−1566.69
298.419
log10
p sat
=2.3813 PA
sat =240.62mm Hg
log10
PB
Sat
=6.87987− 1196.76
25+219.1619
7 | P a g e
Since, mass of condensed water is equal to difference in molar flow rate x molar mass.
mH 2 O =M H 2 O x( y¿ x ai r¿− yout airout )
mH 2 O =0.01802 x (0.0554 x 440.093−0.02∗425.002) = 0.2862 k/h
The amount of moisture condensed is 0.2862 k/h Ans
Solution 3
As given in question,
Acetone (A) Benzene (B) at temperature 25 o C.
We know that the
Antoine equation is derived as log10
p sat
= A− B
T +C
Acetone: Benzene:
A = 7.6313 A = 6.87987
B = 1566.69 B = 1196.76
C = 273.419 C = 219.161
log10
p sat
=7.6313− 1566.69
25+273.419
log10
p sat
=7.6313−1566.69
298.419
log10
p sat
=2.3813 PA
sat =240.62mm Hg
log10
PB
Sat
=6.87987− 1196.76
25+219.1619
7 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Problem Solution
log10
PB
Sat
=6.87987− 1196.76
244.161
log10
PB
Sat
=1.9783
PB
Sat =95.137 mm Hg
Now I must calculate at XA = 0.4
P= X A PA
Sat + X B PB
Sat
P=0.4 x 240.62+0.6 x 95.137
P=96.248+57.0822
P = 153.3302 mm Hg
PYa = XaPASat
YA = 0.4 x 240.62 / 153.33 = 0.6277
P (mm
Hg) x(A) 1-x(A)
109.685
3 0.1
0.219373
1
124.233
6 0.2 0.387367
138.781
9 0.3
0.520139
9
153.330
2 0.4
0.627717
2
167.878
5 0.5
0.716649
2
182.426
8 0.6
0.791396
9
196.975 0.7 0.855103
8 | P a g e
log10
PB
Sat
=6.87987− 1196.76
244.161
log10
PB
Sat
=1.9783
PB
Sat =95.137 mm Hg
Now I must calculate at XA = 0.4
P= X A PA
Sat + X B PB
Sat
P=0.4 x 240.62+0.6 x 95.137
P=96.248+57.0822
P = 153.3302 mm Hg
PYa = XaPASat
YA = 0.4 x 240.62 / 153.33 = 0.6277
P (mm
Hg) x(A) 1-x(A)
109.685
3 0.1
0.219373
1
124.233
6 0.2 0.387367
138.781
9 0.3
0.520139
9
153.330
2 0.4
0.627717
2
167.878
5 0.5
0.716649
2
182.426
8 0.6
0.791396
9
196.975 0.7 0.855103
8 | P a g e
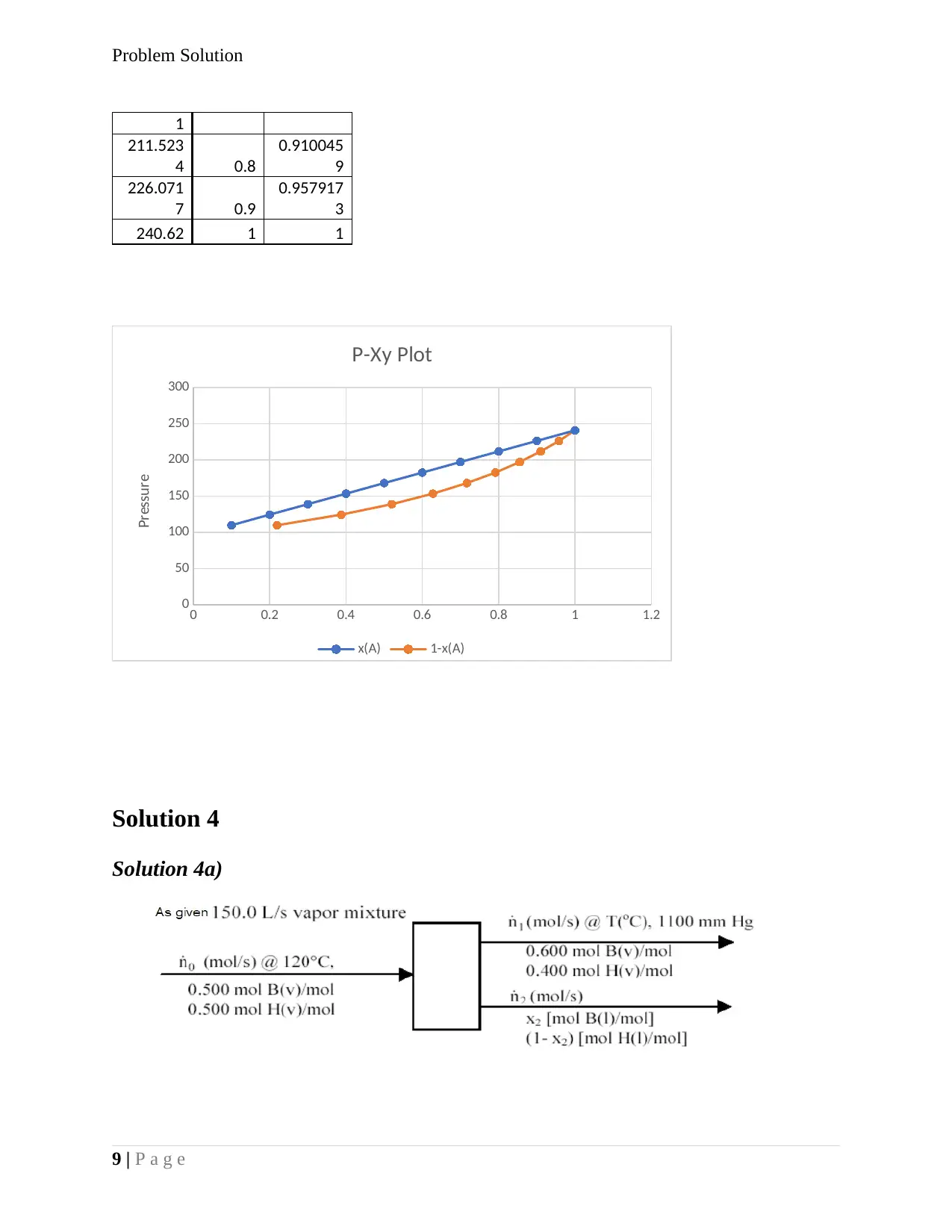
Problem Solution
1
211.523
4 0.8
0.910045
9
226.071
7 0.9
0.957917
3
240.62 1 1
0 0.2 0.4 0.6 0.8 1 1.2
0
50
100
150
200
250
300
P-Xy Plot
x(A) 1-x(A)
Pressure
Solution 4
Solution 4a)
9 | P a g e
1
211.523
4 0.8
0.910045
9
226.071
7 0.9
0.957917
3
240.62 1 1
0 0.2 0.4 0.6 0.8 1 1.2
0
50
100
150
200
250
300
P-Xy Plot
x(A) 1-x(A)
Pressure
Solution 4
Solution 4a)
9 | P a g e

Problem Solution
Solution 4b)
As given in the question, the unknown parameter is n1, n2, x2, P which is 4 in numbers, and in
the same way there are two equations for Raoult’s Law and 2 another equation for balancing the
material
We know that Degree of freedom = independent equation in numbers – unknown parameter in
numbers
Putting the value as given above = Degree of freedom = 4-4 = 0
Since degree of freedom is zero, therefore we have enough parameter information for calculating
pressure and composition of liquid product.
Solution 4c)
As given in question,
Butane Vapour pressure at given temperature (57o C) = pB* = 4417 mm Hg
Hexane Vapour pressure at given temperature (57o C) = pH* = 517.6 mm Hg
As per Roult’s Law,
