FC710 Chemistry Exam: Equilibrium, Atomic Structure, and Reactions
VerifiedAdded on 2022/09/26
|9
|1649
|32
Homework Assignment
AI Summary
This chemistry assignment solution covers several key concepts, including chemical equilibrium, atomic structure, and redox reactions. The first question involves calculating Kc and Kp for a reversible reaction and applying Le Chatelier's principle. The second question focuses on atomic radius and electronic configurations. The third question identifies and explains an electrolytic cell, detailing electrode reactions and Faraday's law. The fourth question involves pH calculations. The final question covers thermochemistry, including calculating enthalpy changes and heat transfer. The document provides detailed solutions and explanations for each part, offering a comprehensive resource for students studying these topics. Desklib offers a platform for students to access similar solved assignments and study materials.

Chemistry 1
CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 2
Question one
From the reversible reaction below
The initial values of moles of CO (nCO) = 0.36, the number of moles of H2 (nH2) = 0.64, the temperature
is 1500C = 423 K . The container volume = 10 litres
At equilibrium the number of moles of CH3OH ( nCH3OH) = 0.12
Part a
Kc
The mole ratio between Co : H2 = 0.36: 0.64.
At equilibrium 0.36-x 0.64-x
Kc = Kn ( ¿
X= 0.12 ( given)
Kn = ( nCH 3 OH
(nCO )¿ ¿
VT ( the totalvolume ) = 10 litres
∆ ng = change in the number of moles = 1-(1+2) = -2
Kc= x
( 0.36−x ) ¿ ¿
Kc= 0.12
( 0.36−0.12 ) ¿ ¿
Kc= 3.125 (100) = 312.5
Part b
Kp = kc (RT¿∆ ng
Kp= ( 312.5) ( 0.0821)( 423¿−2 Temperature = 423 K ( given)
Kp = 312.5
¿ ¿
Question one
From the reversible reaction below
The initial values of moles of CO (nCO) = 0.36, the number of moles of H2 (nH2) = 0.64, the temperature
is 1500C = 423 K . The container volume = 10 litres
At equilibrium the number of moles of CH3OH ( nCH3OH) = 0.12
Part a
Kc
The mole ratio between Co : H2 = 0.36: 0.64.
At equilibrium 0.36-x 0.64-x
Kc = Kn ( ¿
X= 0.12 ( given)
Kn = ( nCH 3 OH
(nCO )¿ ¿
VT ( the totalvolume ) = 10 litres
∆ ng = change in the number of moles = 1-(1+2) = -2
Kc= x
( 0.36−x ) ¿ ¿
Kc= 0.12
( 0.36−0.12 ) ¿ ¿
Kc= 3.125 (100) = 312.5
Part b
Kp = kc (RT¿∆ ng
Kp= ( 312.5) ( 0.0821)( 423¿−2 Temperature = 423 K ( given)
Kp = 312.5
¿ ¿

Chemistry 3
Part c
Since change in temperature is negative, the reaction is exothermic,
i.
If the pressure is increased by keeping temperature constant , the reaction will move in a direction
where partial pressure of the gas decreases and we know that pressure is directly proportional to
number of moles . Thus increasing the pressure , the reaction will move in a direction where the number
of moles decreases . Therefore here, the number of moles of product is less than the number of moles
of reactant thus the reaction will move into the forward direction.
ii.
This reaction is exothermic in nature. Therefore if we increase the temperature, the reaction will move
into backward direction to decrease the temperature.
Question two
Part a
i.
largest atom is Na : elecronic configuration of Na : [Ne] 3s1 ; Mg : [Ne] 3s2 ; Al: [Ne] 3s2 3p1 .
Since with increasing atomic No. there is increse in effectuve nuclear charge, with additional
electrons added to new shell.
ii.
