Genetics of Eye Pigmentation in Drosophila melanogaster Study
VerifiedAdded on 2023/01/17
|11
|2987
|80
Report
AI Summary
This report presents an investigation into the genetics of eye pigmentation in Drosophila melanogaster, focusing on the effects of cinnabar and white eye mutations. The study involved reciprocal crosses between flies with different eye color phenotypes to analyze the inheritance patterns in F1 and F2 generations. The experiment aimed to understand how these genes interact and influence eye color. The results revealed deviations from expected ratios in the F2 generation, indicating that environmental factors and gene interactions played a crucial role in the mutation process. The report discusses the observed phenotypes, the significance of reciprocal crosses, and the implications of the results in terms of gene expression, inheritance, and environmental influences. The chi-squared test showed significant differences between observed and expected numbers. The study highlights the complexity of gene interactions and the influence of environmental factors on genetic expression.

The Effects of Eye Pigmentation in Drosophila melanogaster with Consideration of
Reciprocal Crosses
Biology-Genetics
Reciprocal Crosses
Biology-Genetics
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Abstract
Eye pigmentation process is an essential aspect in assessing mutation process.
Drosophila melanogaster has been used as an effective organism in the assessment of this
pigmentation over the years. Testing of cinnabar female crossed by white males and an
eventual reciprocal process was undertaken. This process entails a critical understanding of
how pigmentations are often crossed to assess pigmentation. The Drosophila melanogaster
produces eye pigmentation while cinnabar allele associated with the protein. This practical
assessed crossing of two genes using the parents’ genotype in order to obtain the F1 and later
F2 generations. Phenotypes produced were the key focus of this study. The hypothesis of the
study entailed an assessment of crossing the white females with the cinnabar males to get the
F1 generation. Further F 2 generations were undertaken with predictions of obtaining wild
type color and cinnabar males. The results obtained indicated that crossing four cinnabar
males and two white females resulted, further Cinnabar males were crossed with white
females, yielding wild type females and white males. In the F2 generation, there are far fewer
white apricots and far more double mutants than expected. (X2=249, d.f.=3, p<0.0001).
Reciprocal process plays a vital role in the overall mutation process. Environmental factors
are critical in gene mutation which could have a significant impact on the occurrence of
dominant and recessive genes as observed in the results. Adjustment son the overall mutation
on the environment is key towards achieving maximum mutation.
Eye pigmentation process is an essential aspect in assessing mutation process.
Drosophila melanogaster has been used as an effective organism in the assessment of this
pigmentation over the years. Testing of cinnabar female crossed by white males and an
eventual reciprocal process was undertaken. This process entails a critical understanding of
how pigmentations are often crossed to assess pigmentation. The Drosophila melanogaster
produces eye pigmentation while cinnabar allele associated with the protein. This practical
assessed crossing of two genes using the parents’ genotype in order to obtain the F1 and later
F2 generations. Phenotypes produced were the key focus of this study. The hypothesis of the
study entailed an assessment of crossing the white females with the cinnabar males to get the
F1 generation. Further F 2 generations were undertaken with predictions of obtaining wild
type color and cinnabar males. The results obtained indicated that crossing four cinnabar
males and two white females resulted, further Cinnabar males were crossed with white
females, yielding wild type females and white males. In the F2 generation, there are far fewer
white apricots and far more double mutants than expected. (X2=249, d.f.=3, p<0.0001).
Reciprocal process plays a vital role in the overall mutation process. Environmental factors
are critical in gene mutation which could have a significant impact on the occurrence of
dominant and recessive genes as observed in the results. Adjustment son the overall mutation
on the environment is key towards achieving maximum mutation.

Introduction
In this experiment, we tested the eye pigmentations of cinnabar with white eyes on
Drosophila melanogaster. We used D. melanogaster because it is a good model organism
due to its short life span and large number of offspring. We tested the eye pigments with a
cinnabar female crossed by white males and did the reciprocal of that. The experiment was
conducted to understand eye pigmentations and how they are transported. For D.
melanogaster, it produces eye pigmentation through pigment pathways, which the cinnabar
allele is a pigment protein which is involved in the actual pigment production, while the white
allele is a transport pigment which transports the pigments into the eye. The brown
pigmentation is given off by chromosomes which is synthesized in the pigment pathway and
gives off the color. There is also the pigment ptendines which gives off the color red and
yellows. While the white pigmentation is due to a lack of pigment in the eyes and the white is
also a transporter which does not produce enzymes, but transports the pigments to the eyes.
