Systematic Review of Hospital Readmissions Among Cancer Patients
VerifiedAdded on 2022/10/14
|16
|15186
|14
Report
AI Summary
This report summarizes a systematic review of hospital readmissions among adult cancer patients in the United States, published in the Oncology Nursing Forum. The review analyzed 56 studies from 2005-2015, revealing significant readmission rates, particularly for patients with bladder, pancreatic, ovarian, or liver cancer. Key predictors of readmission included comorbidities, older age, advanced disease, and longer hospital stays. Common reasons for readmission were gastrointestinal and surgical complications, infections, and dehydration, highlighting nursing-sensitive patient outcomes. The review emphasizes the role of oncology nurses in implementing evidence-based interventions to address these outcomes and prevent readmissions, suggesting proactive management of nausea/vomiting, infection prevention, and nurse-led care coordination, especially for older adults with multiple comorbidities and advanced cancer. The study underscores the need for further research to assess the impact of these interventions and reduce the burden of readmissions in this population.

176 VOL. 44, NO. 2, MARCH 2017 • ONCOLOGY NURSING FORUM
Systematic Review of Hospital Readmissions
Among Patients With Cancer in the United States
Janice F. Bell, PhD, MN, MPH, Robin L. Whitney, RN, PhD, Sarah C. Reed, MSW, MPH,
Hermine Poghosyan, PhD, MPH, Rebecca S. Lash, PhD, MPP, RN,
Katherine K. Kim, PhD, MPH, MBA, Andra Davis, RN, MN, PhD, Richard J. Bold, MD,
and Jill G. Joseph, MD, PhD
ARTICLE
Cancer care has been declared a crisis in the United States because of
the growing demand for services, increasing complexity of treatment,
and dramatically rising costs of care (Institute of Medicine [IOM],
2013). Some 1.6 million individuals are diagnosed with cancer each
year, and the number of cancer survivors is projected to increase
dramatically because of the aging population and improvements in treatment
(American Cancer Society [ACS], 2016; IOM, 2013). By 2020, cancer care costs
are expected to reach $173 billion, reflecting a considerable increase from $72
billion in 2004 (ACS, 2014; Smith & Hillner, 2011). At the same time, national
reports criticize the quality of cancer care, calling for greater patient-centered
focus; improved care coordination, with management of care transitions across
settings; and cost containment through the reduction of preventable healthcare
use (IOM, 2013; Smith & Hillner, 2011).
Programs and policies to reduce hospital readmissions are increasingly viewed
as promising avenues to reduce spending and improve healthcare quality and
efficiency as well as patient experiences (Naylor, Aiken, Kurtzman, Olds, &
Hirschman, 2011; Robert Wood Johnson Foundation [RWJF], 2013; Schoen, Os-
Purpose/Objectives: To review the existing literature on readmission rates, predictors
and reasons for readmission among adults with cancer.
Data Sources: U.S.-based empirical studies reporting readmission rates from January 2
to December 2015 were identified using four online library databases—PubMed, CINAHL®
,
EconLit, and the online bibliography of the National Cancer Institute’s Surveillance Epide
miology and End Results Program. Some articles were identified by the authors outside
the database and bibliography searches.
Data Synthesis: Of the 1,219 abstracts and 271 full-text articles screened, 56 studies
met inclusion criteria. The highest readmission rates were observed in patients with blad
der, pancreatic, ovarian, or liver cancer. Significant predictors of readmission included
comorbidities, older age, advanced disease, and index length of hospital stay. Common
reasons for readmission included gastrointestinal and surgical complications, infection,
and dehydration.
Conclusions: Clinical efforts to reduce the substantial readmission rates among adults
with cancer may target high-rate conditions, infection prevention, proactive managemen
of nausea and vomiting, and nurse-led care coordination interventions for older adult
patients with multiple comorbid conditions and advanced cancer.
Implications for Nursing: Commonly reported reasons for readmission were nursing-s
patient outcomes (NSPOs), amenable to nursing intervention in oncology settings. These
findings underscore the important role oncology nurses play in readmission prevention b
implementing evidence-based interventions to address NSPOs and testing their impact
in future research.
Bell is an associate professor in the Betty
Irene Moore School of Nursing at the Uni-
versity of California (UC) Davis; Whitney is
an associate professor in the Department
of Internal Medicine at UC San Francisco
in Fresno; Reed is a doctoral candidate in
the Betty Irene Moore School of Nursing
at UC Davis; Poghosyan is an assistant
professor in the School of Nursing in
the Bouvé College of Health Sciences at
Northeastern University in Boston, MA;
Lash is a nursing publications manager
in the Department of Nursing Practice,
Research, and Education at UCLA Health
System; Kim is an assistant professor in
the Betty Irene Moore School of Nursing at
UC Davis; Davis is an assistant professor
in the College of Nursing at Washington
State University in Vancouver; Bold is a
physician at the UC Davis Comprehensive
Cancer Center in Sacramento; and Joseph
is an associate dean for research and a
professor in the Betty Irene Moore School
of Nursing at UC Davis.
Bell, Poghosyan, Lash, Kim, Bold, and
Joseph contributed to the conceptualiza-
tion and design. Bell, Whitney, Reed,
Poghosyan, and Lash completed the data
collection. Bell and Poghosyan provided
statistical support. Bell, Whitney, Reed,
Poghosyan, Lash, Kim, and Joseph provid-
ed the analysis. Bell, Whitney, Reed, Lash,
Kim, Davis, Bold, and Joseph contributed
to the manuscript preparation.
Bell can be reached at jfbell@ucdavis.edu,
with copy to editor at ONFEditor@ons.org.
Submitted May 2016. Accepted for publi-
cation July 12, 2016.
Keywords: clinical practice; nursing re-
search quantitative; outcomes research
ONF, 44(2), 176–191.
doi: 10.1011/17.ONF.176-191
Downloaded on 12 05 2018. Single-user license only. Copyright 2018 by the Oncology Nursing Society. For permission to post online, reprint, adapt, or reuse, please email pubpermissions@ons.org
Systematic Review of Hospital Readmissions
Among Patients With Cancer in the United States
Janice F. Bell, PhD, MN, MPH, Robin L. Whitney, RN, PhD, Sarah C. Reed, MSW, MPH,
Hermine Poghosyan, PhD, MPH, Rebecca S. Lash, PhD, MPP, RN,
Katherine K. Kim, PhD, MPH, MBA, Andra Davis, RN, MN, PhD, Richard J. Bold, MD,
and Jill G. Joseph, MD, PhD
ARTICLE
Cancer care has been declared a crisis in the United States because of
the growing demand for services, increasing complexity of treatment,
and dramatically rising costs of care (Institute of Medicine [IOM],
2013). Some 1.6 million individuals are diagnosed with cancer each
year, and the number of cancer survivors is projected to increase
dramatically because of the aging population and improvements in treatment
(American Cancer Society [ACS], 2016; IOM, 2013). By 2020, cancer care costs
are expected to reach $173 billion, reflecting a considerable increase from $72
billion in 2004 (ACS, 2014; Smith & Hillner, 2011). At the same time, national
reports criticize the quality of cancer care, calling for greater patient-centered
focus; improved care coordination, with management of care transitions across
settings; and cost containment through the reduction of preventable healthcare
use (IOM, 2013; Smith & Hillner, 2011).
Programs and policies to reduce hospital readmissions are increasingly viewed
as promising avenues to reduce spending and improve healthcare quality and
efficiency as well as patient experiences (Naylor, Aiken, Kurtzman, Olds, &
Hirschman, 2011; Robert Wood Johnson Foundation [RWJF], 2013; Schoen, Os-
Purpose/Objectives: To review the existing literature on readmission rates, predictors
and reasons for readmission among adults with cancer.
Data Sources: U.S.-based empirical studies reporting readmission rates from January 2
to December 2015 were identified using four online library databases—PubMed, CINAHL®
,
EconLit, and the online bibliography of the National Cancer Institute’s Surveillance Epide
miology and End Results Program. Some articles were identified by the authors outside
the database and bibliography searches.
Data Synthesis: Of the 1,219 abstracts and 271 full-text articles screened, 56 studies
met inclusion criteria. The highest readmission rates were observed in patients with blad
der, pancreatic, ovarian, or liver cancer. Significant predictors of readmission included
comorbidities, older age, advanced disease, and index length of hospital stay. Common
reasons for readmission included gastrointestinal and surgical complications, infection,
and dehydration.
Conclusions: Clinical efforts to reduce the substantial readmission rates among adults
with cancer may target high-rate conditions, infection prevention, proactive managemen
of nausea and vomiting, and nurse-led care coordination interventions for older adult
patients with multiple comorbid conditions and advanced cancer.
Implications for Nursing: Commonly reported reasons for readmission were nursing-s
patient outcomes (NSPOs), amenable to nursing intervention in oncology settings. These
findings underscore the important role oncology nurses play in readmission prevention b
implementing evidence-based interventions to address NSPOs and testing their impact
in future research.
Bell is an associate professor in the Betty
Irene Moore School of Nursing at the Uni-
versity of California (UC) Davis; Whitney is
an associate professor in the Department
of Internal Medicine at UC San Francisco
in Fresno; Reed is a doctoral candidate in
the Betty Irene Moore School of Nursing
at UC Davis; Poghosyan is an assistant
professor in the School of Nursing in
the Bouvé College of Health Sciences at
Northeastern University in Boston, MA;
Lash is a nursing publications manager
in the Department of Nursing Practice,
Research, and Education at UCLA Health
System; Kim is an assistant professor in
the Betty Irene Moore School of Nursing at
UC Davis; Davis is an assistant professor
in the College of Nursing at Washington
State University in Vancouver; Bold is a
physician at the UC Davis Comprehensive
Cancer Center in Sacramento; and Joseph
is an associate dean for research and a
professor in the Betty Irene Moore School
of Nursing at UC Davis.
Bell, Poghosyan, Lash, Kim, Bold, and
Joseph contributed to the conceptualiza-
tion and design. Bell, Whitney, Reed,
Poghosyan, and Lash completed the data
collection. Bell and Poghosyan provided
statistical support. Bell, Whitney, Reed,
Poghosyan, Lash, Kim, and Joseph provid-
ed the analysis. Bell, Whitney, Reed, Lash,
Kim, Davis, Bold, and Joseph contributed
to the manuscript preparation.
Bell can be reached at jfbell@ucdavis.edu,
with copy to editor at ONFEditor@ons.org.
Submitted May 2016. Accepted for publi-
cation July 12, 2016.
Keywords: clinical practice; nursing re-
search quantitative; outcomes research
ONF, 44(2), 176–191.
doi: 10.1011/17.ONF.176-191
Downloaded on 12 05 2018. Single-user license only. Copyright 2018 by the Oncology Nursing Society. For permission to post online, reprint, adapt, or reuse, please email pubpermissions@ons.org
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ONCOLOGY NURSING FORUM • VOL. 44, NO. 2, MARCH 2017 177
born, How, Doty, & Peugh, 2009). Hospital stays are
stressful and inconvenient for patients and their fam-
ilies, and substantially contribute to out-of-pocket
healthcare costs. One aim of the Patient Protection
and Affordable Care Act is to reduce healthcare
spending through improved outpatient management
of chronic disease and reduced hospital readmis-
sions (Carroll & Frakt, 2013; Kocher & Adashi, 2011).
Likewise, the Center for Medicaid and Medicare
Innovation instituted a five-year Community Care
Transitions Program to test models for improving
patient transitions from hospitals to other settings
and avoiding unnecessary readmissions (Agency
for Healthcare Research and Quality [AHRQ], 2014;
Kocher & Adashi, 2011). Such initiatives are built on
the assumption that some readmissions are prevent-
able; the validity of readmission rates as indicators
of healthcare quality depends on this premise (Gold-
field et al., 2008).
Oncology nurses play important roles in prevent-
ing readmission from the moment patients are ad-
mitted to hospitals by identifying and addressing
complications and adverse inpatient events that
may increase readmission risk, assessing patient and
family knowledge, providing education throughout
the hospital stay and in preparation for discharge,
assisting with medication management, support-
ing advanced care planning, and coordinating care
transitions between inpatient and community-based
providers and services (Feigenbaum et al., 2012;
Naylor et al., 2011). Indeed, a growing body of evi-
dence suggests that multicomponent interventions
focused on care transitions and incorporations of
strategies—such as comprehensive discharge plan-
ning and instructions with follow-up, home visits,
individualized care planning, clinical management,
education, and behavioral support—may be effec-
tive in reducing readmission rates (Coleman, Parry,
Chalmers, & Min, 2006; Epstein, Jha, & Orav, 2011;
Feigenbaum et al., 2012; Hansen, Young, Hinami,
Leung, & Williams, 2011; Hari & Rosenzweig, 2012;
Jack et al., 2009; Naylor et al., 2011; Peikes, Chen,
Schore, & Brown, 2009; VanSuch, Naessens, Stroebel,
Huddleston, & Williams, 2006).
Successful nursing interventions to reduce re-
admission depend on identifying groups at risk for
preventable readmission; however, the burden of
readmissions for patients with cancer is not well
described in extant literature, nor is the extent to
which readmissions are preventable in this popula-
tion. To date, cancer-specific readmission rates are
not publicly reported, and the Centers for Medicare
and Medicaid Services (CMS) penalties for readmis-
sions do not apply to cancer hospitals (Horwitz et
al., 2012). In a predictive model of avoidable read-
missions developed at a large academic medical
center (Donzé, Aujesky, Williams, & Schnipper, 2013),
discharge from an oncology service was a significant
risk factor, even when excluding planned readmis-
sions for chemotherapy. Similarly, a Canadian study
(Ji, Abushomar, Chen, Qian, & Gerson, 2012) found
that the all-cause readmission rates of patients with
cancer were higher than the rates of patients with
other conditions. Whether these findings are relevant
to the unique U.S. clinical, payment, and healthcare
policy environment is unknown.
Studies of readmissions among patients with cancer
in the United States are needed to ascertain the extent
of this population’s risk for readmission, to identify
subgroups that might benefit from interventions to
reduce readmissions, and to provide benchmarks
against which to measure the success of such
interventions. Accordingly, this systematic literature
review had three related aims focused on patients
with cancer: (a) to examine the proportion of patients
with cancer who are readmitted to the hospital within
30 days of discharge, (b) to enumerate the reasons
for and predictors of readmissions, and (c) to assess
whether and how current studies identify potentially
preventable readmissions.
Methods
Following the Preferred Reporting Items for System-
atic Reviews and Meta-Analyses (PRISMA) guidelines
(Moher, Liberati, Tetzlaff, & Altman, 2009), the authors
of the current study searched three electronic library
databases (PubMed, CINAHL®
, EconLit) and the online
bibliography of the National Cancer Institute’s Surveil-
lance Epidemiology and End Results (SEER) Program.
The Medical Subject Heading (MeSH) terms patient
readmission and neoplasms or neoplasm metastasis
or carcinoma were employed in the PubMed search.
The keywords readmission(s) or rehospitalization(s)
were used in the EconLit search, which was limited
to publications in analysis of healthcare markets,
health, government policy, regulation, public health,
and health production. The subject headings readmis-
sion and neoplasms were employed in the CINAHL
search. The SEER bibliography search focused on
the keywords readmission(s) or rehospitalization(s) in
abstracts and titles. In addition, the authors identified
relevant articles outside the database and bibliogra-
phy searches.
Inclusion and Exclusion Criteria
The inclusion criteria included (a) peer-reviewed
empirical studies conducted in the United States, (b)
articles published from January 1, 2005, to Decem-
ber 31, 2015, (c) articles with sample sizes of 50 or
born, How, Doty, & Peugh, 2009). Hospital stays are
stressful and inconvenient for patients and their fam-
ilies, and substantially contribute to out-of-pocket
healthcare costs. One aim of the Patient Protection
and Affordable Care Act is to reduce healthcare
spending through improved outpatient management
of chronic disease and reduced hospital readmis-
sions (Carroll & Frakt, 2013; Kocher & Adashi, 2011).
