Technologies to detect the Prion
VerifiedAdded on 2023/01/06
|17
|3009
|57
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Technologies to detect the Prion
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Table of Contents
Abstract............................................................................................................................................1
Introduction......................................................................................................................................1
Table................................................................................................................................................2
Description of five technologies......................................................................................................7
Protein Misfolding Cyclic Amplification (PMCA) Application.................................................7
Immunohistochemical Detection.................................................................................................9
Enzyme-linked immunosorbent assay (ELISA)........................................................................10
The Real-Time Quaking-Induced Conversion (RT-QuIC) assay..............................................11
Streptomycin detection..............................................................................................................12
Conclusion.....................................................................................................................................13
References......................................................................................................................................14
Abstract............................................................................................................................................1
Introduction......................................................................................................................................1
Table................................................................................................................................................2
Description of five technologies......................................................................................................7
Protein Misfolding Cyclic Amplification (PMCA) Application.................................................7
Immunohistochemical Detection.................................................................................................9
Enzyme-linked immunosorbent assay (ELISA)........................................................................10
The Real-Time Quaking-Induced Conversion (RT-QuIC) assay..............................................11
Streptomycin detection..............................................................................................................12
Conclusion.....................................................................................................................................13
References......................................................................................................................................14


Abstract
Prion diseases is comprised with several conditions, where a prion is one of the type of
protein which can be triggered the normal proteins in human brain in order to fold abnormally.
Such diseases can affect both animals and man as well as sometimes can spread to humans
through infected beef and other meat products. Creutzfeldt-Jakob disease (CJD) refers to the
most common form of prion disease which highly affects humans. Therefore, a number of
technologies are demonstrated in this report that are used for detecting prions at early stage, so
that treatments can be given prior for improving quality of life.
Introduction
The Protein has been known for its beneficial roles in the organisms since it was recognized
as early as the 18th century until 1967, when Griffith assumed that some proteins could be
infectious and they are the pathogens in scrapie. Scrapie is a fatal disease that affects the central
nervous system of sheep and goats. Griffith’s hypothesis was unsupported by evidence until
1982 when Prusiner and co-workers had purified the pathogens in the brain of hamsters infected
by scrapie, they found particles consisting of a specific protein that they called a “prion”. Over
the last 30 years the prion hypothesis has proven that the prion is responsible for many
transmissible spongiform encephalopathies (TSEs) or “prion diseases” in humans and mammals,
including Creutzfeldt-Jakob disease (CJD), Kuru and mad cow disease.
A Prion (PrP) is a protein that is naturally synthesized in the body and it is expressed by the gene
PRNP. The main function of Prion is obscure, however, researchers have suggested that it plays
an important role in some essential processes in the brain such as the formation of synapses that
connect between neurons and transport of copper. Mutations in PRNP resulting in abnormal
protein synthesis known as PrPSc. This gene is located in the short (p) arm of chromosome 20 at
position 13.
1
Prion diseases is comprised with several conditions, where a prion is one of the type of
protein which can be triggered the normal proteins in human brain in order to fold abnormally.
Such diseases can affect both animals and man as well as sometimes can spread to humans
through infected beef and other meat products. Creutzfeldt-Jakob disease (CJD) refers to the
most common form of prion disease which highly affects humans. Therefore, a number of
technologies are demonstrated in this report that are used for detecting prions at early stage, so
that treatments can be given prior for improving quality of life.
Introduction
The Protein has been known for its beneficial roles in the organisms since it was recognized
as early as the 18th century until 1967, when Griffith assumed that some proteins could be
infectious and they are the pathogens in scrapie. Scrapie is a fatal disease that affects the central
nervous system of sheep and goats. Griffith’s hypothesis was unsupported by evidence until
1982 when Prusiner and co-workers had purified the pathogens in the brain of hamsters infected
by scrapie, they found particles consisting of a specific protein that they called a “prion”. Over
the last 30 years the prion hypothesis has proven that the prion is responsible for many
transmissible spongiform encephalopathies (TSEs) or “prion diseases” in humans and mammals,
including Creutzfeldt-Jakob disease (CJD), Kuru and mad cow disease.
A Prion (PrP) is a protein that is naturally synthesized in the body and it is expressed by the gene
PRNP. The main function of Prion is obscure, however, researchers have suggested that it plays
an important role in some essential processes in the brain such as the formation of synapses that
connect between neurons and transport of copper. Mutations in PRNP resulting in abnormal
protein synthesis known as PrPSc. This gene is located in the short (p) arm of chromosome 20 at
position 13.