The Mole fraction of Butane = yB = 0.6
In this condition, partial pressure will be = pB = yB(P)
We know that,
Partial pressure = mole fraction x vapour pressure
yB (P) = mole fraction x pB
Or, mole fraction of butane = 0.6P / 4417
Similarly, for hexane
0.4P = (1 – mole fraction of butane) (517.6)
0.4P = 517.6 – 517.6 (0.6P / 4417)
0.4P + 0.0703P = 517.6
P = 1100.57 / 760 atm, = 1.45 atm
10 | P a g e
Solution 4b)
As given in the question, the unknown parameter is n1, n2, x2, P which is 4 in numbers, and in
the same way there are two equations for Raoult’s Law and 2 another equation for balancing the
material
We know that Degree of freedom = independent equation in numbers – unknown parameter in
numbers
Putting the value as given above = Degree of freedom = 4-4 = 0
Since degree of freedom is zero, therefore we have enough parameter information for calculating
pressure and composition of liquid product.
Solution 4c)
As given in question,
Butane Vapour pressure at given temperature (57o C) = pB* = 4417 mm Hg
Hexane Vapour pressure at given temperature (57o C) = pH* = 517.6 mm Hg
As per Roult’s Law,
The Mole fraction of Butane = yB = 0.6
In this condition, partial pressure will be = pB = yB(P)
We know that,
Partial pressure = mole fraction x vapour pressure
yB (P) = mole fraction x pB
Or, mole fraction of butane = 0.6P / 4417
Similarly, for hexane
0.4P = (1 – mole fraction of butane) (517.6)
0.4P = 517.6 – 517.6 (0.6P / 4417)
0.4P + 0.0703P = 517.6
P = 1100.57 / 760 atm, = 1.45 atm
10 | P a g e
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Problem Solution
Now,
Mole fraction of butane = 0.6 x (1100.57 / 4417) = 0.15
Then mole fraction of hexane = 1-0.15 = 0.85
As per Ideal gas law
n = PV / RT
Putting the value as per given and calculated data
N =(1 x 150 )/ (0.0821 x 393)
N = 4.65 mol /s
Now we have to calculate overall material in system balance
N = N1 + N2
4.65 = N1 + N2 …………(i)
Balancing the butane
N(0.5) = 0.6N1 + 0.15N2
4.65 x 0.5 = 0.6 N1 + 0.15N2
N1 = 1.03 mol/s
N2 = 3.62 mol/s
Solution 4d)
Since we know that, fractional condensation of hexane
= mole of Hexane condensed / mole of Hexane fed
= 0.85 / (4.65 * 0.5) = 0.85 / 2.325 = 0.365591 Ans
11 | P a g e
Now,
Mole fraction of butane = 0.6 x (1100.57 / 4417) = 0.15
Then mole fraction of hexane = 1-0.15 = 0.85
As per Ideal gas law
n = PV / RT
Putting the value as per given and calculated data
N =(1 x 150 )/ (0.0821 x 393)
N = 4.65 mol /s
Now we have to calculate overall material in system balance
N = N1 + N2
4.65 = N1 + N2 …………(i)
Balancing the butane
N(0.5) = 0.6N1 + 0.15N2
4.65 x 0.5 = 0.6 N1 + 0.15N2
N1 = 1.03 mol/s
N2 = 3.62 mol/s
Solution 4d)
Since we know that, fractional condensation of hexane
= mole of Hexane condensed / mole of Hexane fed
= 0.85 / (4.65 * 0.5) = 0.85 / 2.325 = 0.365591 Ans
11 | P a g e
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.