Likewise, the Center for Medicaid and Medicare
Innovation instituted a five-year Community Care
Transitions Program to test models for improving
patient transitions from hospitals to other settings
and avoiding unnecessary readmissions (Agency
for Healthcare Research and Quality [AHRQ], 2014;
Kocher & Adashi, 2011). Such initiatives are built on
the assumption that some readmissions are prevent-
able; the validity of readmission rates as indicators
of healthcare quality depends on this premise (Gold-
field et al., 2008).
Oncology nurses play important roles in prevent-
ing readmission from the moment patients are ad-
mitted to hospitals by identifying and addressing
complications and adverse inpatient events that
may increase readmission risk, assessing patient and
family knowledge, providing education throughout
the hospital stay and in preparation for discharge,
assisting with medication management, support-
ing advanced care planning, and coordinating care
transitions between inpatient and community-based
providers and services (Feigenbaum et al., 2012;
Naylor et al., 2011). Indeed, a growing body of evi-
dence suggests that multicomponent interventions
focused on care transitions and incorporations of
strategies—such as comprehensive discharge plan-
ning and instructions with follow-up, home visits,
individualized care planning, clinical management,
education, and behavioral support—may be effec-
tive in reducing readmission rates (Coleman, Parry,
Chalmers, & Min, 2006; Epstein, Jha, & Orav, 2011;
Feigenbaum et al., 2012; Hansen, Young, Hinami,
Leung, & Williams, 2011; Hari & Rosenzweig, 2012;
Jack et al., 2009; Naylor et al., 2011; Peikes, Chen,
Schore, & Brown, 2009; VanSuch, Naessens, Stroebel,
Huddleston, & Williams, 2006).
Successful nursing interventions to reduce re-
admission depend on identifying groups at risk for
preventable readmission; however, the burden of
readmissions for patients with cancer is not well
described in extant literature, nor is the extent to
which readmissions are preventable in this popula-
tion. To date, cancer-specific readmission rates are
not publicly reported, and the Centers for Medicare
and Medicaid Services (CMS) penalties for readmis-
sions do not apply to cancer hospitals (Horwitz et
al., 2012). In a predictive model of avoidable read-
missions developed at a large academic medical
center (Donzé, Aujesky, Williams, & Schnipper, 2013),
discharge from an oncology service was a significant
risk factor, even when excluding planned readmis-
sions for chemotherapy. Similarly, a Canadian study
(Ji, Abushomar, Chen, Qian, & Gerson, 2012) found
that the all-cause readmission rates of patients with
cancer were higher than the rates of patients with
other conditions. Whether these findings are relevant
to the unique U.S. clinical, payment, and healthcare
policy environment is unknown.
Studies of readmissions among patients with cancer
in the United States are needed to ascertain the extent
of this population’s risk for readmission, to identify
subgroups that might benefit from interventions to
reduce readmissions, and to provide benchmarks
against which to measure the success of such
interventions. Accordingly, this systematic literature
review had three related aims focused on patients
with cancer: (a) to examine the proportion of patients
with cancer who are readmitted to the hospital within
30 days of discharge, (b) to enumerate the reasons
for and predictors of readmissions, and (c) to assess
whether and how current studies identify potentially
preventable readmissions.
Methods
Following the Preferred Reporting Items for System-
atic Reviews and Meta-Analyses (PRISMA) guidelines
(Moher, Liberati, Tetzlaff, & Altman, 2009), the authors
of the current study searched three electronic library
databases (PubMed, CINAHL®
, EconLit) and the online
bibliography of the National Cancer Institute’s Surveil-
lance Epidemiology and End Results (SEER) Program.
The Medical Subject Heading (MeSH) terms patient
readmission and neoplasms or neoplasm metastasis
or carcinoma were employed in the PubMed search.
The keywords readmission(s) or rehospitalization(s)
were used in the EconLit search, which was limited
to publications in analysis of healthcare markets,
health, government policy, regulation, public health,
and health production. The subject headings readmis-
sion and neoplasms were employed in the CINAHL
search. The SEER bibliography search focused on
the keywords readmission(s) or rehospitalization(s) in
abstracts and titles. In addition, the authors identified
relevant articles outside the database and bibliogra-
phy searches.
Inclusion and Exclusion Criteria
The inclusion criteria included (a) peer-reviewed
empirical studies conducted in the United States, (b)
articles published from January 1, 2005, to Decem-
ber 31, 2015, (c) articles with sample sizes of 50 or
178 VOL. 44, NO. 2, MARCH 2017 • ONCOLOGY NURSING FORUM
more, and (d) studies that identified the proportion
of readmissions among patients with cancer aged 18
years or older. Articles were excluded if they were
(a) reports of a literature review, meta-analysis,
commentary, or case study; (b) focused solely on
health service use at the end of life, given higher
expected rates of readmissions attributed to con-
founding by progression of disease; or (c) presented
readmission rates that were not exclusive to patients
with cancer.
Screening Process
All citations were managed in EndNote X7, and
duplicates were discarded. A two-stage screening
process was applied to assess whether articles met
inclusion criteria, with all articles screened by the
lead author and at least one other investigator. In
the first stage, the authors searched all EndNote
fields, including titles and abstracts, for the keywords
readmission(s) or rehospitalization(s). Articles were
retrieved and the full text examined if they could
not be included or excluded based on the EndNote
keyword search, as in the case of scanned papers.
In the second stage of the review, the full text of all
included papers from the first stage was obtained and
examined against the inclusion and exclusion criteria
independently by at least two investigators. All the
references of the included articles, meta-analyses,
and review papers identified during the review were
iteratively examined.
Data Abstraction
Included studies were sorted into one
of two groups according to their focus on
a single institution (hospital or medical
center) or multiple institutions. A stan-
dardized abstraction form was developed
to systematically collect and summarize
key data elements from each article. The
authors of the current study calculated
30-day readmission rates for articles pre-
senting readmission rates in time frames
other than 30 days, assuming a constant
rate of readmission over time. This ap-
proach yielded conservative 30-day read-
mission estimates because most readmis-
sions occur within the first 30 days and
decline afterward (Benbassat & Taragin,
2000). Most studies using alternative time
frames reported readmissions within time
frames longer than 30 days. Significant
predictors of readmission from the results
of multivariate regression models were re-
corded, as were the most common reasons
for readmission, if specified in the articles.
Finally, the authors examined the studies
to ascertain whether the readmissions were classified
as potentially preventable and, if so, they recorded the
definition. At least 90% agreement was reached in each
stage of the review, with discrepancies resolved by the
consensus of all participating authors.
Results
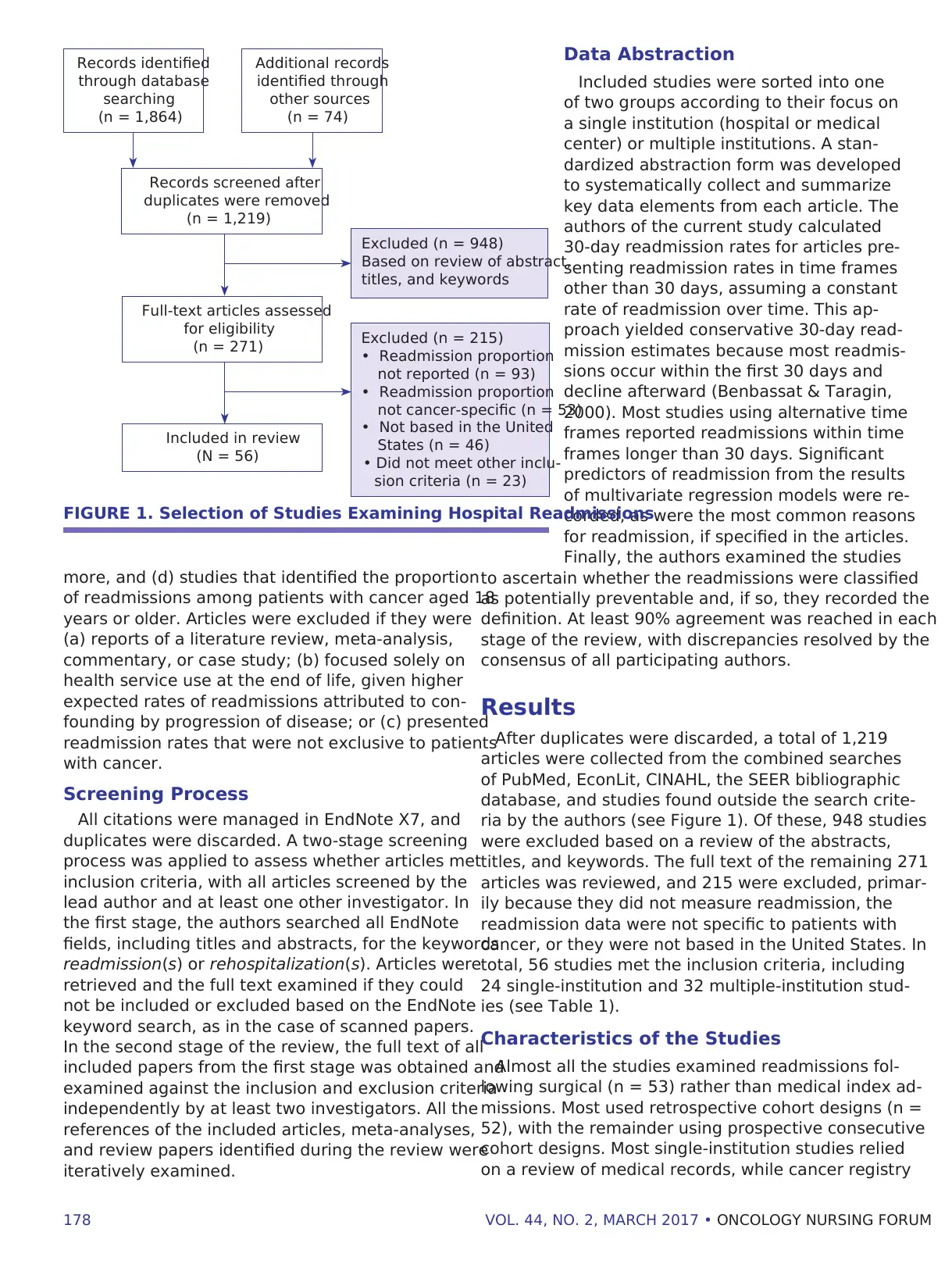
After duplicates were discarded, a total of 1,219
articles were collected from the combined searches
of PubMed, EconLit, CINAHL, the SEER bibliographic
database, and studies found outside the search crite-
ria by the authors (see Figure 1). Of these, 948 studies
were excluded based on a review of the abstracts,
titles, and keywords. The full text of the remaining 271
articles was reviewed, and 215 were excluded, primar-
ily because they did not measure readmission, the
readmission data were not specific to patients with
cancer, or they were not based in the United States. In
total, 56 studies met the inclusion criteria, including
24 single-institution and 32 multiple-institution stud-
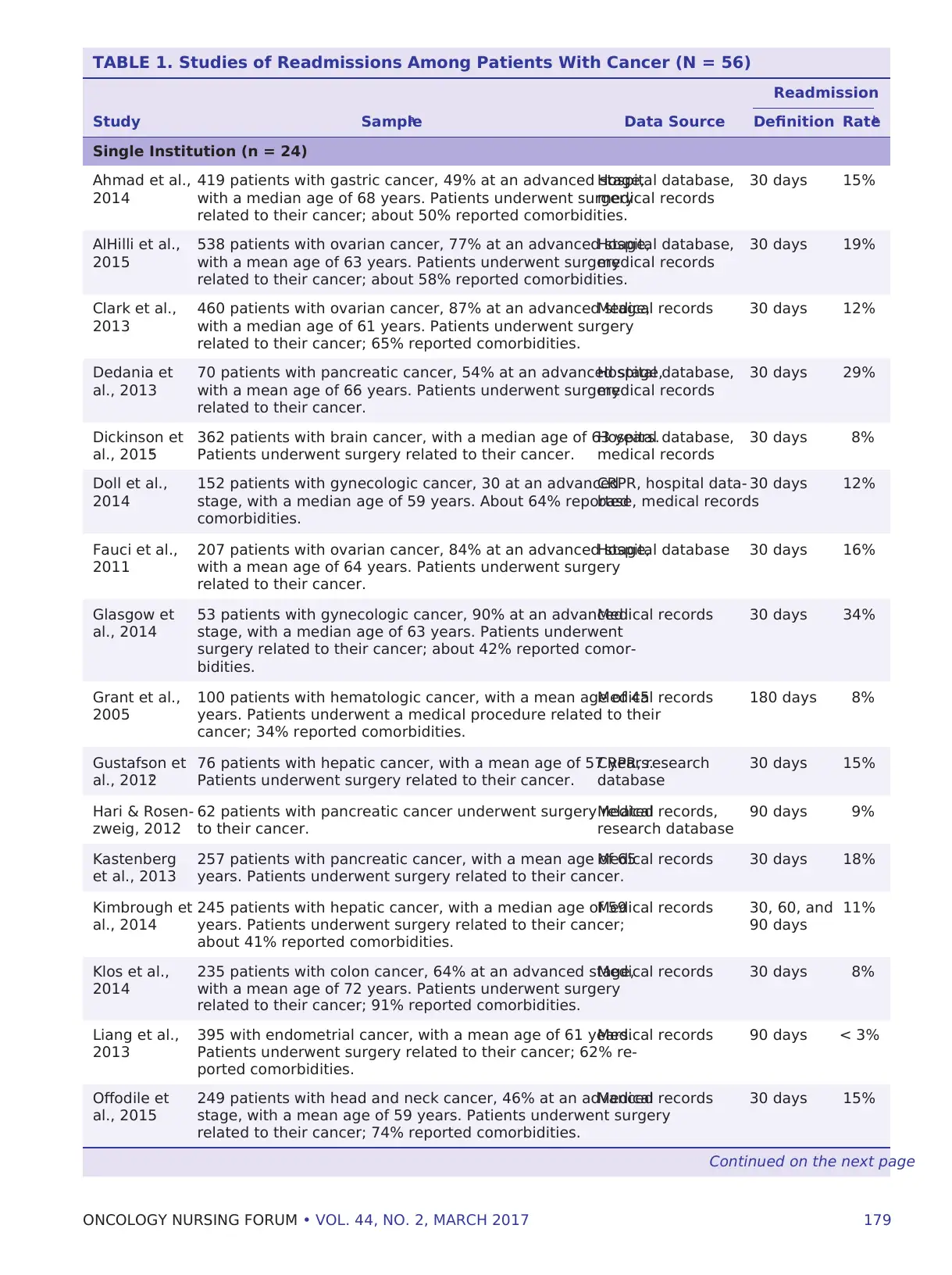
ies (see Table 1).
Characteristics of the Studies
Almost all the studies examined readmissions fol-
lowing surgical (n = 53) rather than medical index ad-
missions. Most used retrospective cohort designs (n =
52), with the remainder using prospective consecutive
cohort designs. Most single-institution studies relied
on a review of medical records, while cancer registry
FIGURE 1. Selection of Studies Examining Hospital Readmissions
Records identified
through database
searching
(n = 1,864)
Additional records
identified through
other sources
(n = 74)
Records screened after
duplicates were removed
(n = 1,219)
Full-text articles assessed
for eligibility
(n = 271)
Included in review
(N = 56)
Excluded (n = 948)
Based on review of abstract,
titles, and keywords
Excluded (n = 215)
• Readmission proportion
not reported (n = 93)
• Readmission proportion
not cancer-specific (n = 53)
• Not based in the United
States (n = 46)
• Did not meet other inclu-
sion criteria (n = 23)
more, and (d) studies that identified the proportion
of readmissions among patients with cancer aged 18
years or older. Articles were excluded if they were
(a) reports of a literature review, meta-analysis,
commentary, or case study; (b) focused solely on
health service use at the end of life, given higher
expected rates of readmissions attributed to con-
founding by progression of disease; or (c) presented
readmission rates that were not exclusive to patients
with cancer.