Largest atom is Rb : elecronic configuration of Na : [Ne] 3s1 ; K : [Ar] 4s1 ; Rb : [Kr] 5s1 ; since new
shell is added down in group
Part b
Al : [Ne] 3s2 3p1 : = Lowest energy orbitals fill first : in n=3 shell lowest energy orbital '3s' filled
first. (The orbital in a subshell are degenerate (same energy), the whole subshell of a given type of
orbital is occupied before going on to adjacent subshell of higher energy). Just 2 electrons are
allowed for every orbital and they have to be of contrasting spin. The most steady electron
configuration in a subshell happens when the optimum number of non-paired electrons occur, all will
have direction of the same spin.
Part c
element symbol electron protons No. of Neutrons
Magnesium Mg 12 12 12
Part c
Since change in temperature is negative, the reaction is exothermic,
i.
If the pressure is increased by keeping temperature constant , the reaction will move in a direction
where partial pressure of the gas decreases and we know that pressure is directly proportional to
number of moles . Thus increasing the pressure , the reaction will move in a direction where the number
of moles decreases . Therefore here, the number of moles of product is less than the number of moles
of reactant thus the reaction will move into the forward direction.
ii.
This reaction is exothermic in nature. Therefore if we increase the temperature, the reaction will move
into backward direction to decrease the temperature.
Question two
Part a
i.
largest atom is Na : elecronic configuration of Na : [Ne] 3s1 ; Mg : [Ne] 3s2 ; Al: [Ne] 3s2 3p1 .
Since with increasing atomic No. there is increse in effectuve nuclear charge, with additional
electrons added to new shell.
ii.
Largest atom is Rb : elecronic configuration of Na : [Ne] 3s1 ; K : [Ar] 4s1 ; Rb : [Kr] 5s1 ; since new
shell is added down in group
Part b
Al : [Ne] 3s2 3p1 : = Lowest energy orbitals fill first : in n=3 shell lowest energy orbital '3s' filled
first. (The orbital in a subshell are degenerate (same energy), the whole subshell of a given type of
orbital is occupied before going on to adjacent subshell of higher energy). Just 2 electrons are
allowed for every orbital and they have to be of contrasting spin. The most steady electron
configuration in a subshell happens when the optimum number of non-paired electrons occur, all will
have direction of the same spin.
Part c
element symbol electron protons No. of Neutrons