The cinnabar gene is located on the 2R chromosome while the white is located on the
X-chromosome. The eye pigmentation of cinnabar is not autonomous in development
(Beadle and Ephrussi 1936) while the white gene is x-linked. For the mutant cells of
cinnabar, the cells with the mutant genotype will not have a mutant phenotype if they are in
an organism or population of cells that have the wild-type genotype. (Beadle and Ephrussi
1936)
We performed our experiment by crossing the parents (p-generation) to get the F1
generation and then crossed the F1 generations to get F2 generation. The F2 generations were
then scored to understand the pigmentations more in depth by looking at the phenotypes
produced and to understand the results when these two eye pigments were crossed.
Our hypothesis when crossing both cinnabar males and white apricot females, we
predicted that our F2 generation females would be wild-type eye color, and our males would
In this experiment, we tested the eye pigmentations of cinnabar with white eyes on
Drosophila melanogaster. We used D. melanogaster because it is a good model organism
due to its short life span and large number of offspring. We tested the eye pigments with a
cinnabar female crossed by white males and did the reciprocal of that. The experiment was
conducted to understand eye pigmentations and how they are transported. For D.
melanogaster, it produces eye pigmentation through pigment pathways, which the cinnabar
allele is a pigment protein which is involved in the actual pigment production, while the white
allele is a transport pigment which transports the pigments into the eye. The brown
pigmentation is given off by chromosomes which is synthesized in the pigment pathway and
gives off the color. There is also the pigment ptendines which gives off the color red and
yellows. While the white pigmentation is due to a lack of pigment in the eyes and the white is
also a transporter which does not produce enzymes, but transports the pigments to the eyes.
The cinnabar gene is located on the 2R chromosome while the white is located on the
X-chromosome. The eye pigmentation of cinnabar is not autonomous in development
(Beadle and Ephrussi 1936) while the white gene is x-linked. For the mutant cells of
cinnabar, the cells with the mutant genotype will not have a mutant phenotype if they are in
an organism or population of cells that have the wild-type genotype. (Beadle and Ephrussi
1936)
We performed our experiment by crossing the parents (p-generation) to get the F1
generation and then crossed the F1 generations to get F2 generation. The F2 generations were
then scored to understand the pigmentations more in depth by looking at the phenotypes
produced and to understand the results when these two eye pigments were crossed.
Our hypothesis when crossing both cinnabar males and white apricot females, we
predicted that our F2 generation females would be wild-type eye color, and our males would
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

be cinnabar. Due to the white apricot gene being on the X-chromosome, we also predicted
that the F2 generation would consist of double mutants (orange). When crossing the white
females with the cinnabar males we predicted that all the F1 generation would be wild-type
eye color.
Materials and Methods
Two strains of Drosophila melanogaster were used in the study, flies with either
cinnabar or white eyes. Both phenotypes for the parental generation were obtained from stock
maintained at UW-Whitewater. The genotypes for the two parents were as follows:
1. cinnabar females (cn/ cn, Xw+/Xw+) and white males (cn+/cn+, Xw/Y)
2. cinnabar males (cn/cn, Xw+/Y) and white females (cn+/cn+, Xw/Xw)
Both the species were reared on a diet that comprised of molasses medium and
standard cornmeal. The medium had supplementary live yeast grains, with the aim of
promoting egg laying. The two parental species were maintained in 25-30ml vials that were
closed with foam plugs and stored in an incubator maintained at 25 degrees Celsius.
Two crosses were performed for the experiment. The first one involved crossing four
cinnabar males and two white-eyed females. The second one involved a reciprocal cross
between parental phenotypes of four cinnabar females and two white-eyed males. All females
used in the parental crosses were confirmed to be virgins, so there would not be a change of a
female sorting sperm for another male from a different mating. One week after establishing
the parental crosses, all vials were checked for larvae and the adults were removed and
discarded in a flask containing 70% EtOH.