Screening Process
All citations were managed in EndNote X7, and
duplicates were discarded. A two-stage screening
process was applied to assess whether articles met
inclusion criteria, with all articles screened by the
lead author and at least one other investigator. In
the first stage, the authors searched all EndNote
fields, including titles and abstracts, for the keywords
readmission(s) or rehospitalization(s). Articles were
retrieved and the full text examined if they could
not be included or excluded based on the EndNote
keyword search, as in the case of scanned papers.
In the second stage of the review, the full text of all
included papers from the first stage was obtained and
examined against the inclusion and exclusion criteria
independently by at least two investigators. All the
references of the included articles, meta-analyses,
and review papers identified during the review were
iteratively examined.
Data Abstraction
Included studies were sorted into one
of two groups according to their focus on
a single institution (hospital or medical
center) or multiple institutions. A stan-
dardized abstraction form was developed
to systematically collect and summarize
key data elements from each article. The
authors of the current study calculated
30-day readmission rates for articles pre-
senting readmission rates in time frames
other than 30 days, assuming a constant
rate of readmission over time. This ap-
proach yielded conservative 30-day read-
mission estimates because most readmis-
sions occur within the first 30 days and
decline afterward (Benbassat & Taragin,
2000). Most studies using alternative time
frames reported readmissions within time
frames longer than 30 days. Significant
predictors of readmission from the results
of multivariate regression models were re-
corded, as were the most common reasons
for readmission, if specified in the articles.
Finally, the authors examined the studies
to ascertain whether the readmissions were classified
as potentially preventable and, if so, they recorded the
definition. At least 90% agreement was reached in each
stage of the review, with discrepancies resolved by the
consensus of all participating authors.
Results
After duplicates were discarded, a total of 1,219
articles were collected from the combined searches
of PubMed, EconLit, CINAHL, the SEER bibliographic
database, and studies found outside the search crite-
ria by the authors (see Figure 1). Of these, 948 studies
were excluded based on a review of the abstracts,
titles, and keywords. The full text of the remaining 271
articles was reviewed, and 215 were excluded, primar-
ily because they did not measure readmission, the
readmission data were not specific to patients with
cancer, or they were not based in the United States. In
total, 56 studies met the inclusion criteria, including
24 single-institution and 32 multiple-institution stud-
ies (see Table 1).
Characteristics of the Studies
Almost all the studies examined readmissions fol-
lowing surgical (n = 53) rather than medical index ad-
missions. Most used retrospective cohort designs (n =
52), with the remainder using prospective consecutive
cohort designs. Most single-institution studies relied
on a review of medical records, while cancer registry
FIGURE 1. Selection of Studies Examining Hospital Readmissions
Records identified
through database
searching
(n = 1,864)
Additional records
identified through
other sources
(n = 74)
Records screened after
duplicates were removed
(n = 1,219)
Full-text articles assessed
for eligibility
(n = 271)
Included in review
(N = 56)
Excluded (n = 948)
Based on review of abstract,
titles, and keywords
Excluded (n = 215)
• Readmission proportion
not reported (n = 93)
• Readmission proportion
not cancer-specific (n = 53)
• Not based in the United
States (n = 46)
• Did not meet other inclu-
sion criteria (n = 23)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
ONCOLOGY NURSING FORUM • VOL. 44, NO. 2, MARCH 2017 179
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)
Readmission
Study Samplea Data Source Definition Rateb
Single Institution (n = 24)
Ahmad et al.,
2014
419 patients with gastric cancer, 49% at an advanced stage,
with a median age of 68 years. Patients underwent surgery
related to their cancer; about 50% reported comorbidities.
Hospital database,
medical records
30 days 15%
AlHilli et al.,
2015
538 patients with ovarian cancer, 77% at an advanced stage,
with a mean age of 63 years. Patients underwent surgery
related to their cancer; about 58% reported comorbidities.
Hospital database,
medical records
30 days 19%
Clark et al.,
2013
460 patients with ovarian cancer, 87% at an advanced stage,
with a median age of 61 years. Patients underwent surgery
related to their cancer; 65% reported comorbidities.
Medical records 30 days 12%
Dedania et
al., 2013
70 patients with pancreatic cancer, 54% at an advanced stage,
with a mean age of 66 years. Patients underwent surgery
related to their cancer.
Hospital database,
medical records
30 days 29%
Dickinson et
al., 2015c
362 patients with brain cancer, with a median age of 63 years.
Patients underwent surgery related to their cancer.
Hospital database,
medical records
30 days 8%
Doll et al.,
2014
152 patients with gynecologic cancer, 30 at an advanced
stage, with a median age of 59 years. About 64% reported
comorbidities.
CRPR, hospital data-
base, medical records
30 days 12%
Fauci et al.,
2011
207 patients with ovarian cancer, 84% at an advanced stage,
with a mean age of 64 years. Patients underwent surgery
related to their cancer.
Hospital database 30 days 16%
Glasgow et
al., 2014
53 patients with gynecologic cancer, 90% at an advanced
stage, with a median age of 63 years. Patients underwent
surgery related to their cancer; about 42% reported comor-
bidities.
Medical records 30 days 34%
Grant et al.,
2005
100 patients with hematologic cancer, with a mean age of 45
years. Patients underwent a medical procedure related to their
cancer; 34% reported comorbidities.
Medical records 180 days 8%
Gustafson et
al., 2012c
76 patients with hepatic cancer, with a mean age of 57 years.
Patients underwent surgery related to their cancer.
CRPR, research
database
30 days 15%
Hari & Rosen-
zweig, 2012
62 patients with pancreatic cancer underwent surgery related
to their cancer.
Medical records,
research database
90 days 9%
Kastenberg
et al., 2013
257 patients with pancreatic cancer, with a mean age of 65
years. Patients underwent surgery related to their cancer.
Medical records 30 days 18%
Kimbrough et
al., 2014
245 patients with hepatic cancer, with a median age of 59
years. Patients underwent surgery related to their cancer;
about 41% reported comorbidities.
Medical records 30, 60, and
90 days
11%
Klos et al.,
2014
235 patients with colon cancer, 64% at an advanced stage,
with a mean age of 72 years. Patients underwent surgery
related to their cancer; 91% reported comorbidities.
Medical records 30 days 8%
Liang et al.,
2013
395 with endometrial cancer, with a mean age of 61 years.
Patients underwent surgery related to their cancer; 62% re-
ported comorbidities.
Medical records 90 days < 3%
Offodile et
al., 2015
249 patients with head and neck cancer, 46% at an advanced
stage, with a mean age of 59 years. Patients underwent surgery
related to their cancer; 74% reported comorbidities.
Medical records 30 days 15%
Continued on the next page
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)
Readmission
Study Samplea Data Source Definition Rateb
Single Institution (n = 24)
Ahmad et al.,
2014
419 patients with gastric cancer, 49% at an advanced stage,
with a median age of 68 years. Patients underwent surgery
related to their cancer; about 50% reported comorbidities.
Hospital database,
medical records
30 days 15%
AlHilli et al.,
2015
538 patients with ovarian cancer, 77% at an advanced stage,
with a mean age of 63 years. Patients underwent surgery
related to their cancer; about 58% reported comorbidities.
Hospital database,
medical records
30 days 19%
Clark et al.,
2013
460 patients with ovarian cancer, 87% at an advanced stage,
with a median age of 61 years. Patients underwent surgery
related to their cancer; 65% reported comorbidities.
Medical records 30 days 12%
Dedania et
al., 2013
70 patients with pancreatic cancer, 54% at an advanced stage,
with a mean age of 66 years. Patients underwent surgery
related to their cancer.
Hospital database,
medical records
30 days 29%
Dickinson et
al., 2015c
362 patients with brain cancer, with a median age of 63 years.
Patients underwent surgery related to their cancer.
Hospital database,
medical records
30 days 8%
Doll et al.,
2014
152 patients with gynecologic cancer, 30 at an advanced
stage, with a median age of 59 years. About 64% reported
comorbidities.
CRPR, hospital data-
base, medical records
30 days 12%
Fauci et al.,
2011
207 patients with ovarian cancer, 84% at an advanced stage,
with a mean age of 64 years. Patients underwent surgery
related to their cancer.
Hospital database 30 days 16%
Glasgow et
al., 2014
53 patients with gynecologic cancer, 90% at an advanced
stage, with a median age of 63 years. Patients underwent
surgery related to their cancer; about 42% reported comor-
bidities.
Medical records 30 days 34%
Grant et al.,
2005
100 patients with hematologic cancer, with a mean age of 45
years. Patients underwent a medical procedure related to their
cancer; 34% reported comorbidities.
Medical records 180 days 8%
Gustafson et
al., 2012c
76 patients with hepatic cancer, with a mean age of 57 years.
Patients underwent surgery related to their cancer.
CRPR, research
database
30 days 15%
Hari & Rosen-
zweig, 2012
62 patients with pancreatic cancer underwent surgery related
to their cancer.
Medical records,
research database
90 days 9%
Kastenberg
et al., 2013
257 patients with pancreatic cancer, with a mean age of 65
years. Patients underwent surgery related to their cancer.
Medical records 30 days 18%
Kimbrough et
al., 2014
245 patients with hepatic cancer, with a median age of 59
years. Patients underwent surgery related to their cancer;
about 41% reported comorbidities.
Medical records 30, 60, and
90 days
11%
Klos et al.,
2014
235 patients with colon cancer, 64% at an advanced stage,
with a mean age of 72 years. Patients underwent surgery
related to their cancer; 91% reported comorbidities.
Medical records 30 days 8%
Liang et al.,
2013
395 with endometrial cancer, with a mean age of 61 years.
Patients underwent surgery related to their cancer; 62% re-
ported comorbidities.
Medical records 90 days < 3%
Offodile et
al., 2015
249 patients with head and neck cancer, 46% at an advanced
stage, with a mean age of 59 years. Patients underwent surgery
related to their cancer; 74% reported comorbidities.
Medical records 30 days 15%
Continued on the next page
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
180 VOL. 44, NO. 2, MARCH 2017 • ONCOLOGY NURSING FORUM
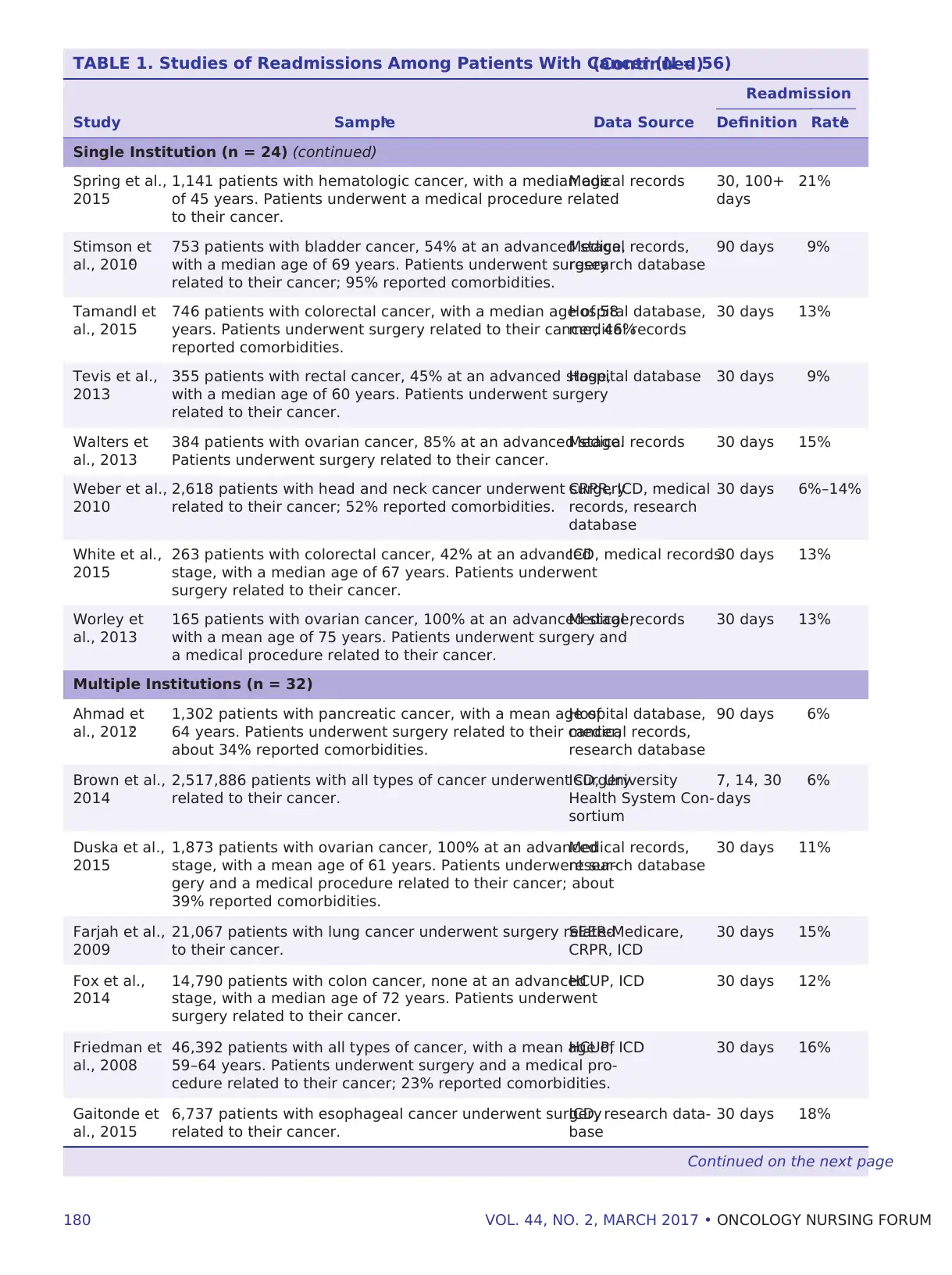
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)(Continued)
Readmission
Study Samplea Data Source Definition Rateb
Single Institution (n = 24) (continued)
Spring et al.,
2015
1,141 patients with hematologic cancer, with a median age
of 45 years. Patients underwent a medical procedure related
to their cancer.
Medical records 30, 100+
days
21%
Stimson et
al., 2010c
753 patients with bladder cancer, 54% at an advanced stage,
with a median age of 69 years. Patients underwent surgery
related to their cancer; 95% reported comorbidities.
Medical records,
research database
90 days 9%
Tamandl et
al., 2015
746 patients with colorectal cancer, with a median age of 58
years. Patients underwent surgery related to their cancer; 46%
reported comorbidities.
Hospital database,
medical records
30 days 13%
Tevis et al.,
2013
355 patients with rectal cancer, 45% at an advanced stage,
with a median age of 60 years. Patients underwent surgery
related to their cancer.
Hospital database 30 days 9%
Walters et
al., 2013
384 patients with ovarian cancer, 85% at an advanced stage.
Patients underwent surgery related to their cancer.
Medical records 30 days 15%
Weber et al.,
2010
2,618 patients with head and neck cancer underwent surgery
related to their cancer; 52% reported comorbidities.
CRPR, ICD, medical
records, research
database
30 days 6%–14%
White et al.,
2015
263 patients with colorectal cancer, 42% at an advanced
stage, with a median age of 67 years. Patients underwent
surgery related to their cancer.
