Ammonia Synthesis from Nitrogen and Hydrogen in Chemical Engineering Contents
11 Pages1192 Words417 Views
Added on 2020-04-15
About This Document
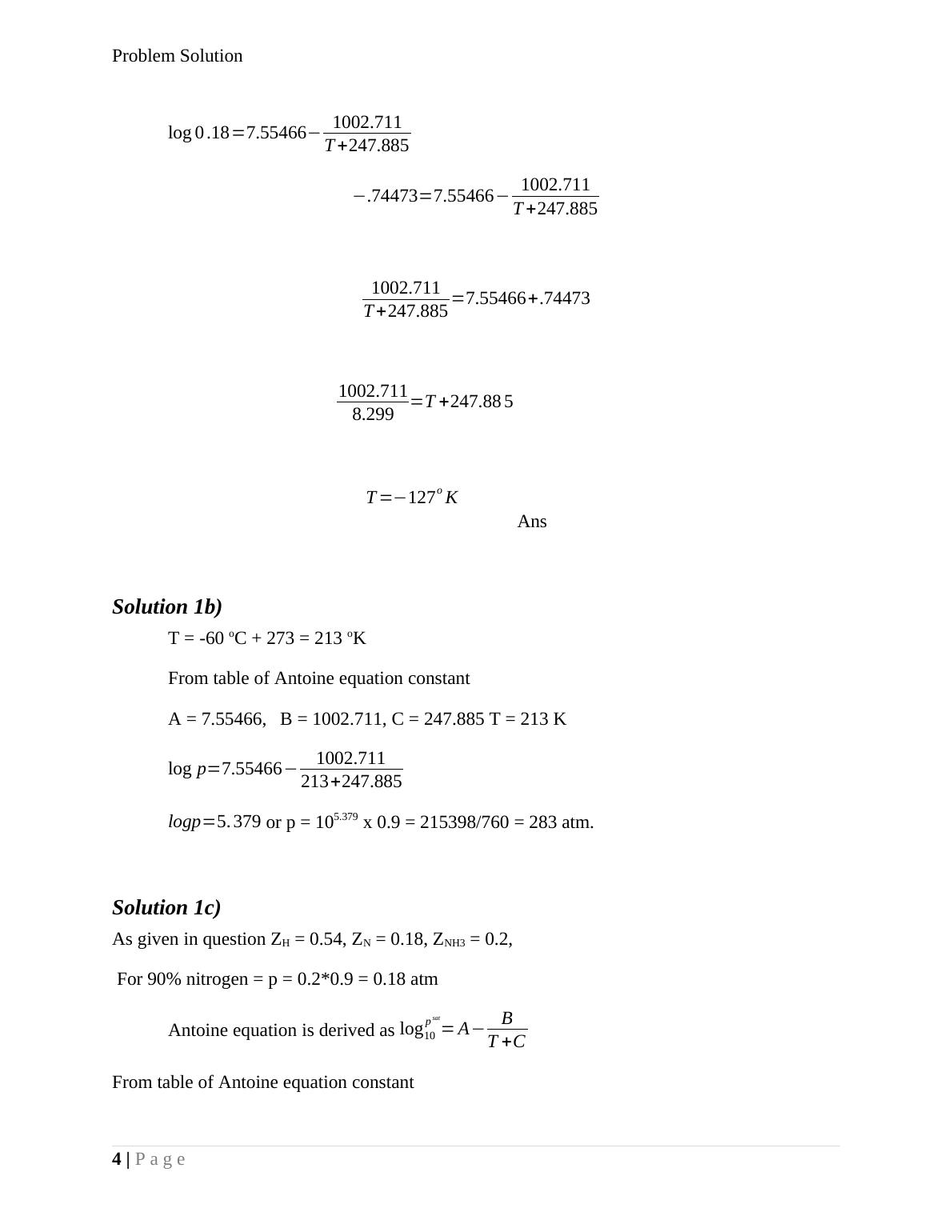
Solution 1 As given in question NH3 = 20 mole%, N2 = 18 mole%, H2 = 54 mole%, Inert = 8 mole% Solution 1a) The given Pressure P = 1 ATM, recovery of ammonia = 0.9 = We know that, PNH3 = yNH3P = 0.2 x 1 = 0.2 atm Since, PNH3 = yNH3P = 0.2 x 1 = 0.2 atm Since partial pressure for ammonia is given = 0.2
Ammonia Synthesis from Nitrogen and Hydrogen in Chemical Engineering Contents
Added on 2020-04-15
ShareRelated Documents
End of preview
Want to access all the pages? Upload your documents or become a member.