For one pair in group three, cinnabar females were crossed with white males. This
cross failed to produce larvae, even when the crosses were attempted a second time. The
second pair was white females crossed with cinnabar males. This cross did produce larvae, so
that the F2 generation would consist of double mutants (orange). When crossing the white
females with the cinnabar males we predicted that all the F1 generation would be wild-type
eye color.
Materials and Methods
Two strains of Drosophila melanogaster were used in the study, flies with either
cinnabar or white eyes. Both phenotypes for the parental generation were obtained from stock
maintained at UW-Whitewater. The genotypes for the two parents were as follows:
1. cinnabar females (cn/ cn, Xw+/Xw+) and white males (cn+/cn+, Xw/Y)
2. cinnabar males (cn/cn, Xw+/Y) and white females (cn+/cn+, Xw/Xw)
Both the species were reared on a diet that comprised of molasses medium and
standard cornmeal. The medium had supplementary live yeast grains, with the aim of
promoting egg laying. The two parental species were maintained in 25-30ml vials that were
closed with foam plugs and stored in an incubator maintained at 25 degrees Celsius.
Two crosses were performed for the experiment. The first one involved crossing four
cinnabar males and two white-eyed females. The second one involved a reciprocal cross
between parental phenotypes of four cinnabar females and two white-eyed males. All females
used in the parental crosses were confirmed to be virgins, so there would not be a change of a
female sorting sperm for another male from a different mating. One week after establishing
the parental crosses, all vials were checked for larvae and the adults were removed and
discarded in a flask containing 70% EtOH.
For one pair in group three, cinnabar females were crossed with white males. This
cross failed to produce larvae, even when the crosses were attempted a second time. The
second pair was white females crossed with cinnabar males. This cross did produce larvae, so
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

the adults were removed and anesthetized, confirmed for the phenotype and gender, then
discarded in the flask with 70% EtOH. These vials were incubated for another week, then the
F1 adults were anesthetized and sorted by gender and phenotype under a dissecting
microscope. The F1 results were supposed to produce wild type females and white males by
crossing the cinnabar males and white females. Dr. Kriska allowed Group three to use the F1
wild type females and some white apricot males because there were no available white males.
After one week establishing the F1 crosses, the bottles were checked for larvae, the
adults were removed and anesthetized, and their numbers and phenotypes confirmed using a
dissecting scope before being disposed of in the flask with 70% EtOH. The following week,
adult F2’s was anesthetized and sorted by gender and phenotype. There was no F2 generation
for the cross between all wild-type flies that were obtained after mating between four
cinnabar males and two white females. On the other hand, the cross conducted between eight
cinnabar males and eight white apricot females of F1 generation resulted in four dissimilar
phenotypes in the F2 generation namely, (i) white apricot, (ii) wild-type, (iii) cinnabar, and
(iv) orange (double mutant).
Prominence of the differences between all the flies obtained in the F2 generation were
determined by using a chi squared test compare the observed and expected numbers of flies
in our four observed categories: wild type, white apricot, cinnabar, and double mutants,
which had a light orange color.
Results
(F1 Results of Original Parental Crosses)
When D. melanogaster was crossed with four cinnabar males and two white females,
the F1 offspring of the F1 generation were seven wild type. However, when three cinnabar
females were crossed with three white apricot males, the F1 generation offspring produced
discarded in the flask with 70% EtOH. These vials were incubated for another week, then the
F1 adults were anesthetized and sorted by gender and phenotype under a dissecting
microscope. The F1 results were supposed to produce wild type females and white males by
crossing the cinnabar males and white females. Dr. Kriska allowed Group three to use the F1
wild type females and some white apricot males because there were no available white males.
After one week establishing the F1 crosses, the bottles were checked for larvae, the
adults were removed and anesthetized, and their numbers and phenotypes confirmed using a
dissecting scope before being disposed of in the flask with 70% EtOH. The following week,
adult F2’s was anesthetized and sorted by gender and phenotype. There was no F2 generation
for the cross between all wild-type flies that were obtained after mating between four
cinnabar males and two white females. On the other hand, the cross conducted between eight
cinnabar males and eight white apricot females of F1 generation resulted in four dissimilar
phenotypes in the F2 generation namely, (i) white apricot, (ii) wild-type, (iii) cinnabar, and
(iv) orange (double mutant).