ICD, medical records30 days 13%
Worley et
al., 2013
165 patients with ovarian cancer, 100% at an advanced stage,
with a mean age of 75 years. Patients underwent surgery and
a medical procedure related to their cancer.
Medical records 30 days 13%
Multiple Institutions (n = 32)
Ahmad et
al., 2012c
1,302 patients with pancreatic cancer, with a mean age of
64 years. Patients underwent surgery related to their cancer;
about 34% reported comorbidities.
Hospital database,
medical records,
research database
90 days 6%
Brown et al.,
2014
2,517,886 patients with all types of cancer underwent surgery
related to their cancer.
ICD, University
Health System Con-
sortium
7, 14, 30
days
6%
Duska et al.,
2015
1,873 patients with ovarian cancer, 100% at an advanced
stage, with a mean age of 61 years. Patients underwent sur-
gery and a medical procedure related to their cancer; about
39% reported comorbidities.
Medical records,
research database
30 days 11%
Farjah et al.,
2009
21,067 patients with lung cancer underwent surgery related
to their cancer.
SEER-Medicare,
CRPR, ICD
30 days 15%
Fox et al.,
2014
14,790 patients with colon cancer, none at an advanced
stage, with a median age of 72 years. Patients underwent
surgery related to their cancer.
HCUP, ICD 30 days 12%
Friedman et
al., 2008
46,392 patients with all types of cancer, with a mean age of
59–64 years. Patients underwent surgery and a medical pro-
cedure related to their cancer; 23% reported comorbidities.
HCUP, ICD 30 days 16%
Gaitonde et
al., 2015
6,737 patients with esophageal cancer underwent surgery
related to their cancer.
ICD, research data-
base
30 days 18%
Continued on the next page
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)(Continued)
Readmission
Study Samplea Data Source Definition Rateb
Single Institution (n = 24) (continued)
Spring et al.,
2015
1,141 patients with hematologic cancer, with a median age
of 45 years. Patients underwent a medical procedure related
to their cancer.
Medical records 30, 100+
days
21%
Stimson et
al., 2010c
753 patients with bladder cancer, 54% at an advanced stage,
with a median age of 69 years. Patients underwent surgery
related to their cancer; 95% reported comorbidities.
Medical records,
research database
90 days 9%
Tamandl et
al., 2015
746 patients with colorectal cancer, with a median age of 58
years. Patients underwent surgery related to their cancer; 46%
reported comorbidities.
Hospital database,
medical records
30 days 13%
Tevis et al.,
2013
355 patients with rectal cancer, 45% at an advanced stage,
with a median age of 60 years. Patients underwent surgery
related to their cancer.
Hospital database 30 days 9%
Walters et
al., 2013
384 patients with ovarian cancer, 85% at an advanced stage.
Patients underwent surgery related to their cancer.
Medical records 30 days 15%
Weber et al.,
2010
2,618 patients with head and neck cancer underwent surgery
related to their cancer; 52% reported comorbidities.
CRPR, ICD, medical
records, research
database
30 days 6%–14%
White et al.,
2015
263 patients with colorectal cancer, 42% at an advanced
stage, with a median age of 67 years. Patients underwent
surgery related to their cancer.
ICD, medical records30 days 13%
Worley et
al., 2013
165 patients with ovarian cancer, 100% at an advanced stage,
with a mean age of 75 years. Patients underwent surgery and
a medical procedure related to their cancer.
Medical records 30 days 13%
Multiple Institutions (n = 32)
Ahmad et
al., 2012c
1,302 patients with pancreatic cancer, with a mean age of
64 years. Patients underwent surgery related to their cancer;
about 34% reported comorbidities.
Hospital database,
medical records,
research database
90 days 6%
Brown et al.,
2014
2,517,886 patients with all types of cancer underwent surgery
related to their cancer.
ICD, University
Health System Con-
sortium
7, 14, 30
days
6%
Duska et al.,
2015
1,873 patients with ovarian cancer, 100% at an advanced
stage, with a mean age of 61 years. Patients underwent sur-
gery and a medical procedure related to their cancer; about
39% reported comorbidities.
Medical records,
research database
30 days 11%
Farjah et al.,
2009
21,067 patients with lung cancer underwent surgery related
to their cancer.
SEER-Medicare,
CRPR, ICD
30 days 15%
Fox et al.,
2014
14,790 patients with colon cancer, none at an advanced
stage, with a median age of 72 years. Patients underwent
surgery related to their cancer.
HCUP, ICD 30 days 12%
Friedman et
al., 2008
46,392 patients with all types of cancer, with a mean age of
59–64 years. Patients underwent surgery and a medical pro-
cedure related to their cancer; 23% reported comorbidities.
HCUP, ICD 30 days 16%
Gaitonde et
al., 2015
6,737 patients with esophageal cancer underwent surgery
related to their cancer.
ICD, research data-
base
30 days 18%
Continued on the next page
ONCOLOGY NURSING FORUM • VOL. 44, NO. 2, MARCH 2017 181
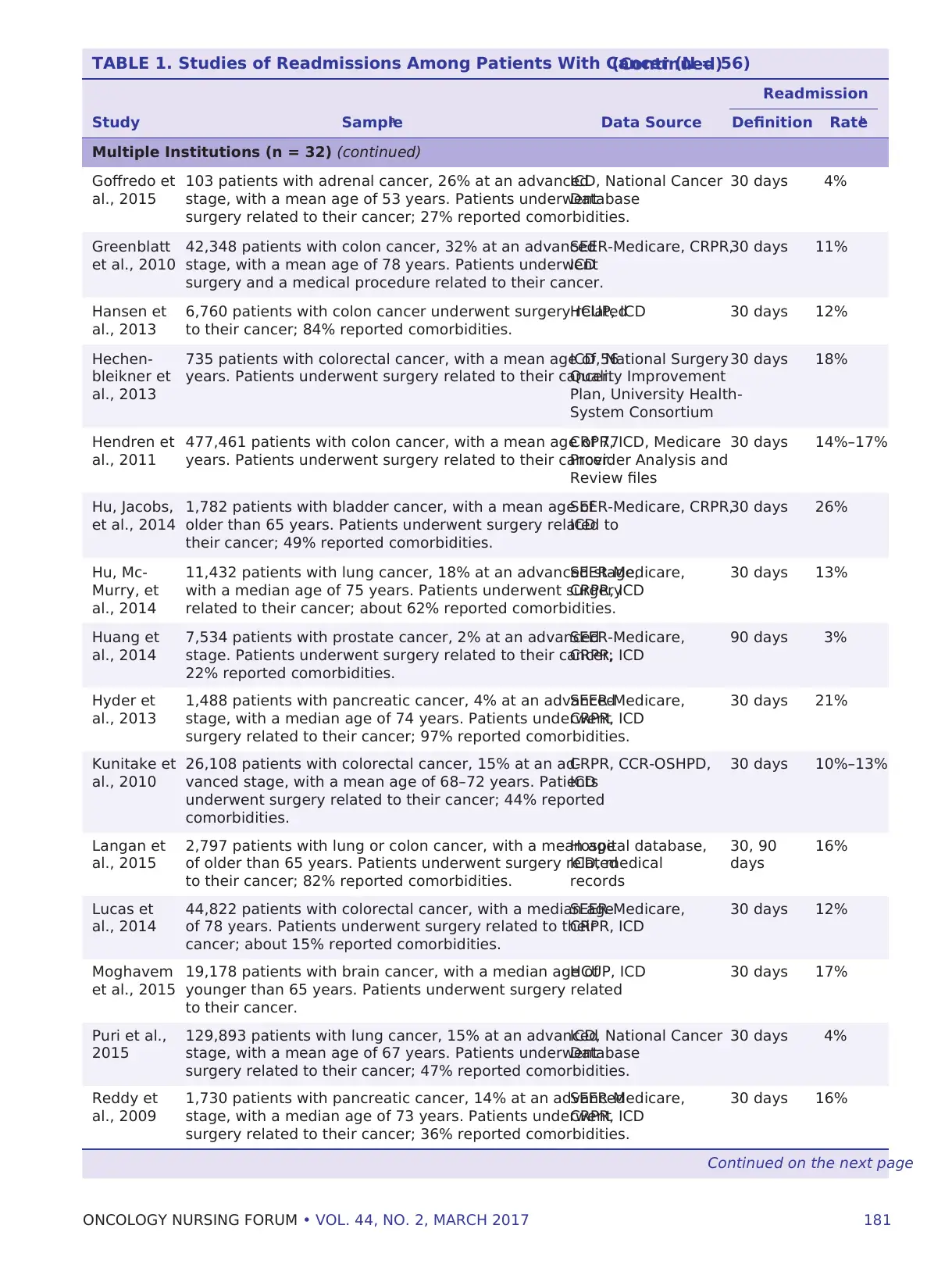
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)(Continued)
Readmission
Study Samplea Data Source Definition Rateb
Multiple Institutions (n = 32) (continued)
Goffredo et
al., 2015
103 patients with adrenal cancer, 26% at an advanced
stage, with a mean age of 53 years. Patients underwent
surgery related to their cancer; 27% reported comorbidities.
ICD, National Cancer
Database
30 days 4%
Greenblatt
et al., 2010
42,348 patients with colon cancer, 32% at an advanced
stage, with a mean age of 78 years. Patients underwent
surgery and a medical procedure related to their cancer.
SEER-Medicare, CRPR,
ICD
30 days 11%
Hansen et
al., 2013
6,760 patients with colon cancer underwent surgery related
to their cancer; 84% reported comorbidities.
HCUP, ICD 30 days 12%
Hechen-
bleikner et
al., 2013
735 patients with colorectal cancer, with a mean age of 56
years. Patients underwent surgery related to their cancer.
ICD, National Surgery
Quality Improvement
Plan, University Health-
System Consortium
30 days 18%
Hendren et
al., 2011
477,461 patients with colon cancer, with a mean age of 77
years. Patients underwent surgery related to their cancer.
CRPR, ICD, Medicare
Provider Analysis and
Review files
30 days 14%–17%
Hu, Jacobs,
et al., 2014
1,782 patients with bladder cancer, with a mean age of
older than 65 years. Patients underwent surgery related to
their cancer; 49% reported comorbidities.
SEER-Medicare, CRPR,
ICD
30 days 26%
Hu, Mc-
Murry, et
al., 2014
11,432 patients with lung cancer, 18% at an advanced stage,
with a median age of 75 years. Patients underwent surgery
related to their cancer; about 62% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 13%
Huang et
al., 2014
7,534 patients with prostate cancer, 2% at an advanced
stage. Patients underwent surgery related to their cancer;
22% reported comorbidities.
SEER-Medicare,
CRPR, ICD
90 days 3%
Hyder et
al., 2013
1,488 patients with pancreatic cancer, 4% at an advanced
stage, with a median age of 74 years. Patients underwent
surgery related to their cancer; 97% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 21%
Kunitake et
al., 2010
26,108 patients with colorectal cancer, 15% at an ad-
vanced stage, with a mean age of 68–72 years. Patients
underwent surgery related to their cancer; 44% reported
comorbidities.
CRPR, CCR-OSHPD,
ICD
30 days 10%–13%
Langan et
al., 2015
2,797 patients with lung or colon cancer, with a mean age
of older than 65 years. Patients underwent surgery related
to their cancer; 82% reported comorbidities.
Hospital database,
ICD, medical
records
30, 90
days
16%
Lucas et
al., 2014
44,822 patients with colorectal cancer, with a median age
of 78 years. Patients underwent surgery related to their
cancer; about 15% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 12%
Moghavem
et al., 2015
19,178 patients with brain cancer, with a median age of
younger than 65 years. Patients underwent surgery related
to their cancer.
HCUP, ICD 30 days 17%
Puri et al.,
2015
129,893 patients with lung cancer, 15% at an advanced
stage, with a mean age of 67 years. Patients underwent
surgery related to their cancer; 47% reported comorbidities.
ICD, National Cancer
Database
30 days 4%
Reddy et
al., 2009
1,730 patients with pancreatic cancer, 14% at an advanced
stage, with a median age of 73 years. Patients underwent
surgery related to their cancer; 36% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 16%
Continued on the next page
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)(Continued)
Readmission
Study Samplea Data Source Definition Rateb
Multiple Institutions (n = 32) (continued)
Goffredo et
al., 2015
103 patients with adrenal cancer, 26% at an advanced
stage, with a mean age of 53 years. Patients underwent
surgery related to their cancer; 27% reported comorbidities.
ICD, National Cancer
Database
30 days 4%
Greenblatt
et al., 2010
42,348 patients with colon cancer, 32% at an advanced
stage, with a mean age of 78 years. Patients underwent
surgery and a medical procedure related to their cancer.
SEER-Medicare, CRPR,
ICD
30 days 11%
Hansen et
al., 2013
6,760 patients with colon cancer underwent surgery related
to their cancer; 84% reported comorbidities.
HCUP, ICD 30 days 12%
Hechen-
bleikner et
al., 2013
735 patients with colorectal cancer, with a mean age of 56
years. Patients underwent surgery related to their cancer.
ICD, National Surgery
Quality Improvement
Plan, University Health-
System Consortium
30 days 18%
Hendren et
al., 2011
477,461 patients with colon cancer, with a mean age of 77
years. Patients underwent surgery related to their cancer.
CRPR, ICD, Medicare
Provider Analysis and
Review files
30 days 14%–17%
Hu, Jacobs,
et al., 2014
1,782 patients with bladder cancer, with a mean age of
older than 65 years. Patients underwent surgery related to
their cancer; 49% reported comorbidities.
SEER-Medicare, CRPR,
ICD
30 days 26%
Hu, Mc-
Murry, et
al., 2014
11,432 patients with lung cancer, 18% at an advanced stage,
with a median age of 75 years. Patients underwent surgery
related to their cancer; about 62% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 13%
Huang et
al., 2014
7,534 patients with prostate cancer, 2% at an advanced
stage. Patients underwent surgery related to their cancer;
22% reported comorbidities.
SEER-Medicare,
CRPR, ICD
90 days 3%
Hyder et
al., 2013
1,488 patients with pancreatic cancer, 4% at an advanced
stage, with a median age of 74 years. Patients underwent
surgery related to their cancer; 97% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 21%
Kunitake et
al., 2010
26,108 patients with colorectal cancer, 15% at an ad-
vanced stage, with a mean age of 68–72 years. Patients
underwent surgery related to their cancer; 44% reported
comorbidities.
CRPR, CCR-OSHPD,
ICD
30 days 10%–13%
Langan et
al., 2015
2,797 patients with lung or colon cancer, with a mean age
of older than 65 years. Patients underwent surgery related
to their cancer; 82% reported comorbidities.
Hospital database,
ICD, medical
records
30, 90
days
16%
Lucas et
al., 2014
44,822 patients with colorectal cancer, with a median age
of 78 years. Patients underwent surgery related to their
cancer; about 15% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 12%
Moghavem
et al., 2015
19,178 patients with brain cancer, with a median age of
younger than 65 years. Patients underwent surgery related
to their cancer.
HCUP, ICD 30 days 17%
Puri et al.,
2015
129,893 patients with lung cancer, 15% at an advanced
stage, with a mean age of 67 years. Patients underwent
surgery related to their cancer; 47% reported comorbidities.