1
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
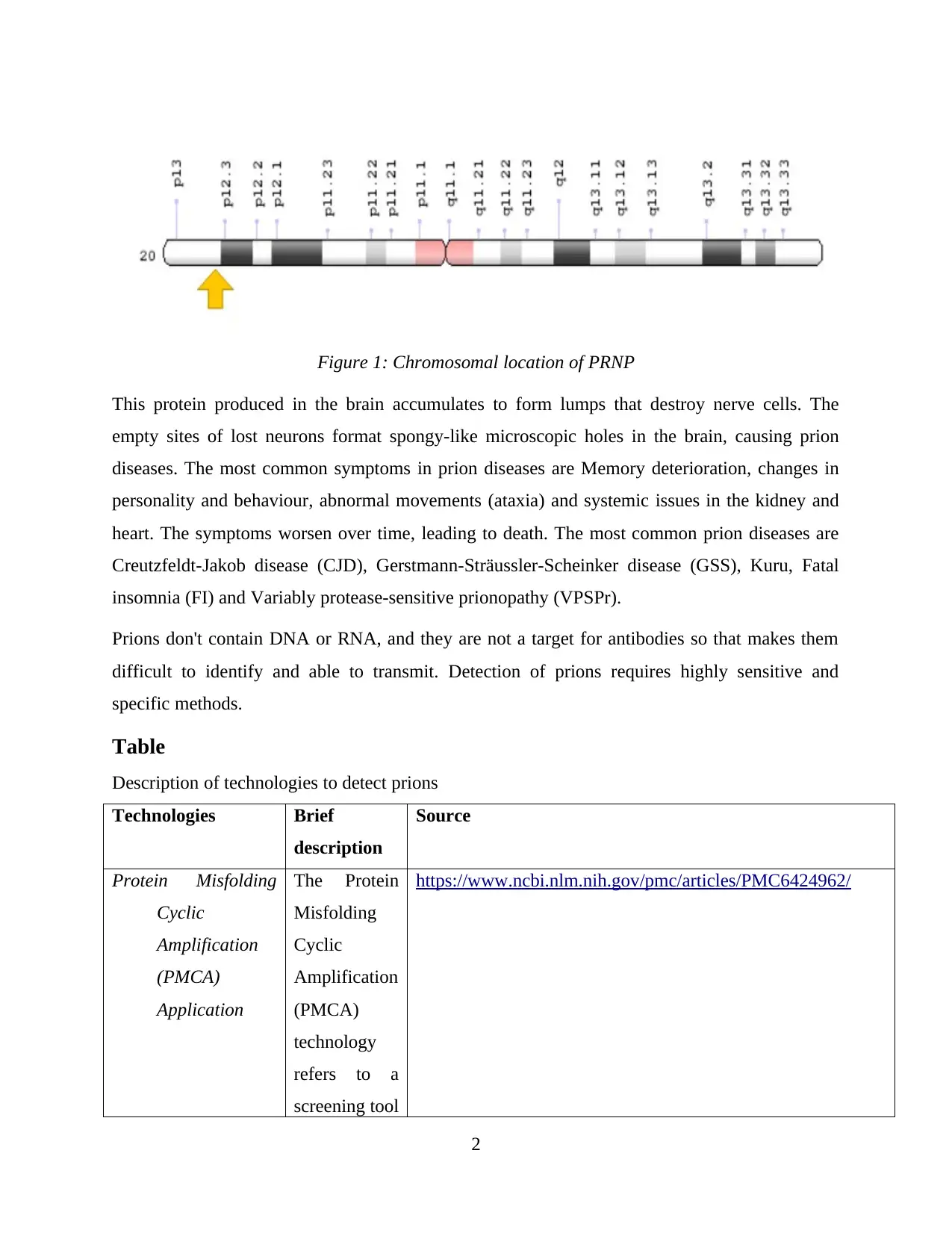
Figure 1: Chromosomal location of PRNP
This protein produced in the brain accumulates to form lumps that destroy nerve cells. The
empty sites of lost neurons format spongy-like microscopic holes in the brain, causing prion
diseases. The most common symptoms in prion diseases are Memory deterioration, changes in
personality and behaviour, abnormal movements (ataxia) and systemic issues in the kidney and
heart. The symptoms worsen over time, leading to death. The most common prion diseases are
Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker disease (GSS), Kuru, Fatal
insomnia (FI) and Variably protease-sensitive prionopathy (VPSPr).
Prions don't contain DNA or RNA, and they are not a target for antibodies so that makes them
difficult to identify and able to transmit. Detection of prions requires highly sensitive and
specific methods.
Table
Description of technologies to detect prions
Technologies Brief
description
Source
Protein Misfolding
Cyclic
Amplification
(PMCA)
Application
The Protein
Misfolding
Cyclic
Amplification
(PMCA)
technology
refers to a
screening tool
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6424962/
2
This protein produced in the brain accumulates to form lumps that destroy nerve cells. The
empty sites of lost neurons format spongy-like microscopic holes in the brain, causing prion
diseases. The most common symptoms in prion diseases are Memory deterioration, changes in
personality and behaviour, abnormal movements (ataxia) and systemic issues in the kidney and
heart. The symptoms worsen over time, leading to death. The most common prion diseases are
Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker disease (GSS), Kuru, Fatal
insomnia (FI) and Variably protease-sensitive prionopathy (VPSPr).
Prions don't contain DNA or RNA, and they are not a target for antibodies so that makes them
difficult to identify and able to transmit. Detection of prions requires highly sensitive and
specific methods.
Table
Description of technologies to detect prions
Technologies Brief
description
Source
Protein Misfolding
Cyclic
Amplification
(PMCA)
Application
The Protein
Misfolding
Cyclic
Amplification
(PMCA)
technology
refers to a
screening tool
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6424962/
2
that is used to
detect the
presence of
bovine (BSE)
and human
(vCJD)
prions within
a human cell
therapy. It is
highly
efficient in
identifying
and
amplifying a
small amount
of PRPSc
present in a
blood or
urine sample
Immunohistochemical
Detection
A technique
Depends on
detection of
PRPSc in
lymphoid
tissues such
as spleen,
palatine
tonsils and
lymph nodes
by
Inoculating
https://jcm.asm.org/content/34/5/1228.short
3
detect the
presence of
bovine (BSE)
and human
(vCJD)
prions within
a human cell
therapy. It is
highly
efficient in
identifying
and
amplifying a
small amount
of PRPSc
present in a
blood or
urine sample
Immunohistochemical
Detection
A technique
Depends on
detection of
PRPSc in
lymphoid
tissues such
as spleen,
palatine
tonsils and
lymph nodes
by
Inoculating
https://jcm.asm.org/content/34/5/1228.short
3
tissue
samples with
anti-peptides
directed
against prions
and synthesis
specific
peptides and
anti-peptide
antisera. IMC
test is used to
detect anti-
peptide
antisera in the
lymphoid
tissues thus
detecting the
prions.
Enzyme-linked
immunosorbent
assay (ELISA)
This
technology
has proven its
high
sensitivity
and
specificity to
detect PrPSc
in brain and
lymphoid
tissues. The
assay is rapid
and reliable
https://onlinelibrary.wiley.com/doi/abs/10.1002/path.1294
4
samples with
anti-peptides
directed
against prions
and synthesis
specific
peptides and
anti-peptide
antisera. IMC
test is used to
detect anti-
peptide
antisera in the
lymphoid
tissues thus
detecting the
prions.
Enzyme-linked
immunosorbent
assay (ELISA)
This
technology
has proven its
high
sensitivity
and
specificity to
detect PrPSc
in brain and
lymphoid
tissues. The
assay is rapid
and reliable
https://onlinelibrary.wiley.com/doi/abs/10.1002/path.1294
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
for human
diagnostic
purposes.