Magnesium Mg 12 12 12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Chemistry 4
Sulphur S 16 16 16
Oxygen 16O 8 8 8
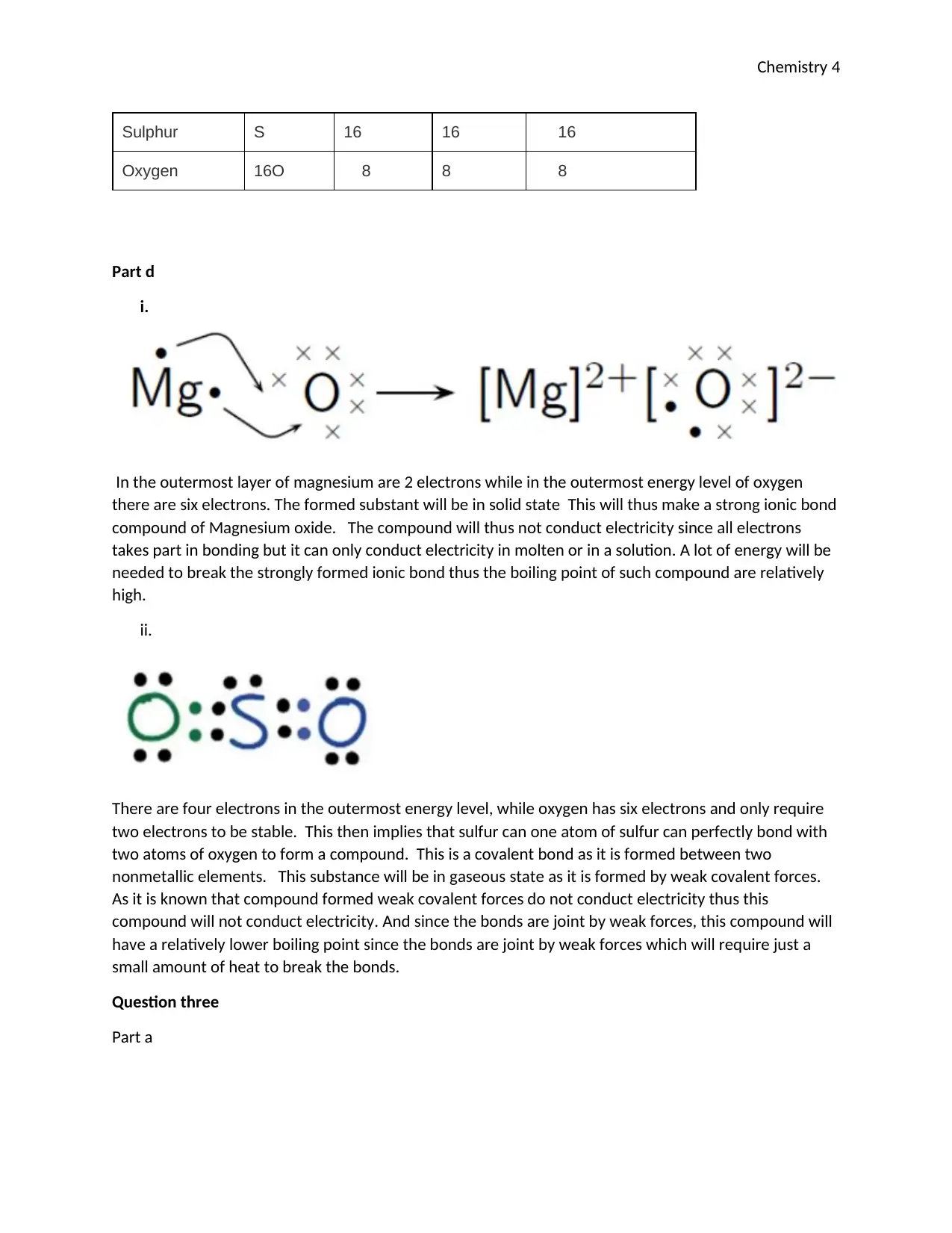
Part d
i.
In the outermost layer of magnesium are 2 electrons while in the outermost energy level of oxygen
there are six electrons. The formed substant will be in solid state This will thus make a strong ionic bond
compound of Magnesium oxide. The compound will thus not conduct electricity since all electrons
takes part in bonding but it can only conduct electricity in molten or in a solution. A lot of energy will be
needed to break the strongly formed ionic bond thus the boiling point of such compound are relatively
high.
ii.
There are four electrons in the outermost energy level, while oxygen has six electrons and only require
two electrons to be stable. This then implies that sulfur can one atom of sulfur can perfectly bond with
two atoms of oxygen to form a compound. This is a covalent bond as it is formed between two
nonmetallic elements. This substance will be in gaseous state as it is formed by weak covalent forces.
As it is known that compound formed weak covalent forces do not conduct electricity thus this
compound will not conduct electricity. And since the bonds are joint by weak forces, this compound will
have a relatively lower boiling point since the bonds are joint by weak forces which will require just a
small amount of heat to break the bonds.
Question three
Part a
Sulphur S 16 16 16
Oxygen 16O 8 8 8
Part d
i.
In the outermost layer of magnesium are 2 electrons while in the outermost energy level of oxygen
there are six electrons. The formed substant will be in solid state This will thus make a strong ionic bond
compound of Magnesium oxide. The compound will thus not conduct electricity since all electrons
takes part in bonding but it can only conduct electricity in molten or in a solution. A lot of energy will be
needed to break the strongly formed ionic bond thus the boiling point of such compound are relatively
high.
ii.
There are four electrons in the outermost energy level, while oxygen has six electrons and only require
two electrons to be stable. This then implies that sulfur can one atom of sulfur can perfectly bond with
two atoms of oxygen to form a compound. This is a covalent bond as it is formed between two
nonmetallic elements. This substance will be in gaseous state as it is formed by weak covalent forces.