ICD, National Cancer
Database
30 days 4%
Reddy et
al., 2009
1,730 patients with pancreatic cancer, 14% at an advanced
stage, with a median age of 73 years. Patients underwent
surgery related to their cancer; 36% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 16%
Continued on the next page
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
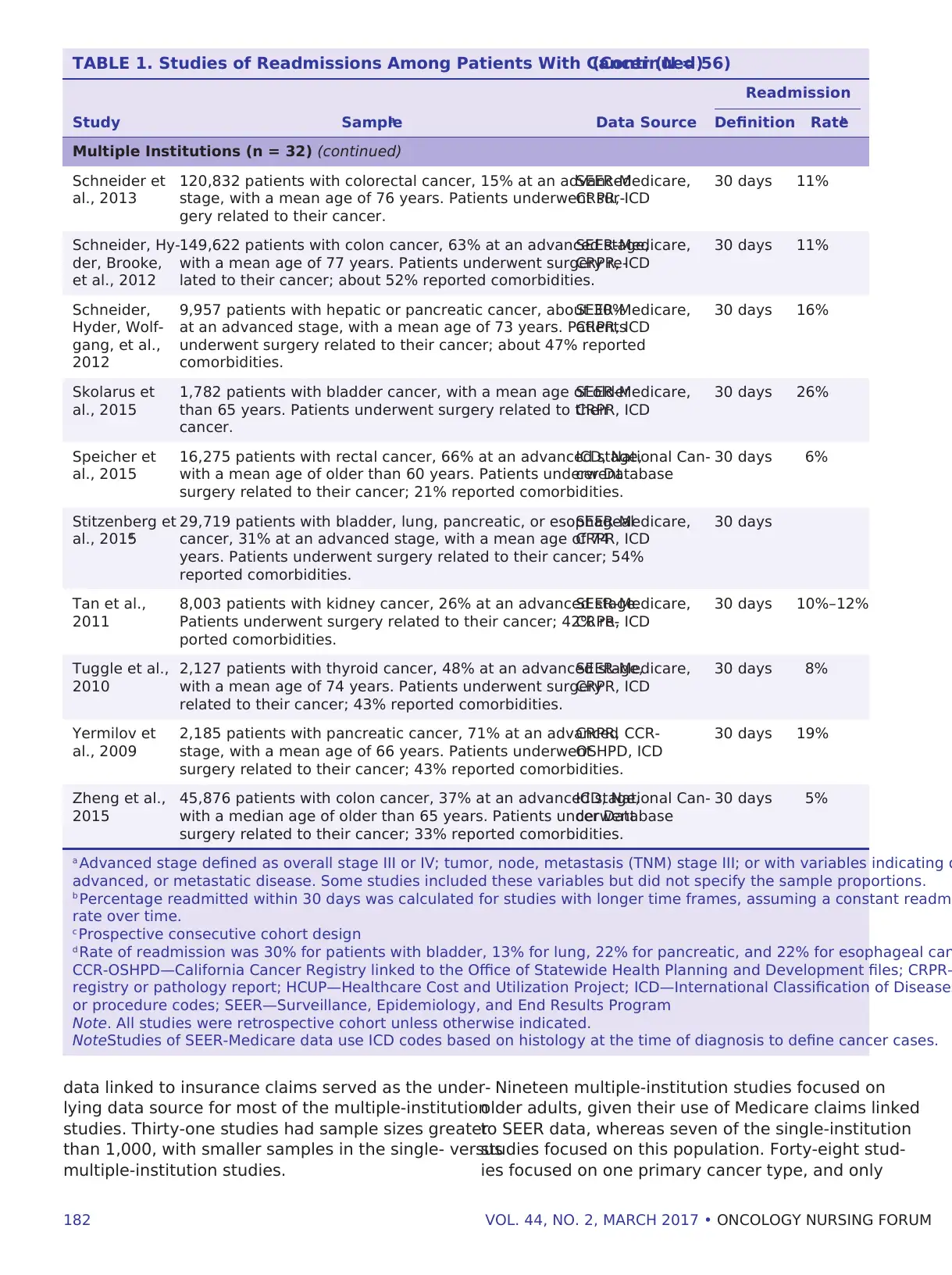
182 VOL. 44, NO. 2, MARCH 2017 • ONCOLOGY NURSING FORUM
data linked to insurance claims served as the under-
lying data source for most of the multiple-institution
studies. Thirty-one studies had sample sizes greater
than 1,000, with smaller samples in the single- versus
multiple-institution studies.
Nineteen multiple-institution studies focused on
older adults, given their use of Medicare claims linked
to SEER data, whereas seven of the single-institution
studies focused on this population. Forty-eight stud-
ies focused on one primary cancer type, and only
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)(Continued)
Readmission
Study Samplea Data Source Definition Rateb
Multiple Institutions (n = 32) (continued)
Schneider et
al., 2013
120,832 patients with colorectal cancer, 15% at an advanced
stage, with a mean age of 76 years. Patients underwent sur-
gery related to their cancer.
SEER-Medicare,
CRPR, ICD
30 days 11%
Schneider, Hy-
der, Brooke,
et al., 2012
149,622 patients with colon cancer, 63% at an advanced stage,
with a mean age of 77 years. Patients underwent surgery re-
lated to their cancer; about 52% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 11%
Schneider,
Hyder, Wolf-
gang, et al.,
2012
9,957 patients with hepatic or pancreatic cancer, about 30%
at an advanced stage, with a mean age of 73 years. Patients
underwent surgery related to their cancer; about 47% reported
comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 16%
Skolarus et
al., 2015
1,782 patients with bladder cancer, with a mean age of older
than 65 years. Patients underwent surgery related to their
cancer.
SEER-Medicare,
CRPR, ICD
30 days 26%
Speicher et
al., 2015
16,275 patients with rectal cancer, 66% at an advanced stage,
with a mean age of older than 60 years. Patients underwent
surgery related to their cancer; 21% reported comorbidities.
ICD, National Can-
cer Database
30 days 6%
Stitzenberg et
al., 2015d
29,719 patients with bladder, lung, pancreatic, or esophageal
cancer, 31% at an advanced stage, with a mean age of 74
years. Patients underwent surgery related to their cancer; 54%
reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days
Tan et al.,
2011
8,003 patients with kidney cancer, 26% at an advanced stage.
Patients underwent surgery related to their cancer; 42% re-
ported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 10%–12%
Tuggle et al.,
2010
2,127 patients with thyroid cancer, 48% at an advanced stage,
with a mean age of 74 years. Patients underwent surgery
related to their cancer; 43% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 8%
Yermilov et
al., 2009
2,185 patients with pancreatic cancer, 71% at an advanced
stage, with a mean age of 66 years. Patients underwent
surgery related to their cancer; 43% reported comorbidities.
CRPR, CCR-
OSHPD, ICD
30 days 19%
Zheng et al.,
2015
45,876 patients with colon cancer, 37% at an advanced stage,
with a median age of older than 65 years. Patients underwent
surgery related to their cancer; 33% reported comorbidities.
ICD, National Can-
cer Database
30 days 5%
a Advanced stage defined as overall stage III or IV; tumor, node, metastasis (TNM) stage III; or with variables indicating d
advanced, or metastatic disease. Some studies included these variables but did not specify the sample proportions.
b Percentage readmitted within 30 days was calculated for studies with longer time frames, assuming a constant readmi
rate over time.
c Prospective consecutive cohort design
d Rate of readmission was 30% for patients with bladder, 13% for lung, 22% for pancreatic, and 22% for esophageal can
CCR-OSHPD—California Cancer Registry linked to the Office of Statewide Health Planning and Development files; CRPR—
registry or pathology report; HCUP—Healthcare Cost and Utilization Project; ICD—International Classification of Diseases
or procedure codes; SEER—Surveillance, Epidemiology, and End Results Program
Note. All studies were retrospective cohort unless otherwise indicated.
Note.Studies of SEER-Medicare data use ICD codes based on histology at the time of diagnosis to define cancer cases.
data linked to insurance claims served as the under-
lying data source for most of the multiple-institution
studies. Thirty-one studies had sample sizes greater
than 1,000, with smaller samples in the single- versus
multiple-institution studies.
Nineteen multiple-institution studies focused on
older adults, given their use of Medicare claims linked
to SEER data, whereas seven of the single-institution
studies focused on this population. Forty-eight stud-
ies focused on one primary cancer type, and only
TABLE 1. Studies of Readmissions Among Patients With Cancer (N = 56)(Continued)
Readmission
Study Samplea Data Source Definition Rateb
Multiple Institutions (n = 32) (continued)
Schneider et
al., 2013
120,832 patients with colorectal cancer, 15% at an advanced
stage, with a mean age of 76 years. Patients underwent sur-
gery related to their cancer.
SEER-Medicare,
CRPR, ICD
30 days 11%
Schneider, Hy-
der, Brooke,
et al., 2012
149,622 patients with colon cancer, 63% at an advanced stage,
with a mean age of 77 years. Patients underwent surgery re-
lated to their cancer; about 52% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 11%
Schneider,
Hyder, Wolf-
gang, et al.,
2012
9,957 patients with hepatic or pancreatic cancer, about 30%
at an advanced stage, with a mean age of 73 years. Patients
underwent surgery related to their cancer; about 47% reported
comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 16%
Skolarus et
al., 2015
1,782 patients with bladder cancer, with a mean age of older
than 65 years. Patients underwent surgery related to their
cancer.
SEER-Medicare,
CRPR, ICD
30 days 26%
Speicher et
al., 2015
16,275 patients with rectal cancer, 66% at an advanced stage,
with a mean age of older than 60 years. Patients underwent
surgery related to their cancer; 21% reported comorbidities.
ICD, National Can-
cer Database
30 days 6%
Stitzenberg et
al., 2015d
29,719 patients with bladder, lung, pancreatic, or esophageal
cancer, 31% at an advanced stage, with a mean age of 74
years. Patients underwent surgery related to their cancer; 54%
reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days
Tan et al.,
2011
8,003 patients with kidney cancer, 26% at an advanced stage.
Patients underwent surgery related to their cancer; 42% re-
ported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 10%–12%
Tuggle et al.,
2010
2,127 patients with thyroid cancer, 48% at an advanced stage,
with a mean age of 74 years. Patients underwent surgery
related to their cancer; 43% reported comorbidities.
SEER-Medicare,
CRPR, ICD
30 days 8%
Yermilov et
al., 2009
2,185 patients with pancreatic cancer, 71% at an advanced
stage, with a mean age of 66 years. Patients underwent
surgery related to their cancer; 43% reported comorbidities.
CRPR, CCR-
OSHPD, ICD
30 days 19%
Zheng et al.,
2015
45,876 patients with colon cancer, 37% at an advanced stage,
with a median age of older than 65 years. Patients underwent
surgery related to their cancer; 33% reported comorbidities.
ICD, National Can-
cer Database
30 days 5%
a Advanced stage defined as overall stage III or IV; tumor, node, metastasis (TNM) stage III; or with variables indicating d
advanced, or metastatic disease. Some studies included these variables but did not specify the sample proportions.
b Percentage readmitted within 30 days was calculated for studies with longer time frames, assuming a constant readmi
rate over time.
c Prospective consecutive cohort design
d Rate of readmission was 30% for patients with bladder, 13% for lung, 22% for pancreatic, and 22% for esophageal can
CCR-OSHPD—California Cancer Registry linked to the Office of Statewide Health Planning and Development files; CRPR—
registry or pathology report; HCUP—Healthcare Cost and Utilization Project; ICD—International Classification of Diseases
or procedure codes; SEER—Surveillance, Epidemiology, and End Results Program
Note. All studies were retrospective cohort unless otherwise indicated.
Note.Studies of SEER-Medicare data use ICD codes based on histology at the time of diagnosis to define cancer cases.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ONCOLOGY NURSING FORUM • VOL. 44, NO. 2, MARCH 2017 183
two studies considered all cancer types. Thirty-three
studies accounted for cancer stage or comorbidities,
albeit with heterogeneous measures across the
studies.
Hospitalization within 30 days of discharge from
an index admission was the most commonly used
readmission definition, appearing in 50 studies. Of the
alternative definitions, most considered readmission
within 90 days, with the remainder using time periods
of as much as a year.
Rates of Readmission
The percentage of patients experiencing readmis-
sion within 30 days ranged from less than 3%–34%
across the reviewed studies. Thirty-five studies
reported readmission rates from 10%–19%, and the
highest rates were reported in studies of patients with
bladder, pancreatic, hematologic, and ovarian can-
cers. The lowest 30-day readmission rates were the
author-calculated rates, which had been presented
within longer time frames in the original studies.
Significant Predictors of Readmission
Across the studies with multivariable models (n =
30) examining predictors of readmission (see Table
2), comorbidities were consistently associated with
higher rates of readmission. Most studies controlled
for gender, with men having higher readmission rates
than women. Other patient factors associated with
significantly higher rates of readmission included
older age; more advanced disease as measured by
cancer stage, tumor size, or lymph node involvement;
low socioeconomic status; unmarried status; African
American (compared to Caucasian) and non-Hispanic
race/ethnicity; and dual eligible insurance status.
Residence in low population areas, rural areas, or the
Midwest or South was also associated with higher
readmission rates.
Surgical factors, such as postoperative complica-
tions and operative methods, were associated with
higher readmission rates, as were longer and shorter
index hospital stays and high and low hospital volume.
Other characteristics of the index hospitalization as-
sociated with higher rates included having a medical
(versus surgical) discharging physician, greater travel
distance, discharge to a place other than home, and
emergent admission.
Top Reported Reasons for Readmission
Of the studies reviewed, 31 reported reasons for
readmission, based primarily on ICD-9 CM codes for
the principal diagnosis. A tally of the top five reported
reasons for readmission (see Table 3) included gastro-
intestinal complications (e.g., nausea, vomiting, diar-
rhea, ileus), infection, nutritional complications (e.g.,
malnutrition, dehydration, failure to thrive), surgical
complications, and cardiopulmonary complications.
Other reasons included genitourinary complications,
disease progression or recurrence, coagulation disor-
ders, and pain.
Definitions of Preventability
Eleven studies considered whether readmissions
were potentially preventable (AlHilli et al., 2015;
Brown, Burgess, Li, Canter, & Bold, 2014; Fox, Tyler,
Vashi, Hsia, & Saxe, 2014; Glasgow, Shields, Vogel,
Teoh, & Argenta, 2014; Grant, Cooke, Bhatia, & For-
man, 2005; Hansen, Fox, Gross, & Bruun, 2013; Hech-
enbleikner et al., 2013; Hynes et al., 2004; Moghavem,
Morrison, Ratliff, & Hernandez-Boussard, 2015; Puri
et al., 2015; Tuggle, Park, Roman, Udelsman, & Sosa,
2010); only one study (Brown et al., 2014) evaluated
individual cases to assess their preventability. Brown
et al. (2014) concluded that 33% of readmissions
within seven days of the index hospitalization were
for issues deemed potentially preventable by the
authors, including nausea, vomiting, dehydration,
and postoperative pain, with improved discharge
follow-up, care coordination, and palliative care. Most
studies conceptualized readmissions as planned ver-
sus unplanned, using this dichotomy to identify and
exclude planned readmissions for chemotherapy,
radiotherapy, or rehabilitation (AlHilli et al., 2015;
Brown et al., 2014; Fox et al., 2014; Glasgow et al.,
2014; Hansen et al., 2013; Hechenbleikner et al., 2013;
Puri et al., 2015; Tuggle et al., 2010). Extensions to this
conceptualization included focusing on readmission
diagnosis related to initial admission (Brown et al.,
2014) and including only readmissions originating in
the emergency department (Fox et al., 2014).
In one study (Hynes et al., 2004), an expert panel
used an iterative consensus process to identify diag-
nosis codes for surgical complications (within 30–365
days of surgery) that could result in readmission;
however, the article did not specify whether these
complications were deemed potentially preventable.
In another study (Grant et al., 2005), readmissions
were conceptualized as unscheduled versus sched-
uled, again without an explicit definition, although
this categorization could have been determined
by the researchers through medical chart review.
Moghavem et al. (2015) examined “unplanned read-
missions” but did not provide a definition or other-
wise explain how these readmissions were identified.
Discussion
This systematic review of 56 studies indicated that
30-day hospital readmission rates among patients with
cancer were comparable to and sometimes exceeded
two studies considered all cancer types. Thirty-three
studies accounted for cancer stage or comorbidities,
albeit with heterogeneous measures across the
studies.