Prominence of the differences between all the flies obtained in the F2 generation were
determined by using a chi squared test compare the observed and expected numbers of flies
in our four observed categories: wild type, white apricot, cinnabar, and double mutants,
which had a light orange color.
Results
(F1 Results of Original Parental Crosses)
When D. melanogaster was crossed with four cinnabar males and two white females,
the F1 offspring of the F1 generation were seven wild type. However, when three cinnabar
females were crossed with three white apricot males, the F1 generation offspring produced
five wild-type females (red) and three cinnabar males. Cinnabar males were crossed with
white females. This cross would result in wild type females and white males. Cinnabar
females were crossed with white apricot males. This cross would result in wild-type females
and males.
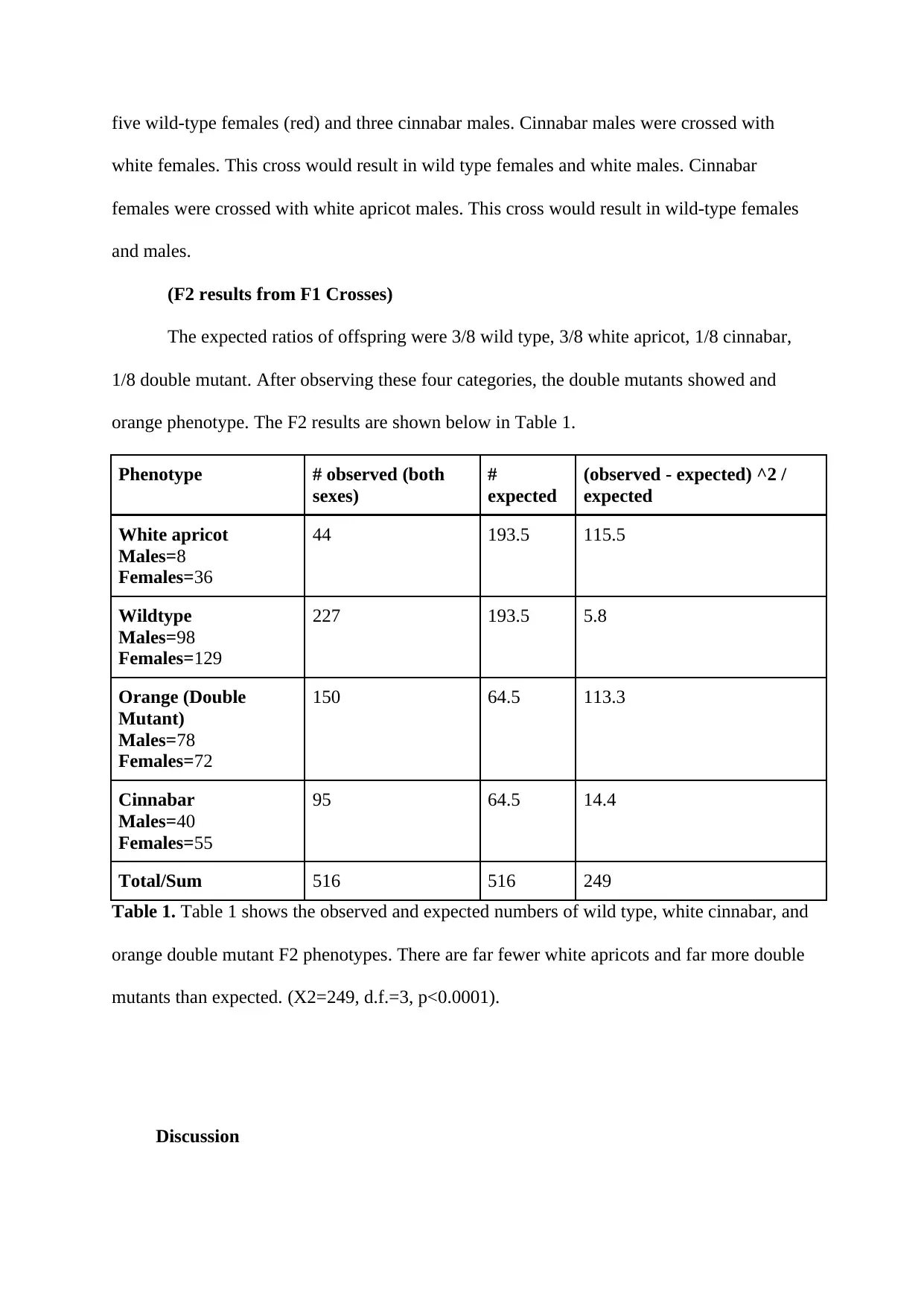
(F2 results from F1 Crosses)
The expected ratios of offspring were 3/8 wild type, 3/8 white apricot, 1/8 cinnabar,
1/8 double mutant. After observing these four categories, the double mutants showed and
orange phenotype. The F2 results are shown below in Table 1.