As it is known that compound formed weak covalent forces do not conduct electricity thus this
compound will not conduct electricity. And since the bonds are joint by weak forces, this compound will
have a relatively lower boiling point since the bonds are joint by weak forces which will require just a
small amount of heat to break the bonds.
Question three
Part a
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 5
The type of electrochemical cell illustrated above is electrolytic cell. This is a cell which drives a non-
spontaneous redox reaction via the usage of electrical energy. In other words electrolytic cell
converts electronic energy into chemical potential energy.
Part b
electrode oxidation or reduction charge of electrode The half reaction equation
anode oxidation positive
2I- 2e- + I2
cathode reduction negative
2H2O + 2e- H2 + 2OH-
Part c
The overall redox reaction is given below
2I- (aq) + 2H2O (l) I2 (s) + H2 (g)+ 2OH- (aq)
Part d
The potassium iodide aqueous solution with phenolphtalein mixture in the early stage will have
slightly yellow in color. As the electrolysis continues Iodine (I2) will form at the anode in dark pink
color. And also solution will turn pink around cathode because of the formation of potassium
hydroxide.
Part e
From the first law of Faraday
W= M × I ×t
96500 ×hf
Where W is the weight of the substance deposited
M is the molar mass of the substance
I is the current passed in amps
t is time given in econds
hf is h factor = number of electron gain or loss
The total molar mass = 39+127 = 166g/mol
Current (I) = 10 amp
Time (t) = 1.5 hours = 5400 seconds
The type of electrochemical cell illustrated above is electrolytic cell. This is a cell which drives a non-
spontaneous redox reaction via the usage of electrical energy. In other words electrolytic cell
converts electronic energy into chemical potential energy.
Part b
electrode oxidation or reduction charge of electrode The half reaction equation
anode oxidation positive
2I- 2e- + I2
cathode reduction negative
2H2O + 2e- H2 + 2OH-
Part c
The overall redox reaction is given below
2I- (aq) + 2H2O (l) I2 (s) + H2 (g)+ 2OH- (aq)
Part d
The potassium iodide aqueous solution with phenolphtalein mixture in the early stage will have
slightly yellow in color. As the electrolysis continues Iodine (I2) will form at the anode in dark pink
color. And also solution will turn pink around cathode because of the formation of potassium
hydroxide.
Part e
From the first law of Faraday
W= M × I ×t
96500 ×hf
Where W is the weight of the substance deposited
M is the molar mass of the substance
I is the current passed in amps
t is time given in econds
hf is h factor = number of electron gain or loss
The total molar mass = 39+127 = 166g/mol
Current (I) = 10 amp
Time (t) = 1.5 hours = 5400 seconds

Chemistry 6
hf=1
w= 166× 10 ×5400
96500 ×1 = 92.89g
Question four
Part a
The molar mass of HCl = 36.5
Moles of HCl = Mass
Molar mass
Moles of HCl = 1.24
36.5
Moles = 0.03397 moles
Molar mass = Moles
Volume
Molar mass = 0.03397
0.5 = 0.0679
pH= - log (0.0679)
pH= 1.16
Part b
Part c
The second dissociation constant value is very low , thus we can assume the second dissociation of HC
O3
−¿ ¿
Part d
H2CO3 = 1.24g
Formula of H2CO3 = 62
hf=1
w= 166× 10 ×5400
96500 ×1 = 92.89g
Question four
Part a
The molar mass of HCl = 36.5
Moles of HCl = Mass
Molar mass
Moles of HCl = 1.24
36.5
Moles = 0.03397 moles
Molar mass = Moles
Volume
Molar mass = 0.03397
0.5 = 0.0679
pH= - log (0.0679)
pH= 1.16
Part b
Part c
The second dissociation constant value is very low , thus we can assume the second dissociation of HC
O3
−¿ ¿
Part d
H2CO3 = 1.24g
Formula of H2CO3 = 62
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chemistry 7
Moles = Mass
Molar mass
Moles = 1.24
62 = 0.02 moles
Molarity = moles
volume
0,02
0.5 = 0.04 moles
[ H+¿¿] = √ Ka1 c= √ 4.3 × 0.04 ×10−7
[ H+¿¿] = 0.000131
Just like the above calculation of pH
pH = - log [ H+¿¿] = - log ( 0.000131 = 3.883
Part e
To maintain the pH of human blood H2CO3 and HCO play very significant roles
When acid is added to blood , the ions of HCO3 will neutralize the H3O+ forming H2CO3 and H2= this
prevents the acidity of blood
HCO3- + H3O+ H2CO3 + H2O
When the base is added to blood , H2CO3 react with hydroxyl ions forming HCO- and H2O , preventing
the pH of blood from becoming a base.