Hospitalization within 30 days of discharge from
an index admission was the most commonly used
readmission definition, appearing in 50 studies. Of the
alternative definitions, most considered readmission
within 90 days, with the remainder using time periods
of as much as a year.
Rates of Readmission
The percentage of patients experiencing readmis-
sion within 30 days ranged from less than 3%–34%
across the reviewed studies. Thirty-five studies
reported readmission rates from 10%–19%, and the
highest rates were reported in studies of patients with
bladder, pancreatic, hematologic, and ovarian can-
cers. The lowest 30-day readmission rates were the
author-calculated rates, which had been presented
within longer time frames in the original studies.
Significant Predictors of Readmission
Across the studies with multivariable models (n =
30) examining predictors of readmission (see Table
2), comorbidities were consistently associated with
higher rates of readmission. Most studies controlled
for gender, with men having higher readmission rates
than women. Other patient factors associated with
significantly higher rates of readmission included
older age; more advanced disease as measured by
cancer stage, tumor size, or lymph node involvement;
low socioeconomic status; unmarried status; African
American (compared to Caucasian) and non-Hispanic
race/ethnicity; and dual eligible insurance status.
Residence in low population areas, rural areas, or the
Midwest or South was also associated with higher
readmission rates.
Surgical factors, such as postoperative complica-
tions and operative methods, were associated with
higher readmission rates, as were longer and shorter
index hospital stays and high and low hospital volume.
Other characteristics of the index hospitalization as-
sociated with higher rates included having a medical
(versus surgical) discharging physician, greater travel
distance, discharge to a place other than home, and
emergent admission.
Top Reported Reasons for Readmission
Of the studies reviewed, 31 reported reasons for
readmission, based primarily on ICD-9 CM codes for
the principal diagnosis. A tally of the top five reported
reasons for readmission (see Table 3) included gastro-
intestinal complications (e.g., nausea, vomiting, diar-
rhea, ileus), infection, nutritional complications (e.g.,
malnutrition, dehydration, failure to thrive), surgical
complications, and cardiopulmonary complications.
Other reasons included genitourinary complications,
disease progression or recurrence, coagulation disor-
ders, and pain.
Definitions of Preventability
Eleven studies considered whether readmissions
were potentially preventable (AlHilli et al., 2015;
Brown, Burgess, Li, Canter, & Bold, 2014; Fox, Tyler,
Vashi, Hsia, & Saxe, 2014; Glasgow, Shields, Vogel,
Teoh, & Argenta, 2014; Grant, Cooke, Bhatia, & For-
man, 2005; Hansen, Fox, Gross, & Bruun, 2013; Hech-
enbleikner et al., 2013; Hynes et al., 2004; Moghavem,
Morrison, Ratliff, & Hernandez-Boussard, 2015; Puri
et al., 2015; Tuggle, Park, Roman, Udelsman, & Sosa,
2010); only one study (Brown et al., 2014) evaluated
individual cases to assess their preventability. Brown
et al. (2014) concluded that 33% of readmissions
within seven days of the index hospitalization were
for issues deemed potentially preventable by the
authors, including nausea, vomiting, dehydration,
and postoperative pain, with improved discharge
follow-up, care coordination, and palliative care. Most
studies conceptualized readmissions as planned ver-
sus unplanned, using this dichotomy to identify and
exclude planned readmissions for chemotherapy,
radiotherapy, or rehabilitation (AlHilli et al., 2015;
Brown et al., 2014; Fox et al., 2014; Glasgow et al.,
2014; Hansen et al., 2013; Hechenbleikner et al., 2013;
Puri et al., 2015; Tuggle et al., 2010). Extensions to this
conceptualization included focusing on readmission
diagnosis related to initial admission (Brown et al.,
2014) and including only readmissions originating in
the emergency department (Fox et al., 2014).
In one study (Hynes et al., 2004), an expert panel
used an iterative consensus process to identify diag-
nosis codes for surgical complications (within 30–365
days of surgery) that could result in readmission;
however, the article did not specify whether these
complications were deemed potentially preventable.
In another study (Grant et al., 2005), readmissions
were conceptualized as unscheduled versus sched-
uled, again without an explicit definition, although
this categorization could have been determined
by the researchers through medical chart review.
Moghavem et al. (2015) examined “unplanned read-
missions” but did not provide a definition or other-
wise explain how these readmissions were identified.
Discussion
This systematic review of 56 studies indicated that
30-day hospital readmission rates among patients with
cancer were comparable to and sometimes exceeded

184 VOL. 44, NO. 2, MARCH 2017 • ONCOLOGY NURSING FORUM
those of patients with cardiovascular (15%), cardiore-
spiratory (21%), and general medical (18%) conditions
(Horwitz et al., 2012; Macartney, Stacey, Carley, & Har-
rison, 2012; Van Walraven, Bennett, Jennings, Austin,
& Forster, 2011). The wide range of readmission rates
in this population is likely attributable to the heteroge-
neity of cancer case definitions, settings, and popula-
tions across the available literature—factors that also
complicate the comparison of rates across studies.
Collectively, the reviewed articles do not include
cancer-specific rates for all cancer types, pointing to
the need for future population-based research to more
fully enumerate cancer readmission rates.
The reported rates, particularly from single-institution
studies, may underestimate the true burden of read-
missions among patients with cancer because not all
studies in this review accounted for readmission to
different facilities. Readmissions do not always occur
at the index admitting facility; for instance, in a study
of patients discharged after pancreaticoduodenec-
tomy (Yermilov et al., 2009), 47% were readmitted to
different hospitals. This issue may be particularly sa-
lient if patients receiving ongoing care from relatively
distant regional cancer facilities seek local readmis-
sion for symptoms, such as pain or dehydration,
which may be effectively treated in ambulatory care
settings or with care management interventions. In
addition, some individuals may elect to seek care at
alternative hospitals because of perceived or actual
deficiencies in care during the index admission (RWJF,
2013), resulting in underestimates of readmissions
from poor quality care.
The studies focused almost exclusively on readmis-
sions following surgical procedures; few examined
readmissions following index admissions for nonsur-
gical indications, although one study (Brown et al.,
2014) reported that discharge by a physician with a
medical versus surgical specialty was a significant
predictor of readmission. The authors of the current
study would have preferred to present results sepa-
rately for readmissions following index medical ver-
sus surgical admissions; however, few studies focused
on readmissions following medical index admissions.
Studies or readmission after index hospitalizations
for medical indications are required to understand
differences in the reasons and risk factors for re-
admissions following index medical versus surgical
admissions. Such a focus is of particular importance,
as the results of the current review suggest that the
rates of readmission may be higher following an index
medical admission (Brown, Bornstein, & Wilcox, 2012;
Schneider et al., 2013).
The exemption of cancer specialty hospitals from
CMS readmission penalties and the exclusion of
medical oncology admissions from the hospitalwide,
all-cause unplanned readmission rate (Horwitz et al.,
2012) create the impression that the reasons and risk
factors for readmissions among patients with cancer
may differ from those of other inpatient groups. How-
ever, the authors found that many sociodemographic
predictors of readmission among patients with cancer
are consistent with those reported in other work
(Kansagara et al., 2011). Cancer-specific variables
(e.g., disease stage, treatment, operative method)
also had significant independent effects. The reasons
for readmissions across the studies were broadly
categorized, with the most reported complications
(e.g., gastrointestinal, infection, nutritional, surgical)
arguably preventable. Future research is needed to
better understand potentially preventable healthcare
use among patients with cancer, and to more fully
examine readmissions after medical procedures and
their underlying reasons.
As others have noted (Van Walraven et al., 2011),
the value of hospital readmissions as quality indica-
tors depends on the ability to identify the propor-
tion of avoidable readmissions. In one large study of
Medicare beneficiaries with the highest costs (Carroll
& Frakt, 2013), only 10% of spending was attributed
to preventable hospital (re)admissions or emergency
care, suggesting that a focus on readmission may
not yield the savings some have anticipated. The
extent to which this finding applies to readmission
among patients with cancer is unknown. Most of the
studies that considered the issue of preventability in
this review did so only indirectly. In fact, none of the
studies presented rates for the presumed preventable
readmissions as a proportion of all oncology readmis-
sions. Instead, they presented summary readmission
rates for only those hospitalizations meeting their
definition of potentially preventable. Although Brown
et al. (2014) concluded that 33% of readmissions
within seven days were because of issues deemed
potentially preventable, the rate was presented for
a subset already restricted to readmissions meeting
the University Health Consortium definition of related
readmissions, all of which are considered potentially
preventable (Hechenbleikner et al., 2013). Accord-
ingly, the rates presented in these studies cannot be
interpreted as the proportion of preventable readmis-
sions for patients with cancer.
The infrequent consideration of the preventability
of readmissions among patients with cancer may re-
flect the lack of consensus in the literature, generally,
about how to identify preventable healthcare use. A
review by Van Walraven et al. (2011) suggested that
5%–79% of readmissions for all conditions, including
cancer, may be preventable, with the wide-ranging
estimates resulting from the use of subjective criteria
to determine preventability. None of the reviewed
those of patients with cardiovascular (15%), cardiore-
spiratory (21%), and general medical (18%) conditions
(Horwitz et al., 2012; Macartney, Stacey, Carley, & Har-
rison, 2012; Van Walraven, Bennett, Jennings, Austin,
& Forster, 2011). The wide range of readmission rates
in this population is likely attributable to the heteroge-
neity of cancer case definitions, settings, and popula-
tions across the available literature—factors that also
complicate the comparison of rates across studies.
Collectively, the reviewed articles do not include
cancer-specific rates for all cancer types, pointing to
the need for future population-based research to more
fully enumerate cancer readmission rates.
The reported rates, particularly from single-institution
studies, may underestimate the true burden of read-
missions among patients with cancer because not all
studies in this review accounted for readmission to
different facilities. Readmissions do not always occur
at the index admitting facility; for instance, in a study
of patients discharged after pancreaticoduodenec-
tomy (Yermilov et al., 2009), 47% were readmitted to
different hospitals. This issue may be particularly sa-
lient if patients receiving ongoing care from relatively
distant regional cancer facilities seek local readmis-
sion for symptoms, such as pain or dehydration,
which may be effectively treated in ambulatory care
settings or with care management interventions. In
addition, some individuals may elect to seek care at
alternative hospitals because of perceived or actual
deficiencies in care during the index admission (RWJF,
2013), resulting in underestimates of readmissions
from poor quality care.
The studies focused almost exclusively on readmis-
sions following surgical procedures; few examined
readmissions following index admissions for nonsur-
gical indications, although one study (Brown et al.,
2014) reported that discharge by a physician with a
medical versus surgical specialty was a significant
predictor of readmission. The authors of the current
study would have preferred to present results sepa-
rately for readmissions following index medical ver-
sus surgical admissions; however, few studies focused
on readmissions following medical index admissions.
Studies or readmission after index hospitalizations
for medical indications are required to understand
differences in the reasons and risk factors for re-
admissions following index medical versus surgical
admissions. Such a focus is of particular importance,
as the results of the current review suggest that the
rates of readmission may be higher following an index
medical admission (Brown, Bornstein, & Wilcox, 2012;
Schneider et al., 2013).
The exemption of cancer specialty hospitals from
CMS readmission penalties and the exclusion of
medical oncology admissions from the hospitalwide,
all-cause unplanned readmission rate (Horwitz et al.,
2012) create the impression that the reasons and risk
factors for readmissions among patients with cancer
may differ from those of other inpatient groups. How-
ever, the authors found that many sociodemographic
predictors of readmission among patients with cancer
are consistent with those reported in other work
(Kansagara et al., 2011). Cancer-specific variables
(e.g., disease stage, treatment, operative method)
also had significant independent effects. The reasons
for readmissions across the studies were broadly
categorized, with the most reported complications
(e.g., gastrointestinal, infection, nutritional, surgical)
arguably preventable. Future research is needed to
better understand potentially preventable healthcare
use among patients with cancer, and to more fully
examine readmissions after medical procedures and
their underlying reasons.
As others have noted (Van Walraven et al., 2011),
the value of hospital readmissions as quality indica-
tors depends on the ability to identify the propor-
tion of avoidable readmissions. In one large study of
Medicare beneficiaries with the highest costs (Carroll
& Frakt, 2013), only 10% of spending was attributed
to preventable hospital (re)admissions or emergency
care, suggesting that a focus on readmission may
not yield the savings some have anticipated. The
extent to which this finding applies to readmission
among patients with cancer is unknown. Most of the
studies that considered the issue of preventability in
this review did so only indirectly. In fact, none of the
studies presented rates for the presumed preventable
readmissions as a proportion of all oncology readmis-
sions. Instead, they presented summary readmission
rates for only those hospitalizations meeting their
definition of potentially preventable. Although Brown
et al. (2014) concluded that 33% of readmissions
within seven days were because of issues deemed
potentially preventable, the rate was presented for
a subset already restricted to readmissions meeting
the University Health Consortium definition of related
readmissions, all of which are considered potentially
preventable (Hechenbleikner et al., 2013). Accord-
ingly, the rates presented in these studies cannot be
interpreted as the proportion of preventable readmis-
sions for patients with cancer.