When the
ELISA plate
is coated with
MAb 11G5,
it will
effectively
capture the
PrP species
and then the
PRPSc can be
distinguished
using
different anti-
PrP MAb.
The Real-Time
Quaking-Induced
Conversion (RT-
QuIC) assay
It is the most
sensitive and
specific assay
to detect the
prion with up
to 97%
sensitivity
and 100%
specificity.
RT-QuiC is
promising
and reliable
as a
diagnostic
https://www.hindawi.com/journals/bmri/2017/5413936/
5
diagnostic
purposes.
When the
ELISA plate
is coated with
MAb 11G5,
it will
effectively
capture the
PrP species
and then the
PRPSc can be
distinguished
using
different anti-
PrP MAb.
The Real-Time
Quaking-Induced
Conversion (RT-
QuIC) assay
It is the most
sensitive and
specific assay
to detect the
prion with up
to 97%
sensitivity
and 100%
specificity.
RT-QuiC is
promising
and reliable
as a
diagnostic
https://www.hindawi.com/journals/bmri/2017/5413936/
5
procedure for
genetic prion
human
diseases.
Streptomycin
detection
The ability of
streptomycin
for forming
the multi-
molecular
aggregates
with PrP i.e.
pathogenic
prion proteins
including
recovery of
the same, by
precipitation
through a
low-speed
centrifugation
step can be
demonstrated.
Such novel
properties of
the
streptomycin
make the
same as a
useful
substance,
which results
https://www.semanticscholar.org/paper/Rapid-diagnosis-of-
human-prion-disease-using-with-Quadrio-Ugnon-Caf
%C3%A9/44ba7d8b1de9213a04457df86226f53533cb1730
6
genetic prion
human
diseases.
Streptomycin
detection
The ability of
streptomycin
for forming
the multi-
molecular
aggregates
with PrP i.e.
pathogenic
prion proteins
including
recovery of
the same, by
precipitation
through a
low-speed
centrifugation
step can be
demonstrated.
Such novel
properties of
the
streptomycin
make the
same as a
useful
substance,
which results
https://www.semanticscholar.org/paper/Rapid-diagnosis-of-
human-prion-disease-using-with-Quadrio-Ugnon-Caf
%C3%A9/44ba7d8b1de9213a04457df86226f53533cb1730
6

in increasing
the sensitivity
of laboratory
diagnostic
techniques to
detect prion
infections in
humans and
animals.
Description of five technologies
Protein Misfolding Cyclic Amplification (PMCA) Application
The Protein Misfolding Cyclic Amplification (PMCA) technology refers to a screening
tool that is used to detect the presence of bovine (BSE) and human (vCJD) prions within a
human cell therapy. It is highly efficient in identifying and amplifying a small amount of PRPSc
present in a blood or urine sample. In fact, prion diseases require a long incubation period before
symptoms appear and that enables PRPSc to accumulate in the brain and causes pathogenesis.
Prions are mainly responsible for developing a group of fatal-neurodegenerative diseases that
affect mammalian species including humans. Hereby, sole component of an infectious agent
considers as a misfolded form i.e. PrPSc of host-encoded with prion protein. Therefore, when PrP
folds into its non-infectious and natural conformation (also represented as PrPC) then it causes
disease due to accumulation. This accumulation PrPSc is developed via self-templated conversion
of PrPC to PrPSc. Further, initial seeds of PrPSc spontaneously can be developed, that raise diseases
like sporadic Creutzfeldt Jakob Disease (sCJD). Hereditary mutations, several rare, in the gene
encoding increase the likelihood of formation of PrPSc from PrP. In this regard, distinct mutations
lead to cause various diseases such as syndrome of Gerstmann–Sträussler–Scheinker, familial
fatal familial insomnia and CJD. Alternatively, via contaminated materials, PrPSc can easily be
transmitted. For an instance, in humans, a variant CJD is generally caused due to intake of beef
products from cattle, that infected with BSE (bovine spongiform encephalopathy) prions.
7
the sensitivity
of laboratory
diagnostic
techniques to
detect prion
infections in
humans and
animals.
Description of five technologies
Protein Misfolding Cyclic Amplification (PMCA) Application
The Protein Misfolding Cyclic Amplification (PMCA) technology refers to a screening
tool that is used to detect the presence of bovine (BSE) and human (vCJD) prions within a
human cell therapy. It is highly efficient in identifying and amplifying a small amount of PRPSc
present in a blood or urine sample. In fact, prion diseases require a long incubation period before
symptoms appear and that enables PRPSc to accumulate in the brain and causes pathogenesis.
Prions are mainly responsible for developing a group of fatal-neurodegenerative diseases that
affect mammalian species including humans. Hereby, sole component of an infectious agent
considers as a misfolded form i.e. PrPSc of host-encoded with prion protein. Therefore, when PrP
folds into its non-infectious and natural conformation (also represented as PrPC) then it causes
disease due to accumulation. This accumulation PrPSc is developed via self-templated conversion
of PrPC to PrPSc. Further, initial seeds of PrPSc spontaneously can be developed, that raise diseases
like sporadic Creutzfeldt Jakob Disease (sCJD). Hereditary mutations, several rare, in the gene
encoding increase the likelihood of formation of PrPSc from PrP. In this regard, distinct mutations
lead to cause various diseases such as syndrome of Gerstmann–Sträussler–Scheinker, familial
fatal familial insomnia and CJD. Alternatively, via contaminated materials, PrPSc can easily be
transmitted. For an instance, in humans, a variant CJD is generally caused due to intake of beef
products from cattle, that infected with BSE (bovine spongiform encephalopathy) prions.
7
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Similarly, human to human transmission can also be occurred that causes iCJD (iatrogenic CJD)
in medical procedures.
The sensitivity of detecting prions in the early stage is necessary to avoid transmission by
organs transplant and blood transfusion A study has been done to detect the presence of PRPSc in
a serial dilution of RML-infected cells starting from 1 cell to 100,000 cells. Using Western blots
of RKM7-RML cell dilutions and NBH as a migration control.