H2CO3+ OH- HCO3- +H2O
Moles = Mass
Molar mass
Moles = 1.24
62 = 0.02 moles
Molarity = moles
volume
0,02
0.5 = 0.04 moles
[ H+¿¿] = √ Ka1 c= √ 4.3 × 0.04 ×10−7
[ H+¿¿] = 0.000131
Just like the above calculation of pH
pH = - log [ H+¿¿] = - log ( 0.000131 = 3.883
Part e
To maintain the pH of human blood H2CO3 and HCO play very significant roles
When acid is added to blood , the ions of HCO3 will neutralize the H3O+ forming H2CO3 and H2= this
prevents the acidity of blood
HCO3- + H3O+ H2CO3 + H2O
When the base is added to blood , H2CO3 react with hydroxyl ions forming HCO- and H2O , preventing
the pH of blood from becoming a base.
H2CO3+ OH- HCO3- +H2O
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 8
Reaction which maintains human pH occurs the pH of a range between 6.9 and 7.8 . At point the body
may stop operating normally because the cell damage and
Question five
Part a
C+O2 CO2 ∆ H0 c=−394 kj/mol
H2+O2 H2O ∆ H0 f =−286 kj /mol
C6H6+7.5O2 6CO2+3H2O ∆ H0 c=−3273 kj/mol
Part b
6C+3H2 C6H6 ∆ H0 c
6C+6H2 6CO2 ∆ H0 c = -394 x 6 = -2364kJ/mol
3H2+ 1.5 O2 3H2O ∆ H0 c = -286 x 3 = -858 kJ/mol
Through reversing equations
6C+3H2 C6H6+7.5O2 ∆ H0 c = 3273kJ/mol
6C+3H2 C6H6∆ H0 f = ∆ H 1+∆ H 2+∆ H 3 = -2364-858+3273 = 51kJ/mol
Part c
The mass of water = 500g and the density of water is 1g/cm3
q= MS∆ T = 500g x 4.18Jg-1k-1 x ( 37-25)
if 1 mole of benzene is burned to give 3273 kJ/mol of heat
therefore the moles of benzene giving 25.8kJ of heat = 25.08
3273 = 0.00766 moles
Reaction which maintains human pH occurs the pH of a range between 6.9 and 7.8 . At point the body
may stop operating normally because the cell damage and
Question five
Part a
C+O2 CO2 ∆ H0 c=−394 kj/mol
H2+O2 H2O ∆ H0 f =−286 kj /mol
C6H6+7.5O2 6CO2+3H2O ∆ H0 c=−3273 kj/mol
Part b
6C+3H2 C6H6 ∆ H0 c
6C+6H2 6CO2 ∆ H0 c = -394 x 6 = -2364kJ/mol
3H2+ 1.5 O2 3H2O ∆ H0 c = -286 x 3 = -858 kJ/mol
Through reversing equations
6C+3H2 C6H6+7.5O2 ∆ H0 c = 3273kJ/mol
6C+3H2 C6H6∆ H0 f = ∆ H 1+∆ H 2+∆ H 3 = -2364-858+3273 = 51kJ/mol
Part c
The mass of water = 500g and the density of water is 1g/cm3
q= MS∆ T = 500g x 4.18Jg-1k-1 x ( 37-25)
if 1 mole of benzene is burned to give 3273 kJ/mol of heat
therefore the moles of benzene giving 25.8kJ of heat = 25.08
3273 = 0.00766 moles

Chemistry 9
Thus mass of benzene = 0.00766 x 78 g/mol
Mass of benzene = 0.60g
References
Barry, N.P. and Sadler, P.J., 2013. Exploration of the medical periodic table: towards new
targets. Chemical communications, 49(45), pp.5106-5131.