The infrequent consideration of the preventability
of readmissions among patients with cancer may re-
flect the lack of consensus in the literature, generally,
about how to identify preventable healthcare use. A
review by Van Walraven et al. (2011) suggested that
5%–79% of readmissions for all conditions, including
cancer, may be preventable, with the wide-ranging
estimates resulting from the use of subjective criteria
to determine preventability. None of the reviewed
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
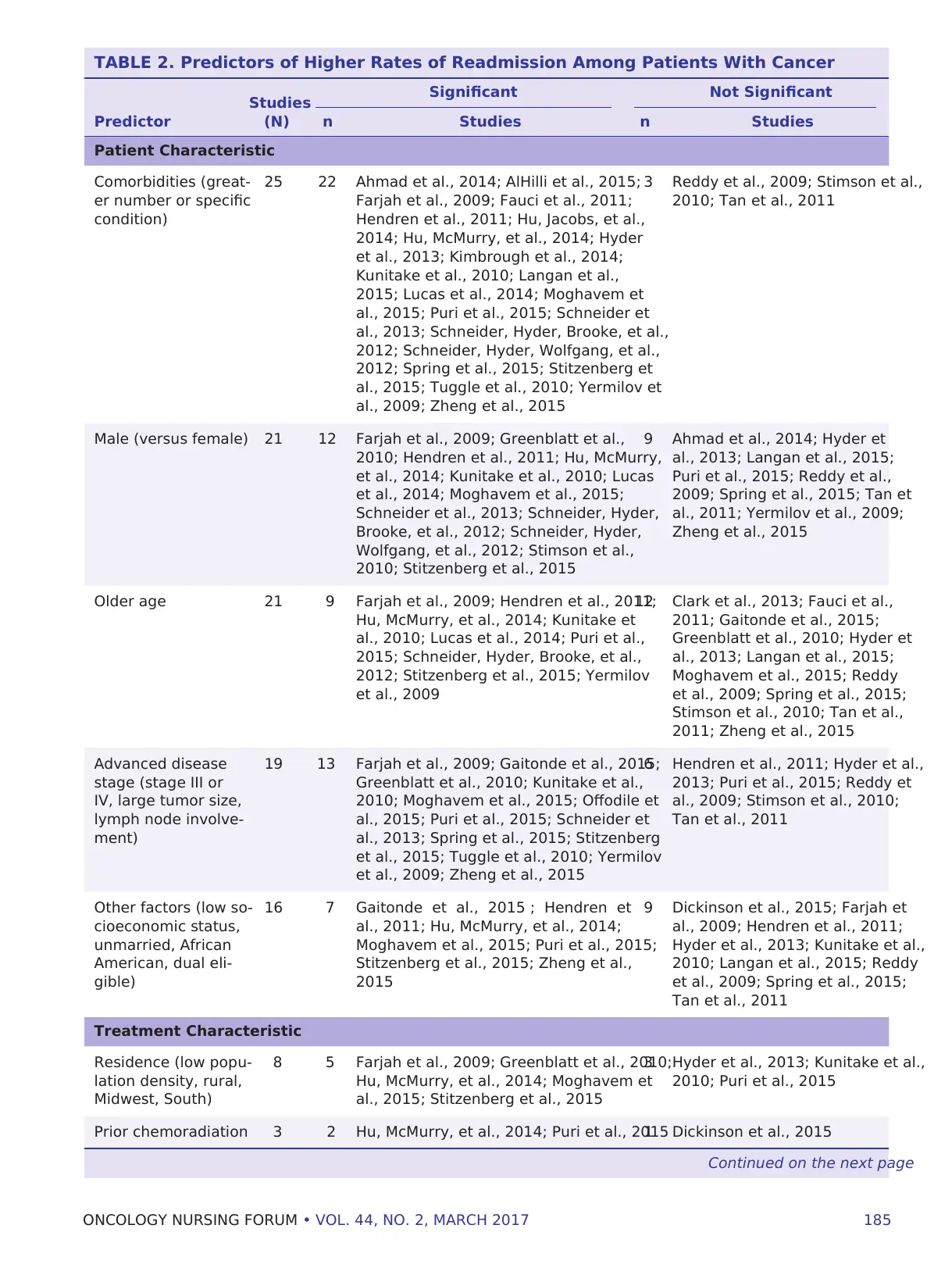
ONCOLOGY NURSING FORUM • VOL. 44, NO. 2, MARCH 2017 185
TABLE 2. Predictors of Higher Rates of Readmission Among Patients With Cancer
Studies
(N)
Significant Not Significant
Predictor n Studies n Studies
Patient Characteristic
Comorbidities (great-
er number or specific
condition)
25 22 Ahmad et al., 2014; AlHilli et al., 2015;
Farjah et al., 2009; Fauci et al., 2011;
Hendren et al., 2011; Hu, Jacobs, et al.,
2014; Hu, McMurry, et al., 2014; Hyder
et al., 2013; Kimbrough et al., 2014;
Kunitake et al., 2010; Langan et al.,
2015; Lucas et al., 2014; Moghavem et
al., 2015; Puri et al., 2015; Schneider et
al., 2013; Schneider, Hyder, Brooke, et al.,
2012; Schneider, Hyder, Wolfgang, et al.,
2012; Spring et al., 2015; Stitzenberg et
al., 2015; Tuggle et al., 2010; Yermilov et
al., 2009; Zheng et al., 2015
3 Reddy et al., 2009; Stimson et al.,
2010; Tan et al., 2011
Male (versus female) 21 12 Farjah et al., 2009; Greenblatt et al.,
2010; Hendren et al., 2011; Hu, McMurry,
et al., 2014; Kunitake et al., 2010; Lucas
et al., 2014; Moghavem et al., 2015;
Schneider et al., 2013; Schneider, Hyder,
Brooke, et al., 2012; Schneider, Hyder,
Wolfgang, et al., 2012; Stimson et al.,
2010; Stitzenberg et al., 2015
9 Ahmad et al., 2014; Hyder et
al., 2013; Langan et al., 2015;
Puri et al., 2015; Reddy et al.,
2009; Spring et al., 2015; Tan et
al., 2011; Yermilov et al., 2009;
Zheng et al., 2015
Older age 21 9 Farjah et al., 2009; Hendren et al., 2011;
Hu, McMurry, et al., 2014; Kunitake et
al., 2010; Lucas et al., 2014; Puri et al.,
2015; Schneider, Hyder, Brooke, et al.,
2012; Stitzenberg et al., 2015; Yermilov
et al., 2009
12 Clark et al., 2013; Fauci et al.,
2011; Gaitonde et al., 2015;
Greenblatt et al., 2010; Hyder et
al., 2013; Langan et al., 2015;
Moghavem et al., 2015; Reddy
et al., 2009; Spring et al., 2015;
Stimson et al., 2010; Tan et al.,
2011; Zheng et al., 2015
Advanced disease
stage (stage III or
IV, large tumor size,
lymph node involve-
ment)
19 13 Farjah et al., 2009; Gaitonde et al., 2015;
Greenblatt et al., 2010; Kunitake et al.,
2010; Moghavem et al., 2015; Offodile et
al., 2015; Puri et al., 2015; Schneider et
al., 2013; Spring et al., 2015; Stitzenberg
et al., 2015; Tuggle et al., 2010; Yermilov
et al., 2009; Zheng et al., 2015
6 Hendren et al., 2011; Hyder et al.,
2013; Puri et al., 2015; Reddy et
al., 2009; Stimson et al., 2010;
Tan et al., 2011
Other factors (low so-
cioeconomic status,
unmarried, African
American, dual eli-
gible)
16 7 Gaitonde et al., 2015 ; Hendren et
al., 2011; Hu, McMurry, et al., 2014;
Moghavem et al., 2015; Puri et al., 2015;
Stitzenberg et al., 2015; Zheng et al.,
2015
9 Dickinson et al., 2015; Farjah et
al., 2009; Hendren et al., 2011;
Hyder et al., 2013; Kunitake et al.,
2010; Langan et al., 2015; Reddy
et al., 2009; Spring et al., 2015;
Tan et al., 2011
Treatment Characteristic
Residence (low popu-
lation density, rural,
Midwest, South)
8 5 Farjah et al., 2009; Greenblatt et al., 2010;
Hu, McMurry, et al., 2014; Moghavem et
al., 2015; Stitzenberg et al., 2015
3 Hyder et al., 2013; Kunitake et al.,
2010; Puri et al., 2015
Prior chemoradiation 3 2 Hu, McMurry, et al., 2014; Puri et al., 20151 Dickinson et al., 2015
Continued on the next page
TABLE 2. Predictors of Higher Rates of Readmission Among Patients With Cancer
Studies
(N)
Significant Not Significant
Predictor n Studies n Studies
Patient Characteristic
Comorbidities (great-
er number or specific
condition)
25 22 Ahmad et al., 2014; AlHilli et al., 2015;
Farjah et al., 2009; Fauci et al., 2011;
Hendren et al., 2011; Hu, Jacobs, et al.,
2014; Hu, McMurry, et al., 2014; Hyder
et al., 2013; Kimbrough et al., 2014;
Kunitake et al., 2010; Langan et al.,
2015; Lucas et al., 2014; Moghavem et
al., 2015; Puri et al., 2015; Schneider et
al., 2013; Schneider, Hyder, Brooke, et al.,
2012; Schneider, Hyder, Wolfgang, et al.,
2012; Spring et al., 2015; Stitzenberg et
al., 2015; Tuggle et al., 2010; Yermilov et
al., 2009; Zheng et al., 2015
3 Reddy et al., 2009; Stimson et al.,
2010; Tan et al., 2011
Male (versus female) 21 12 Farjah et al., 2009; Greenblatt et al.,
2010; Hendren et al., 2011; Hu, McMurry,
et al., 2014; Kunitake et al., 2010; Lucas
et al., 2014; Moghavem et al., 2015;
Schneider et al., 2013; Schneider, Hyder,
Brooke, et al., 2012; Schneider, Hyder,
Wolfgang, et al., 2012; Stimson et al.,
2010; Stitzenberg et al., 2015
9 Ahmad et al., 2014; Hyder et
al., 2013; Langan et al., 2015;
Puri et al., 2015; Reddy et al.,
2009; Spring et al., 2015; Tan et
al., 2011; Yermilov et al., 2009;
Zheng et al., 2015
Older age 21 9 Farjah et al., 2009; Hendren et al., 2011;
Hu, McMurry, et al., 2014; Kunitake et
al., 2010; Lucas et al., 2014; Puri et al.,
2015; Schneider, Hyder, Brooke, et al.,
2012; Stitzenberg et al., 2015; Yermilov
et al., 2009
12 Clark et al., 2013; Fauci et al.,
2011; Gaitonde et al., 2015;
Greenblatt et al., 2010; Hyder et
al., 2013; Langan et al., 2015;
Moghavem et al., 2015; Reddy
et al., 2009; Spring et al., 2015;
Stimson et al., 2010; Tan et al.,
2011; Zheng et al., 2015
Advanced disease
stage (stage III or
IV, large tumor size,
lymph node involve-
ment)
19 13 Farjah et al., 2009; Gaitonde et al., 2015;
Greenblatt et al., 2010; Kunitake et al.,
2010; Moghavem et al., 2015; Offodile et
al., 2015; Puri et al., 2015; Schneider et
al., 2013; Spring et al., 2015; Stitzenberg
et al., 2015; Tuggle et al., 2010; Yermilov
et al., 2009; Zheng et al., 2015
6 Hendren et al., 2011; Hyder et al.,
2013; Puri et al., 2015; Reddy et
al., 2009; Stimson et al., 2010;
Tan et al., 2011
Other factors (low so-
cioeconomic status,
unmarried, African
American, dual eli-
gible)
16 7 Gaitonde et al., 2015 ; Hendren et
al., 2011; Hu, McMurry, et al., 2014;
Moghavem et al., 2015; Puri et al., 2015;
Stitzenberg et al., 2015; Zheng et al.,
2015
9 Dickinson et al., 2015; Farjah et
al., 2009; Hendren et al., 2011;
Hyder et al., 2013; Kunitake et al.,
2010; Langan et al., 2015; Reddy
et al., 2009; Spring et al., 2015;
Tan et al., 2011
Treatment Characteristic
Residence (low popu-
lation density, rural,
Midwest, South)
8 5 Farjah et al., 2009; Greenblatt et al., 2010;
Hu, McMurry, et al., 2014; Moghavem et
al., 2015; Stitzenberg et al., 2015
3 Hyder et al., 2013; Kunitake et al.,
2010; Puri et al., 2015
Prior chemoradiation 3 2 Hu, McMurry, et al., 2014; Puri et al., 20151 Dickinson et al., 2015
Continued on the next page
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
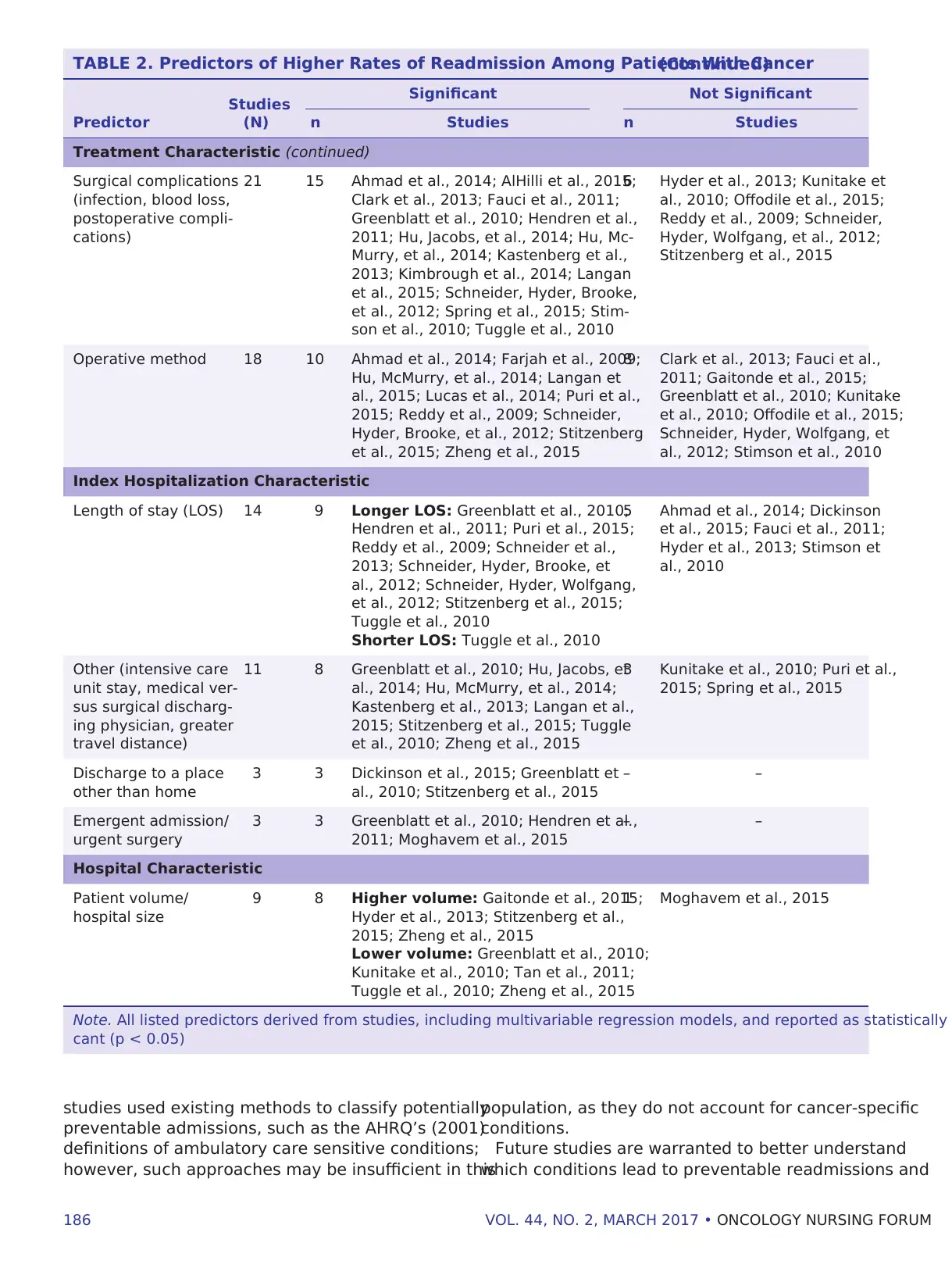
186 VOL. 44, NO. 2, MARCH 2017 • ONCOLOGY NURSING FORUM
studies used existing methods to classify potentially
preventable admissions, such as the AHRQ’s (2001)
definitions of ambulatory care sensitive conditions;
however, such approaches may be insufficient in this
population, as they do not account for cancer-specific
conditions.