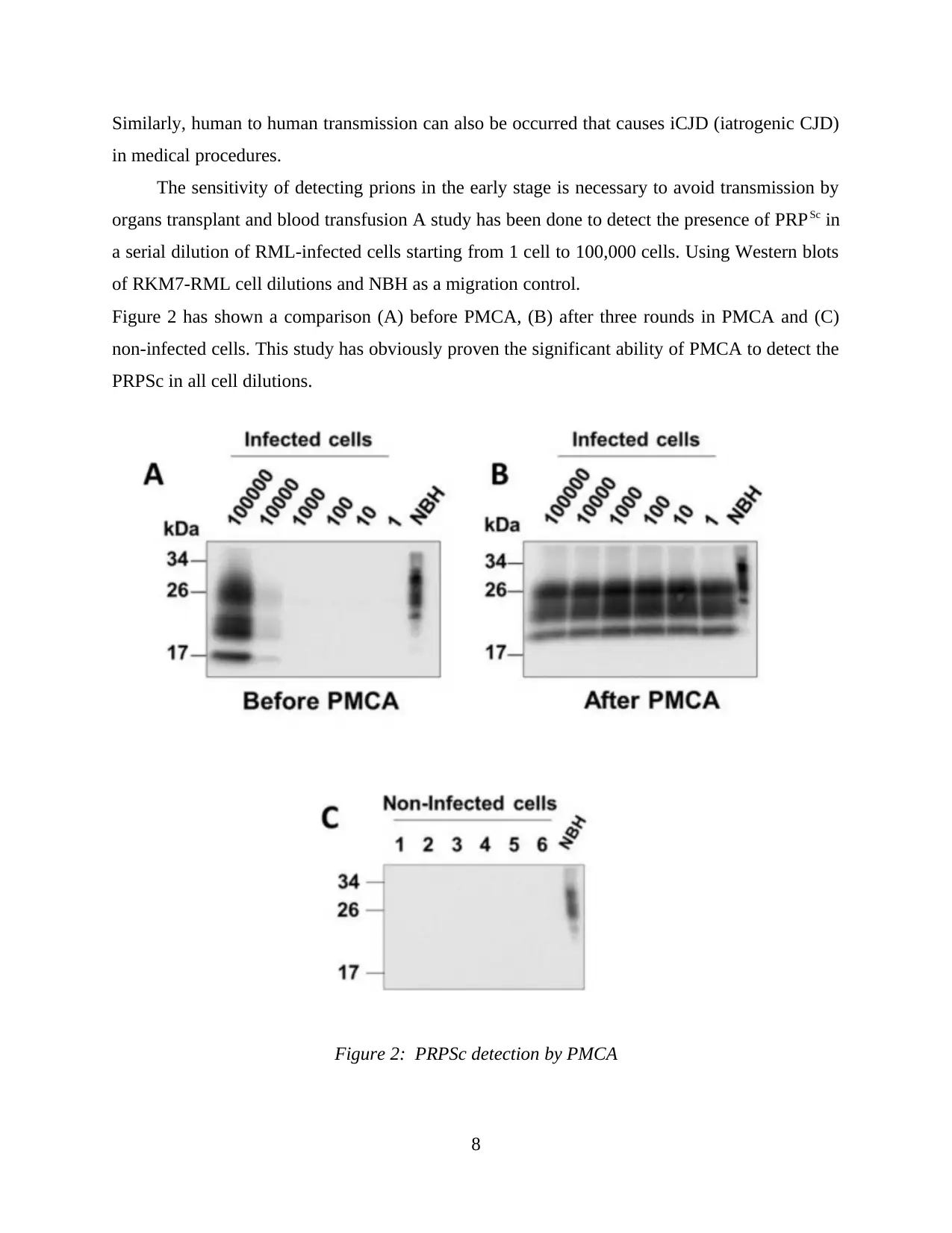
Figure 2 has shown a comparison (A) before PMCA, (B) after three rounds in PMCA and (C)
non-infected cells. This study has obviously proven the significant ability of PMCA to detect the
PRPSc in all cell dilutions.
Figure 2: PRPSc detection by PMCA
8
in medical procedures.
The sensitivity of detecting prions in the early stage is necessary to avoid transmission by
organs transplant and blood transfusion A study has been done to detect the presence of PRPSc in
a serial dilution of RML-infected cells starting from 1 cell to 100,000 cells. Using Western blots
of RKM7-RML cell dilutions and NBH as a migration control.
Figure 2 has shown a comparison (A) before PMCA, (B) after three rounds in PMCA and (C)
non-infected cells. This study has obviously proven the significant ability of PMCA to detect the
PRPSc in all cell dilutions.
Figure 2: PRPSc detection by PMCA
8
Immunohistochemical Detection
A technique Depends on detection of PRPSc in lymphoid tissues such as spleen, palatine
tonsils and lymph nodes by Inoculating tissue samples with anti-peptides directed against prions
and synthesis specific peptides and anti-peptide antisera. In other words, Immunohistochemistry
(IHC) technology is used for detecting haptens or antigens in cells of a tissue section through
exploiting the antibodies binding principle precisely to antigens within biological tissues.
Hereby, antibody-antigen binding is visualized in various manners like Horseradish Peroxidase
(HRP) or Alkaline Phosphatase (AP) enzymes commonly used for catalysing a colour-producing
reaction. IHC is more widely used in various clinical laboratories because it helps in visualising
the distribution and localization of specific cellular components within cells and in proper tissue
context. There are numerous IHC methods that can be used to localize antigens. The method
selected should include consideration of parameters such as the specimen types and assay
sensitivity. Immunohistochemical test is used to detect anti-peptide antisera in the lymphoid
tissues thus detecting the prions.
The table below has illustrated results of immunohistochemical test in lymphoid tissues of
scrapie-affected sheep.