Phenotype # observed (both
sexes)
#
expected
(observed - expected) ^2 /
expected
White apricot
Males=8
Females=36
44 193.5 115.5
Wildtype
Males=98
Females=129
227 193.5 5.8
Orange (Double
Mutant)
Males=78
Females=72
150 64.5 113.3
Cinnabar
Males=40
Females=55
95 64.5 14.4
Total/Sum 516 516 249
Table 1. Table 1 shows the observed and expected numbers of wild type, white cinnabar, and
orange double mutant F2 phenotypes. There are far fewer white apricots and far more double
mutants than expected. (X2=249, d.f.=3, p<0.0001).
Discussion
white females. This cross would result in wild type females and white males. Cinnabar
females were crossed with white apricot males. This cross would result in wild-type females
and males.
(F2 results from F1 Crosses)
The expected ratios of offspring were 3/8 wild type, 3/8 white apricot, 1/8 cinnabar,
1/8 double mutant. After observing these four categories, the double mutants showed and
orange phenotype. The F2 results are shown below in Table 1.
Phenotype # observed (both
sexes)
#
expected
(observed - expected) ^2 /
expected
White apricot
Males=8
Females=36
44 193.5 115.5
Wildtype
Males=98
Females=129
227 193.5 5.8
Orange (Double
Mutant)
Males=78
Females=72
150 64.5 113.3
Cinnabar
Males=40
Females=55
95 64.5 14.4
Total/Sum 516 516 249
Table 1. Table 1 shows the observed and expected numbers of wild type, white cinnabar, and
orange double mutant F2 phenotypes. There are far fewer white apricots and far more double
mutants than expected. (X2=249, d.f.=3, p<0.0001).
Discussion
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The results obtained from this assessment illustrate that the crossing of the P
generation led to wild type phenotype of both females and males. This was quite contrary to
the expectations of this study being sought. The hypothesis tested attempted to assess the
cross on the parents between the cinnabes and white females. In all variation conducted, there
was the presence of wild type F1 generation offspring. The wild type flies – red eyes carrier
the white allele and white-eyed flies carry the white allele. The development of the brown
pigment obtained from the pigment pathways is responsible for the color development of the
F1 generation wild type. Despite this fact, some of the F1 generations generated other
different offspring of cinnabar males. Crossing the cinnabar males which its location is
situated on the 2 R chromosome and the autonomous, occurrence of would type and white
males were obtained due to the effect the white gene is located on the X chromosome thus
being homozygous to the offspring (Hardon & Mitchell 1951). More often F1 have unique
mutation obtained from mutation of inheritance from parents’ gremlin. The F1 stage results in
dominant mutations obtained from the parent's gene. The presence of mutant cells gene
situated on the cinnabar do not allow for the production of mutant phenotypes hence giving
off different colors of wild type males and white makes. This pathway depicted shows
different mutations; similar phenotypes often occur as a result of different mutations.