Future studies are warranted to better understand
which conditions lead to preventable readmissions and
TABLE 2. Predictors of Higher Rates of Readmission Among Patients With Cancer(Continued)
Studies
(N)
Significant Not Significant
Predictor n Studies n Studies
Treatment Characteristic (continued)
Surgical complications
(infection, blood loss,
postoperative compli-
cations)
21 15 Ahmad et al., 2014; AlHilli et al., 2015;
Clark et al., 2013; Fauci et al., 2011;
Greenblatt et al., 2010; Hendren et al.,
2011; Hu, Jacobs, et al., 2014; Hu, Mc-
Murry, et al., 2014; Kastenberg et al.,
2013; Kimbrough et al., 2014; Langan
et al., 2015; Schneider, Hyder, Brooke,
et al., 2012; Spring et al., 2015; Stim-
son et al., 2010; Tuggle et al., 2010
6 Hyder et al., 2013; Kunitake et
al., 2010; Offodile et al., 2015;
Reddy et al., 2009; Schneider,
Hyder, Wolfgang, et al., 2012;
Stitzenberg et al., 2015
Operative method 18 10 Ahmad et al., 2014; Farjah et al., 2009;
Hu, McMurry, et al., 2014; Langan et
al., 2015; Lucas et al., 2014; Puri et al.,
2015; Reddy et al., 2009; Schneider,
Hyder, Brooke, et al., 2012; Stitzenberg
et al., 2015; Zheng et al., 2015
8 Clark et al., 2013; Fauci et al.,
2011; Gaitonde et al., 2015;
Greenblatt et al., 2010; Kunitake
et al., 2010; Offodile et al., 2015;
Schneider, Hyder, Wolfgang, et
al., 2012; Stimson et al., 2010
Index Hospitalization Characteristic
Length of stay (LOS) 14 9 Longer LOS: Greenblatt et al., 2010;
Hendren et al., 2011; Puri et al., 2015;
Reddy et al., 2009; Schneider et al.,
2013; Schneider, Hyder, Brooke, et
al., 2012; Schneider, Hyder, Wolfgang,
et al., 2012; Stitzenberg et al., 2015;
Tuggle et al., 2010
Shorter LOS: Tuggle et al., 2010
5 Ahmad et al., 2014; Dickinson
et al., 2015; Fauci et al., 2011;
Hyder et al., 2013; Stimson et
al., 2010
Other (intensive care
unit stay, medical ver-
sus surgical discharg-
ing physician, greater
travel distance)
11 8 Greenblatt et al., 2010; Hu, Jacobs, et
al., 2014; Hu, McMurry, et al., 2014;
Kastenberg et al., 2013; Langan et al.,
2015; Stitzenberg et al., 2015; Tuggle
et al., 2010; Zheng et al., 2015
3 Kunitake et al., 2010; Puri et al.,
2015; Spring et al., 2015
Discharge to a place
other than home
3 3 Dickinson et al., 2015; Greenblatt et
al., 2010; Stitzenberg et al., 2015
– –
Emergent admission/
urgent surgery
3 3 Greenblatt et al., 2010; Hendren et al.,
2011; Moghavem et al., 2015
– –
Hospital Characteristic
Patient volume/
hospital size
9 8 Higher volume: Gaitonde et al., 2015;
Hyder et al., 2013; Stitzenberg et al.,
2015; Zheng et al., 2015
Lower volume: Greenblatt et al., 2010;
Kunitake et al., 2010; Tan et al., 2011;
Tuggle et al., 2010; Zheng et al., 2015
1 Moghavem et al., 2015
Note. All listed predictors derived from studies, including multivariable regression models, and reported as statistically
cant (p < 0.05)
studies used existing methods to classify potentially
preventable admissions, such as the AHRQ’s (2001)
definitions of ambulatory care sensitive conditions;
however, such approaches may be insufficient in this
population, as they do not account for cancer-specific
conditions.
Future studies are warranted to better understand
which conditions lead to preventable readmissions and
TABLE 2. Predictors of Higher Rates of Readmission Among Patients With Cancer(Continued)
Studies
(N)
Significant Not Significant
Predictor n Studies n Studies
Treatment Characteristic (continued)
Surgical complications
(infection, blood loss,
postoperative compli-
cations)
21 15 Ahmad et al., 2014; AlHilli et al., 2015;
Clark et al., 2013; Fauci et al., 2011;
Greenblatt et al., 2010; Hendren et al.,
2011; Hu, Jacobs, et al., 2014; Hu, Mc-
Murry, et al., 2014; Kastenberg et al.,
2013; Kimbrough et al., 2014; Langan
et al., 2015; Schneider, Hyder, Brooke,
et al., 2012; Spring et al., 2015; Stim-
son et al., 2010; Tuggle et al., 2010
6 Hyder et al., 2013; Kunitake et
al., 2010; Offodile et al., 2015;
Reddy et al., 2009; Schneider,
Hyder, Wolfgang, et al., 2012;
Stitzenberg et al., 2015
Operative method 18 10 Ahmad et al., 2014; Farjah et al., 2009;
Hu, McMurry, et al., 2014; Langan et
al., 2015; Lucas et al., 2014; Puri et al.,
2015; Reddy et al., 2009; Schneider,
Hyder, Brooke, et al., 2012; Stitzenberg
et al., 2015; Zheng et al., 2015
8 Clark et al., 2013; Fauci et al.,
2011; Gaitonde et al., 2015;
Greenblatt et al., 2010; Kunitake
et al., 2010; Offodile et al., 2015;
Schneider, Hyder, Wolfgang, et
al., 2012; Stimson et al., 2010
Index Hospitalization Characteristic
Length of stay (LOS) 14 9 Longer LOS: Greenblatt et al., 2010;
Hendren et al., 2011; Puri et al., 2015;
Reddy et al., 2009; Schneider et al.,
2013; Schneider, Hyder, Brooke, et
al., 2012; Schneider, Hyder, Wolfgang,
et al., 2012; Stitzenberg et al., 2015;
Tuggle et al., 2010
Shorter LOS: Tuggle et al., 2010
5 Ahmad et al., 2014; Dickinson
et al., 2015; Fauci et al., 2011;
Hyder et al., 2013; Stimson et
al., 2010
Other (intensive care
unit stay, medical ver-
sus surgical discharg-
ing physician, greater
travel distance)
11 8 Greenblatt et al., 2010; Hu, Jacobs, et
al., 2014; Hu, McMurry, et al., 2014;
Kastenberg et al., 2013; Langan et al.,
2015; Stitzenberg et al., 2015; Tuggle
et al., 2010; Zheng et al., 2015
3 Kunitake et al., 2010; Puri et al.,
2015; Spring et al., 2015
Discharge to a place
other than home
3 3 Dickinson et al., 2015; Greenblatt et
al., 2010; Stitzenberg et al., 2015
– –
Emergent admission/
urgent surgery
3 3 Greenblatt et al., 2010; Hendren et al.,
2011; Moghavem et al., 2015
– –
Hospital Characteristic
Patient volume/
hospital size
9 8 Higher volume: Gaitonde et al., 2015;
Hyder et al., 2013; Stitzenberg et al.,
2015; Zheng et al., 2015
Lower volume: Greenblatt et al., 2010;
Kunitake et al., 2010; Tan et al., 2011;
Tuggle et al., 2010; Zheng et al., 2015
1 Moghavem et al., 2015
Note. All listed predictors derived from studies, including multivariable regression models, and reported as statistically
cant (p < 0.05)

ONCOLOGY NURSING FORUM • VOL. 44, NO. 2, MARCH 2017 187
whether discharge follow-up, care coordination, and
palliative care interventions can reduce readmission
rates among patients with cancer. Such efforts are
consistent with national recommendations that hospi-
tal staff interview patients and caregivers to elicit the
“story behind the story” to better understand their ex-
periences of communication, coordination, or logistical
barriers leading to readmission (AHRQ, 2014).
The authors opted not to grade the quality of the
evidence in this review for several reasons. First, they
reported unadjusted readmission rates rather the
measured effects of any exposure or intervention.
Second, none of the studies could be rated as produc-
ing the highest quality evidence because random-
ized, controlled trials were inapplicable. Third, they
separated single- versus multiple-institution studies,
which could be viewed as lower versus higher quality
evidence, respectively.
Limitations
Given the reliance on secondary analysis of extant
administrative or clinical data, most of the reviewed
studies included risk of bias. Administrative data
may underreport untreated comorbid conditions or
those reimbursed as part of the hospital stay (e.g.,
substance abuse, mental health conditions) and,
subsequently, underestimate the effects of these
conditions on readmissions. In addition, most of the
studies lacked variables to adequately measure so-
cioeconomic status, social support, self-care ability,
transportation, health literacy, receipt of timely or
ongoing follow-up care, or the quality of discharge
instructions. Accordingly, the effect of these variables
on readmission is unknown, although they may be
just as important as those reported, or perhaps even
more salient. Also, most of the studies either excluded
or did not describe the proportion of individuals who
died within 30 days of hospital discharge, therefore in-
troducing bias from semicompeting risk (i.e., reduced
readmission rates attributable to death), which may
be particularly applicable to patients with advanced
cancer.
Interpretation of the findings from this review is
subject to additional limitations. The abstraction and
classification are subject to interpretation, although
this subjectivity was mitigated through a dual review
and consensus process. The authors may have inad-
vertently missed relevant publications that included
readmission rates among patients with cancer in
their review; however, additional studies changing
TABLE 3. Leading Reported Reasons for Patient Readmission
Variable
Studies
(N) Studies Reporting Finding
Gastrointestinal com-
plications (ileus, colitis,
nausea, vomiting, and
diarrhea)
24 Ahmad et al., 2014; AlHilli et al., 2015; Brown et al., 2014; Clark et al., 2013; Fauci e
al., 2011; Glasgow et al., 2014; Grant et al., 2005; Greenblatt et al., 2010; Gustafson
al., 2012; Hansen et al., 2013; Hari & Rosenzweig, 2012; Hu, Jacobs, et al., 2014; Hu
McMurry, et al., 2014; Hyder et al., 2013; Kimbrough et al., 2014; Langan et al., 201
Liang et al., 2013; Offodile et al., 2015; Schneider et al., 2013; Stimson et al., 2010;
Tamandl et al., 2015; White et al., 2015; Worley et al., 2013; Yermilov et al., 2009
Infection (fever, cellulitis,
septicemia)
21 AlHilli et al., 2015; Brown et al., 2014; Dickinson et al., 2015; Grant et al., 2005; Gre
blatt et al., 2010; Hansen et al., 2013; Hari & Rosenzweig, 2012; Hu, Jacobs, et al., 2
Hu, McMurry, et al., 2014; Kastenberg et al., 2013; Kimbrough et al., 2014; Kunitake
al., 2010; Liang et al., 2013; Moghavem et al., 2015; Offodile et al., 2015; Schneider
et al., 2013; Schneider, Hyder, Brooke, et al., 2012; Tamandl et al., 2015; White et a
2015; Worley et al., 2013; Yermilov et al., 2009
Nutritional complications
(dehydration, malnutri-
tion, failure to thrive)
17 Ahmad et al., 2014; AlHilli et al., 2015; Brown et al., 2014; Glasgow et al., 2014; Gra
et al., 2005; Hansen et al., 2013; Hari & Rosenzweig, 2012; Hu, Jacobs et al., 2014;
McMurry et al., 2014; Hyder et al., 2013; Kimbrough et al., 2014; Schneider et al., 2
Schneider, Hyder, Brooke, et al., 2012; Stimson et al., 2010, White et al., 2015; Wor
et al, 2013; Yermilov et al., 2009
Surgical complications
(blood loss, postopera-
tive complications)
13 Ahmad et al., 2014; Clark et al., 2013; Fauci et al., 2011; Greenblatt et al., 2010; Ha
et al., 2013; Langan et al., 2015; Moghavem et al., 2015; Offodile et al., 2015; Redd
et al., 2009; Schneider et al., 2012; Tuggle et al., 2010; White et al., 2015; Yermilov
al., 2009
Cardiopulmonary com-
plications (respiratory
complaints, pneumonia)
11 Ahmad et al., 2014; Fauci et al., 2011; Greenblatt et al., 2010; Hu, McMurry, et al., 2
Hyder et al., 2013; Kimbrough et al., 2014; Moghavem et al., 2015; Langan et al., 20
Tamandl et al., 2015; Tuggle et al., 2010; White et al., 2015
whether discharge follow-up, care coordination, and
palliative care interventions can reduce readmission
rates among patients with cancer. Such efforts are
consistent with national recommendations that hospi-
tal staff interview patients and caregivers to elicit the
“story behind the story” to better understand their ex-
periences of communication, coordination, or logistical
barriers leading to readmission (AHRQ, 2014).
The authors opted not to grade the quality of the
evidence in this review for several reasons. First, they
reported unadjusted readmission rates rather the
measured effects of any exposure or intervention.
Second, none of the studies could be rated as produc-
ing the highest quality evidence because random-
ized, controlled trials were inapplicable. Third, they
separated single- versus multiple-institution studies,
which could be viewed as lower versus higher quality
evidence, respectively.
Limitations
Given the reliance on secondary analysis of extant
administrative or clinical data, most of the reviewed
studies included risk of bias. Administrative data
may underreport untreated comorbid conditions or
those reimbursed as part of the hospital stay (e.g.,
substance abuse, mental health conditions) and,
subsequently, underestimate the effects of these
conditions on readmissions. In addition, most of the
studies lacked variables to adequately measure so-
cioeconomic status, social support, self-care ability,
transportation, health literacy, receipt of timely or
ongoing follow-up care, or the quality of discharge
instructions. Accordingly, the effect of these variables
on readmission is unknown, although they may be
just as important as those reported, or perhaps even
more salient. Also, most of the studies either excluded
or did not describe the proportion of individuals who
died within 30 days of hospital discharge, therefore in-
troducing bias from semicompeting risk (i.e., reduced
readmission rates attributable to death), which may
be particularly applicable to patients with advanced
cancer.
Interpretation of the findings from this review is
subject to additional limitations. The abstraction and
classification are subject to interpretation, although
this subjectivity was mitigated through a dual review
and consensus process. The authors may have inad-
vertently missed relevant publications that included
readmission rates among patients with cancer in
their review; however, additional studies changing
TABLE 3. Leading Reported Reasons for Patient Readmission
Variable
Studies
(N) Studies Reporting Finding
Gastrointestinal com-
plications (ileus, colitis,
nausea, vomiting, and
diarrhea)
24 Ahmad et al., 2014; AlHilli et al., 2015; Brown et al., 2014; Clark et al., 2013; Fauci e
al., 2011; Glasgow et al., 2014; Grant et al., 2005; Greenblatt et al., 2010; Gustafson
al., 2012; Hansen et al., 2013; Hari & Rosenzweig, 2012; Hu, Jacobs, et al., 2014; Hu
McMurry, et al., 2014; Hyder et al., 2013; Kimbrough et al., 2014; Langan et al., 201
Liang et al., 2013; Offodile et al., 2015; Schneider et al., 2013; Stimson et al., 2010;
Tamandl et al., 2015; White et al., 2015; Worley et al., 2013; Yermilov et al., 2009
Infection (fever, cellulitis,
septicemia)
21 AlHilli et al., 2015; Brown et al., 2014; Dickinson et al., 2015; Grant et al., 2005; Gre
blatt et al., 2010; Hansen et al., 2013; Hari & Rosenzweig, 2012; Hu, Jacobs, et al., 2
Hu, McMurry, et al., 2014; Kastenberg et al., 2013; Kimbrough et al., 2014; Kunitake
al., 2010; Liang et al., 2013; Moghavem et al., 2015; Offodile et al., 2015; Schneider
et al., 2013; Schneider, Hyder, Brooke, et al., 2012; Tamandl et al., 2015; White et a
2015; Worley et al., 2013; Yermilov et al., 2009
Nutritional complications
(dehydration, malnutri-
tion, failure to thrive)
17 Ahmad et al., 2014; AlHilli et al., 2015; Brown et al., 2014; Glasgow et al., 2014; Gra
et al., 2005; Hansen et al., 2013; Hari & Rosenzweig, 2012; Hu, Jacobs et al., 2014;
McMurry et al., 2014; Hyder et al., 2013; Kimbrough et al., 2014; Schneider et al., 2
Schneider, Hyder, Brooke, et al., 2012; Stimson et al., 2010, White et al., 2015; Wor
et al, 2013; Yermilov et al., 2009
Surgical complications
(blood loss, postopera-
tive complications)
13 Ahmad et al., 2014; Clark et al., 2013; Fauci et al., 2011; Greenblatt et al., 2010; Ha
et al., 2013; Langan et al., 2015; Moghavem et al., 2015; Offodile et al., 2015; Redd
et al., 2009; Schneider et al., 2012; Tuggle et al., 2010; White et al., 2015; Yermilov
al., 2009
Cardiopulmonary com-
plications (respiratory
complaints, pneumonia)
11 Ahmad et al., 2014; Fauci et al., 2011; Greenblatt et al., 2010; Hu, McMurry, et al., 2
Hyder et al., 2013; Kimbrough et al., 2014; Moghavem et al., 2015; Langan et al., 20
Tamandl et al., 2015; Tuggle et al., 2010; White et al., 2015
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