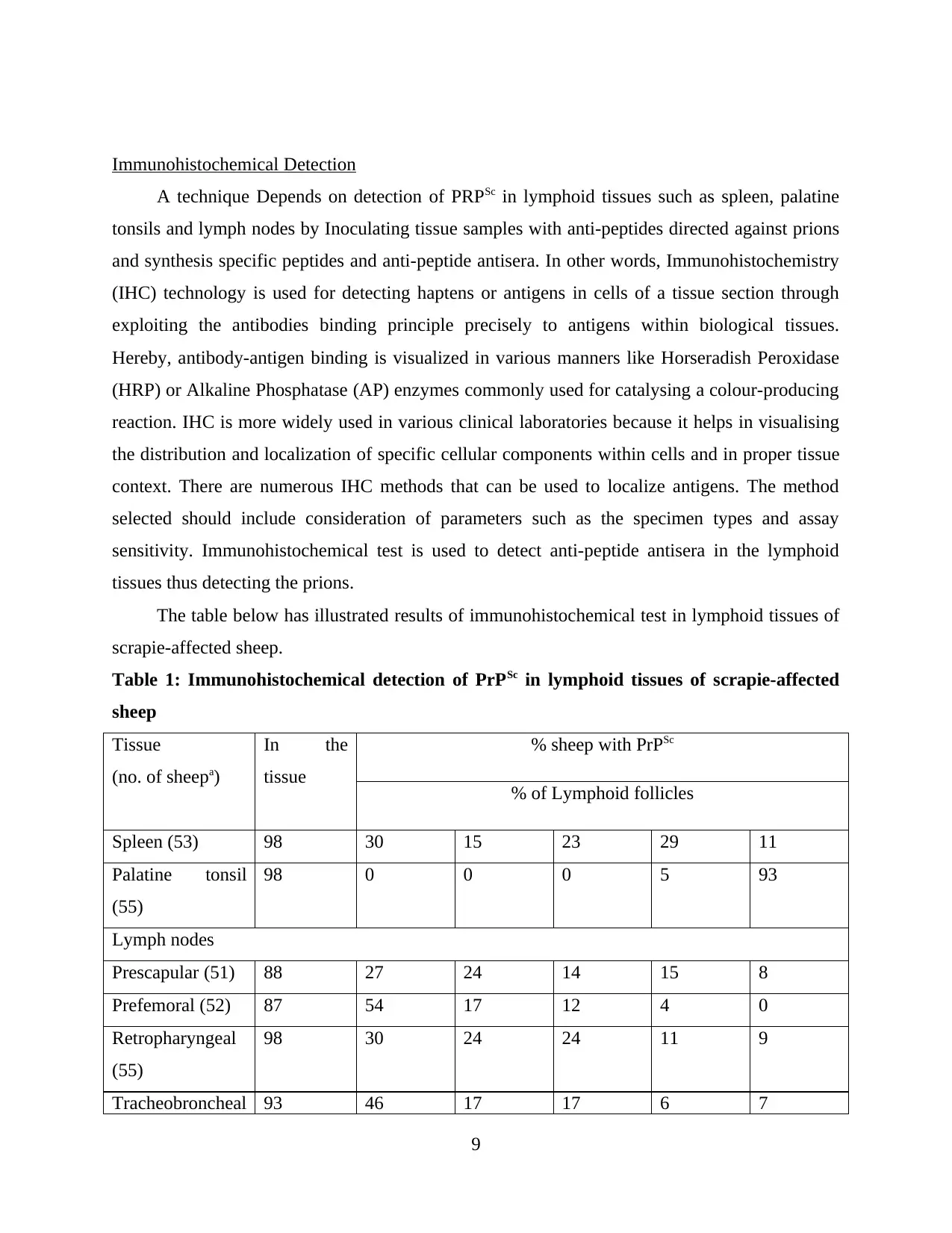
Table 1: Immunohistochemical detection of PrPSc in lymphoid tissues of scrapie-affected
sheep
Tissue
(no. of sheepa)
In the
tissue
% sheep with PrPSc
% of Lymphoid follicles
Spleen (53) 98 30 15 23 29 11
Palatine tonsil
(55)
98 0 0 0 5 93
Lymph nodes
Prescapular (51) 88 27 24 14 15 8
Prefemoral (52) 87 54 17 12 4 0
Retropharyngeal
(55)
98 30 24 24 11 9
Tracheobroncheal 93 46 17 17 6 7
9
A technique Depends on detection of PRPSc in lymphoid tissues such as spleen, palatine
tonsils and lymph nodes by Inoculating tissue samples with anti-peptides directed against prions
and synthesis specific peptides and anti-peptide antisera. In other words, Immunohistochemistry
(IHC) technology is used for detecting haptens or antigens in cells of a tissue section through
exploiting the antibodies binding principle precisely to antigens within biological tissues.
Hereby, antibody-antigen binding is visualized in various manners like Horseradish Peroxidase
(HRP) or Alkaline Phosphatase (AP) enzymes commonly used for catalysing a colour-producing
reaction. IHC is more widely used in various clinical laboratories because it helps in visualising
the distribution and localization of specific cellular components within cells and in proper tissue
context. There are numerous IHC methods that can be used to localize antigens. The method
selected should include consideration of parameters such as the specimen types and assay
sensitivity. Immunohistochemical test is used to detect anti-peptide antisera in the lymphoid
tissues thus detecting the prions.
The table below has illustrated results of immunohistochemical test in lymphoid tissues of
scrapie-affected sheep.
Table 1: Immunohistochemical detection of PrPSc in lymphoid tissues of scrapie-affected
sheep
Tissue
(no. of sheepa)
In the
tissue
% sheep with PrPSc
% of Lymphoid follicles
Spleen (53) 98 30 15 23 29 11
Palatine tonsil
(55)
98 0 0 0 5 93
Lymph nodes
Prescapular (51) 88 27 24 14 15 8
Prefemoral (52) 87 54 17 12 4 0
Retropharyngeal
(55)
98 30 24 24 11 9
Tracheobroncheal 93 46 17 17 6 7
9

(54)
Mesenteric (55) 98 24 22 31 12 9
here, a represents number of sheep does not always equal 55 because some tissue sections
repeatedly got detached from glass slides after pre-treatment.