Dal Corso, A., 2014. Pseudopotentials periodic table: From H to Pu. Computational Materials Science, 95,
pp.337-350.
Hadjiafxenti, A., Gunduz, I.E., Kyratsi, T., Doumanidis, C.C. and Rebholz, C., 2013. Exothermic reaction
characteristics of continuously ball-milled Al/Ni powder compacts. Vacuum, 96, pp.73-78.
Ko, M., Yoon, H.C., Yoo, H., Oh, J.H., Yang, H. and Do, Y.R., 2017. Highly Efficient Green Zn Ag In
S/Zn In S/ZnS QDs by a Strong Exothermic Reaction for Down ‐Converted Green and Tripackage
White LEDs. Advanced Functional Materials, 27(4), p.1602638.
Pyykkö, P., 2011. A suggested periodic table up to Z≤ 172, based on Dirac–Fock calculations on atoms
and ions. Physical Chemistry Chemical Physics, 13(1), pp.161-168.
Scerri, E., 2019. The periodic table: its story and its significance. Oxford University Press.
Won, C.W., Nersisyan, H.H. and Won, H.I., 2010. Titanium powder prepared by a rapid exothermic
reaction. Chemical Engineering Journal, 157(1), pp.270-275.
Yang, Y., Xu, D. and Zhang, K., 2012. Effect of nanostructures on the exothermic reaction and ignition of
Al/CuOx based energetic materials. Journal of Materials Science, 47(3), pp.1296-1305.
Zhou, L., Piekiel, N., Chowdhury, S. and Zachariah, M.R., 2010. Time-resolved mass spectrometry of the
exothermic reaction between nanoaluminum and metal oxides: the role of oxygen release. The Journal of
Physical Chemistry C, 114(33), pp.14269-14275.
Thus mass of benzene = 0.00766 x 78 g/mol
Mass of benzene = 0.60g
References
Barry, N.P. and Sadler, P.J., 2013. Exploration of the medical periodic table: towards new
targets. Chemical communications, 49(45), pp.5106-5131.
Dal Corso, A., 2014. Pseudopotentials periodic table: From H to Pu. Computational Materials Science, 95,
pp.337-350.
Hadjiafxenti, A., Gunduz, I.E., Kyratsi, T., Doumanidis, C.C. and Rebholz, C., 2013. Exothermic reaction
characteristics of continuously ball-milled Al/Ni powder compacts. Vacuum, 96, pp.73-78.
Ko, M., Yoon, H.C., Yoo, H., Oh, J.H., Yang, H. and Do, Y.R., 2017. Highly Efficient Green Zn Ag In
S/Zn In S/ZnS QDs by a Strong Exothermic Reaction for Down ‐Converted Green and Tripackage
White LEDs. Advanced Functional Materials, 27(4), p.1602638.
Pyykkö, P., 2011. A suggested periodic table up to Z≤ 172, based on Dirac–Fock calculations on atoms
and ions. Physical Chemistry Chemical Physics, 13(1), pp.161-168.
Scerri, E., 2019. The periodic table: its story and its significance. Oxford University Press.
Won, C.W., Nersisyan, H.H. and Won, H.I., 2010. Titanium powder prepared by a rapid exothermic
reaction. Chemical Engineering Journal, 157(1), pp.270-275.
Yang, Y., Xu, D. and Zhang, K., 2012. Effect of nanostructures on the exothermic reaction and ignition of
Al/CuOx based energetic materials. Journal of Materials Science, 47(3), pp.1296-1305.
Zhou, L., Piekiel, N., Chowdhury, S. and Zachariah, M.R., 2010. Time-resolved mass spectrometry of the
exothermic reaction between nanoaluminum and metal oxides: the role of oxygen release. The Journal of
Physical Chemistry C, 114(33), pp.14269-14275.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.