Through performing this test, it has been evaluated that Scrapie in sheep is a
transmissible spongiform encephalopathy (TSE). At the molecular level prion or TSEs diseases,
generally characterized through accumulation of PrPSc (pathogenic prion protein), as a modified
form of host-encoded PrP (prion protein). It has been evaluated that scrapie in mammals and
human beings is a neurodegenerative disease with the vacuolation of neuropil, neurons, gliosis
and neuronal loss. As per prion hypothesis, transmissible agent of entire prion diseases, is
composed largely of PrPSc. Although, verified disease without detection of any PrPSc or with
revealing of small amounts of it, has been also described. Hereby, prion protein i.e. PrPC in
normal form is found in a wider range of tissues and can be expressed in peripheral nervous as
well as lymphoid tissue, which seems to be more prerequisite for spread and transfer of
infectivity to the CNS (central nervous system).
Enzyme-linked immunosorbent assay (ELISA)
This technology has proven its high sensitivity and specificity to detect PrPSc in brain and
lymphoid tissues. The assay is rapid and reliable for human diagnostic purposes. When the
ELISA plate is coated with MAb 11G5, it will effectively capture the PrP species and then the
PRPSc can be distinguished using different anti-PrP MAb. As per protein-only hypothesis, central
event within pathogenesis of prion disease refers to a process of conversion of a normal cellular
PrPC a prion protein into a pathogenic and abnormal scrapie isoform or PrPSc. It is believed
generally that PrPC species in human brain are present into three major glycoforms, that are
diglycosylated, unglycosylated and monoglycosylated. Using a panel of anti-PrPC MAbs
(monoclonal antibodies) that react with several regions of the PrPC, it has been found that in
normal human brain, PrPC is far more complex as compared with three-band pattern of MAb
3F4. By 2-D immunoblotting, seven major species of the PrPC is observed that could be
subdivided within multiple subspecies, that are generated due to differences in N-linked
glycosylation and site of truncation. Further, it has been concluded on the basis of such evidence,
most of the smaller species of PrP are N-terminally truncated despite of PrP species of
10
Mesenteric (55) 98 24 22 31 12 9
here, a represents number of sheep does not always equal 55 because some tissue sections
repeatedly got detached from glass slides after pre-treatment.
Through performing this test, it has been evaluated that Scrapie in sheep is a
transmissible spongiform encephalopathy (TSE). At the molecular level prion or TSEs diseases,
generally characterized through accumulation of PrPSc (pathogenic prion protein), as a modified
form of host-encoded PrP (prion protein). It has been evaluated that scrapie in mammals and
human beings is a neurodegenerative disease with the vacuolation of neuropil, neurons, gliosis
and neuronal loss. As per prion hypothesis, transmissible agent of entire prion diseases, is
composed largely of PrPSc. Although, verified disease without detection of any PrPSc or with
revealing of small amounts of it, has been also described. Hereby, prion protein i.e. PrPC in
normal form is found in a wider range of tissues and can be expressed in peripheral nervous as
well as lymphoid tissue, which seems to be more prerequisite for spread and transfer of
infectivity to the CNS (central nervous system).
Enzyme-linked immunosorbent assay (ELISA)
This technology has proven its high sensitivity and specificity to detect PrPSc in brain and
lymphoid tissues. The assay is rapid and reliable for human diagnostic purposes. When the
ELISA plate is coated with MAb 11G5, it will effectively capture the PrP species and then the
PRPSc can be distinguished using different anti-PrP MAb. As per protein-only hypothesis, central
event within pathogenesis of prion disease refers to a process of conversion of a normal cellular
PrPC a prion protein into a pathogenic and abnormal scrapie isoform or PrPSc. It is believed
generally that PrPC species in human brain are present into three major glycoforms, that are
diglycosylated, unglycosylated and monoglycosylated. Using a panel of anti-PrPC MAbs
(monoclonal antibodies) that react with several regions of the PrPC, it has been found that in
normal human brain, PrPC is far more complex as compared with three-band pattern of MAb
3F4. By 2-D immunoblotting, seven major species of the PrPC is observed that could be
subdivided within multiple subspecies, that are generated due to differences in N-linked
glycosylation and site of truncation. Further, it has been concluded on the basis of such evidence,
most of the smaller species of PrP are N-terminally truncated despite of PrP species of
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

unglycosylated or monoglycosylated. Along with this, it has been established that an ELISA
refers as appropriate and most beneficial primary diagnostic screening tool for detecting the
human prion diseases.
The Real-Time Quaking-Induced Conversion (RT-QuIC) assay
It is the most sensitive and specific assay to detect the prion with up to 97% sensitivity and
100% specificity. It has been developed to be involved in several areas of prion research. In
addition, RT-QuiC assay requiers nasal samples to be performed and that is much easier and
safer compared with blood samples which are exposed to contamination and brain tissue samples
are difficult to obtain. RT-QuiC is promising and reliable as a diagnostic procedure for genetic
prion human diseases. The RT-QuiC application includes vibration and incubation cycles that
break down prion masses producing PRPSc acts here as a seed. The vibration boosts produce
more seeds that are marked by specific markers and then they are detected by the fluorescence
reading.
PrP (rPrP-sen) as expressed in E. coli as a soluble recombinant can be used as a substrate
in QUIC assays, for amplifying the minute amounts of the PrPSc. Hereby, utilisation of a
dedicated shaker, such reaction can be enhanced through vigorous intermittent shaking that
induces rPrP-sen for aggregating and forming the fibrils. One of the most advantages part of
using QUIC is that agitation or shaking could be performed easily as well as consistently than
sonication, that considers as a major problem of varied delivery of the vibrational energy to
samples. Further, by combining QUIC technology with ThT or thioflavin T fluorescence dye,
time can be minimized easily for monitoring amyloid formation and detect protease-resistant
rPrP fibrils (rPrP-res).
11
refers as appropriate and most beneficial primary diagnostic screening tool for detecting the
human prion diseases.
The Real-Time Quaking-Induced Conversion (RT-QuIC) assay
It is the most sensitive and specific assay to detect the prion with up to 97% sensitivity and
100% specificity. It has been developed to be involved in several areas of prion research. In
addition, RT-QuiC assay requiers nasal samples to be performed and that is much easier and
safer compared with blood samples which are exposed to contamination and brain tissue samples
are difficult to obtain. RT-QuiC is promising and reliable as a diagnostic procedure for genetic
prion human diseases. The RT-QuiC application includes vibration and incubation cycles that
break down prion masses producing PRPSc acts here as a seed. The vibration boosts produce
more seeds that are marked by specific markers and then they are detected by the fluorescence
reading.
PrP (rPrP-sen) as expressed in E. coli as a soluble recombinant can be used as a substrate
in QUIC assays, for amplifying the minute amounts of the PrPSc. Hereby, utilisation of a
dedicated shaker, such reaction can be enhanced through vigorous intermittent shaking that
induces rPrP-sen for aggregating and forming the fibrils. One of the most advantages part of
using QUIC is that agitation or shaking could be performed easily as well as consistently than
sonication, that considers as a major problem of varied delivery of the vibrational energy to
samples. Further, by combining QUIC technology with ThT or thioflavin T fluorescence dye,
time can be minimized easily for monitoring amyloid formation and detect protease-resistant
rPrP fibrils (rPrP-res).
11
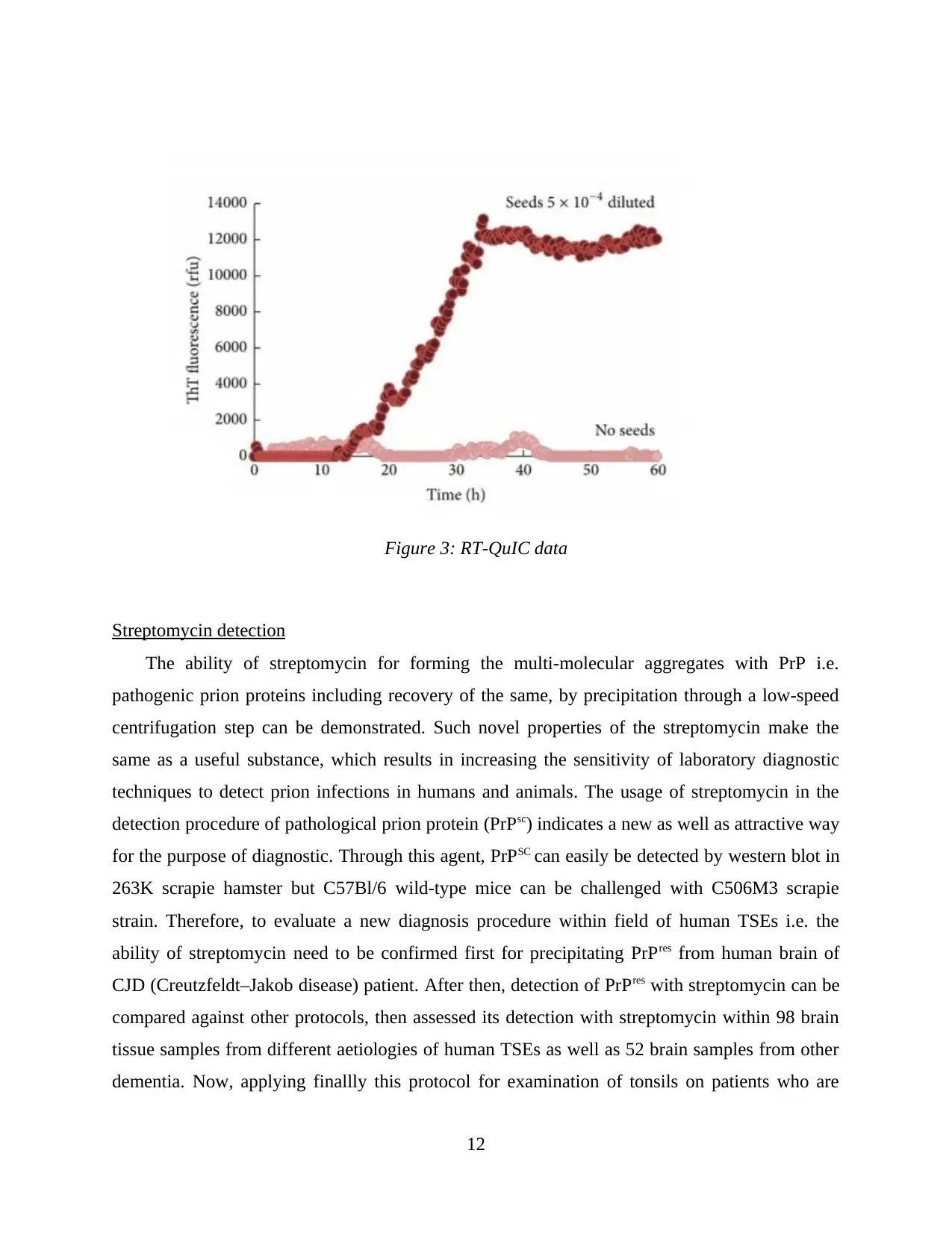
Figure 3: RT-QuIC data
Streptomycin detection
The ability of streptomycin for forming the multi-molecular aggregates with PrP i.e.
pathogenic prion proteins including recovery of the same, by precipitation through a low-speed
centrifugation step can be demonstrated. Such novel properties of the streptomycin make the
same as a useful substance, which results in increasing the sensitivity of laboratory diagnostic
techniques to detect prion infections in humans and animals. The usage of streptomycin in the
detection procedure of pathological prion protein (PrPsc) indicates a new as well as attractive way
for the purpose of diagnostic. Through this agent, PrPSC can easily be detected by western blot in
263K scrapie hamster but C57Bl/6 wild-type mice can be challenged with C506M3 scrapie
strain. Therefore, to evaluate a new diagnosis procedure within field of human TSEs i.e. the
ability of streptomycin need to be confirmed first for precipitating PrPres from human brain of
CJD (Creutzfeldt–Jakob disease) patient. After then, detection of PrPres with streptomycin can be
compared against other protocols, then assessed its detection with streptomycin within 98 brain
tissue samples from different aetiologies of human TSEs as well as 52 brain samples from other
dementia. Now, applying finallly this protocol for examination of tonsils on patients who are
12
Streptomycin detection
The ability of streptomycin for forming the multi-molecular aggregates with PrP i.e.
pathogenic prion proteins including recovery of the same, by precipitation through a low-speed
centrifugation step can be demonstrated. Such novel properties of the streptomycin make the
same as a useful substance, which results in increasing the sensitivity of laboratory diagnostic
techniques to detect prion infections in humans and animals. The usage of streptomycin in the
detection procedure of pathological prion protein (PrPsc) indicates a new as well as attractive way
for the purpose of diagnostic. Through this agent, PrPSC can easily be detected by western blot in
263K scrapie hamster but C57Bl/6 wild-type mice can be challenged with C506M3 scrapie
strain. Therefore, to evaluate a new diagnosis procedure within field of human TSEs i.e. the
ability of streptomycin need to be confirmed first for precipitating PrPres from human brain of
CJD (Creutzfeldt–Jakob disease) patient. After then, detection of PrPres with streptomycin can be
compared against other protocols, then assessed its detection with streptomycin within 98 brain
tissue samples from different aetiologies of human TSEs as well as 52 brain samples from other
dementia. Now, applying finallly this protocol for examination of tonsils on patients who are
12

suspected of variant CJD (v-CJD), aid in obtaining the sensitivity and specificity with the
streptomycin protocol through 100% on brain tissue.
Conclusion
It has been concluded from all over report that prion diseases can be occurred when normal
prion protein is found on many cell surface. This would result in causing abnormal and clump
issues in the brain, that might damage the brain. The abnormal accumulation of protein within
human brain may cause memory impairment, difficulties in movement, personality changes and
sometime even death of patient. Therefore, development of technologies like streptomycin, RT-
QuIC assay, ELISA and Immunohistochemical Detection etc. helps in easily diagnosing the
diseases.
13
streptomycin protocol through 100% on brain tissue.
Conclusion
It has been concluded from all over report that prion diseases can be occurred when normal
prion protein is found on many cell surface. This would result in causing abnormal and clump
issues in the brain, that might damage the brain. The abnormal accumulation of protein within
human brain may cause memory impairment, difficulties in movement, personality changes and
sometime even death of patient. Therefore, development of technologies like streptomycin, RT-
QuIC assay, ELISA and Immunohistochemical Detection etc. helps in easily diagnosing the
diseases.
13
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

References
Books and Journals
Adam Lyon and et. al., 2019. Application of PMCA to screen for prion infection in a human cell
line used to produce biological therapeutics. Online Available at: <
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6424962/>.
Alvin S. Das and Wen-Quan Zou. 2016. Prions: Beyond a Single Protein. Online Available at:<
https://pubmed.ncbi.nlm.nih.gov/27226089/ >.
Federal disease control - Scrapie. 2002. Online Available at: <
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC339408/>.
Hae-Eun Kang and et. al., 2017. Prion Diagnosis: Application of Real-Time Quaking-Induced
Conversion. Online Available at:<
https://www.hindawi.com/journals/bmri/2017/5413936/ >.
Isabelle Quadrio and et. al., 2009. Rapid diagnosis of human prion disease using streptomycin
with tonsil and brain tissues. Online Available at:<
https://www.semanticscholar.org/paper/Rapid-diagnosis-of-human-prion-disease-using-
with-Quadrio-Ugnon-Caf%C3%A9/44ba7d8b1de9213a04457df86226f53533cb1730 >.
Kang, H. E. et al., (2017) Novel Microbial Diagnostic Methods for Clinical, Environmental, and
Food Samples. Biomed Research International 2017,1-8.
L J van Keulen, and et. al., 2020. Immunohistochemical detection of prion protein in lymphoid
tissues of sheep with natural scrapie. Online Available at: <
https://jcm.asm.org/content/34/5/1228.short>.
Mark D. Zabel and Crystal Reid. 2015. A brief history of prions. Online Available at:<
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626585/ >.
Shin-Chung Kang and et. al., 2003. Guanidine hydrochloride extraction and detection of prion
proteins in mouse and hamster prion diseases by ELISA. Online Available at:<
https://onlinelibrary.wiley.com/doi/abs/10.1002/path.1294>.
14
Books and Journals
Adam Lyon and et. al., 2019. Application of PMCA to screen for prion infection in a human cell
line used to produce biological therapeutics. Online Available at: <
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6424962/>.
Alvin S. Das and Wen-Quan Zou. 2016. Prions: Beyond a Single Protein. Online Available at:<
https://pubmed.ncbi.nlm.nih.gov/27226089/ >.
Federal disease control - Scrapie. 2002. Online Available at: <
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC339408/>.
Hae-Eun Kang and et. al., 2017. Prion Diagnosis: Application of Real-Time Quaking-Induced
Conversion. Online Available at:<
https://www.hindawi.com/journals/bmri/2017/5413936/ >.
Isabelle Quadrio and et. al., 2009. Rapid diagnosis of human prion disease using streptomycin
with tonsil and brain tissues. Online Available at:<
https://www.semanticscholar.org/paper/Rapid-diagnosis-of-human-prion-disease-using-
with-Quadrio-Ugnon-Caf%C3%A9/44ba7d8b1de9213a04457df86226f53533cb1730 >.
Kang, H. E. et al., (2017) Novel Microbial Diagnostic Methods for Clinical, Environmental, and
Food Samples. Biomed Research International 2017,1-8.
L J van Keulen, and et. al., 2020. Immunohistochemical detection of prion protein in lymphoid
tissues of sheep with natural scrapie. Online Available at: <
https://jcm.asm.org/content/34/5/1228.short>.
Mark D. Zabel and Crystal Reid. 2015. A brief history of prions. Online Available at:<
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626585/ >.
Shin-Chung Kang and et. al., 2003. Guanidine hydrochloride extraction and detection of prion
proteins in mouse and hamster prion diseases by ELISA. Online Available at:<
https://onlinelibrary.wiley.com/doi/abs/10.1002/path.1294>.
14
1 out of 17
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