In the second assessment, the study tested various crosses of cinnabar males and
white apricot females, with of production of wild type eye color females and males being the
cinnabar. Further, due to X –chromosome on the apricot gene, F2 prediction was expected to
consist of double mutants’ genes having an orange color. The results yielded far more than
the expected results. White apricot having X chromosome having 8 males and 36 females
were obtained against the expected value of 193.5, while the wild type and organ double
mutant were overexpressed at p-value 0.001 as observed in table 1, the expected output was
widely affected by various factors during the production process. Factors often affect
generation led to wild type phenotype of both females and males. This was quite contrary to
the expectations of this study being sought. The hypothesis tested attempted to assess the
cross on the parents between the cinnabes and white females. In all variation conducted, there
was the presence of wild type F1 generation offspring. The wild type flies – red eyes carrier
the white allele and white-eyed flies carry the white allele. The development of the brown
pigment obtained from the pigment pathways is responsible for the color development of the
F1 generation wild type. Despite this fact, some of the F1 generations generated other
different offspring of cinnabar males. Crossing the cinnabar males which its location is
situated on the 2 R chromosome and the autonomous, occurrence of would type and white
males were obtained due to the effect the white gene is located on the X chromosome thus
being homozygous to the offspring (Hardon & Mitchell 1951). More often F1 have unique
mutation obtained from mutation of inheritance from parents’ gremlin. The F1 stage results in
dominant mutations obtained from the parent's gene. The presence of mutant cells gene
situated on the cinnabar do not allow for the production of mutant phenotypes hence giving
off different colors of wild type males and white makes. This pathway depicted shows
different mutations; similar phenotypes often occur as a result of different mutations.
In the second assessment, the study tested various crosses of cinnabar males and
white apricot females, with of production of wild type eye color females and males being the
cinnabar. Further, due to X –chromosome on the apricot gene, F2 prediction was expected to
consist of double mutants’ genes having an orange color. The results yielded far more than
the expected results. White apricot having X chromosome having 8 males and 36 females
were obtained against the expected value of 193.5, while the wild type and organ double
mutant were overexpressed at p-value 0.001 as observed in table 1, the expected output was
widely affected by various factors during the production process. Factors often affect
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

reproduction rate and thus affecting the growth development. Eggs hatched during the cross
over did not attain the needed stable conditions for the development and reproduction to
occur effectively in the mutations process (Kamleh et al., 2009).
The double mutants were far much more than expected while the white apricots
were far much less. This could be attributed to too few males in the white apricot affecting its
production levels and fewer males in the cinnabar also this lead to more females being
produced (Mertenes & Hammersmith, 2007). The chi-square obtained indicating the
difference occurring between the different mutations and phenotypes offsprings of F2
generation are occurring by chance. Thus the effect of the reciprocal process in mutation
could have a role to play. The effect of the reciprocal process undertaken in this task could
have an impact on the overall gene variation of the F2 phenotypes. The role of the parental
sex gene on the overall F2 phenotype could have been influenced through the subjection of
environmental factors limiting its expression. Female offspring g carrying the mutant white
eye allele but do not offer phenotypically thus indicating recessive gene (Kim, Kim & Yim,
2013).
Assessing parental mutation allows for crossing for the desired trait. The phenotypes
occurrence was attributed to overexpression and underexpression of the parental genotypes
genes leading to alteration in the crossing process. Further environmental changes could have
an effect on the overall progeny mutation process (Reame, Knecht & Chovnk, 1991). In the
reciprocal process, the male offspring which are white eyed often tend to give red-eyed
female offspring. The presence of recessive genes has led to the development of cinnabar
and orange double mutants which have been overexpressed while recessive genes are
associated with lower expressions of white apricot and wild type females and males
respectively (Ziegler, 2003).
over did not attain the needed stable conditions for the development and reproduction to
occur effectively in the mutations process (Kamleh et al., 2009).
The double mutants were far much more than expected while the white apricots
were far much less. This could be attributed to too few males in the white apricot affecting its
production levels and fewer males in the cinnabar also this lead to more females being
produced (Mertenes & Hammersmith, 2007). The chi-square obtained indicating the
difference occurring between the different mutations and phenotypes offsprings of F2
generation are occurring by chance. Thus the effect of the reciprocal process in mutation
could have a role to play. The effect of the reciprocal process undertaken in this task could
have an impact on the overall gene variation of the F2 phenotypes. The role of the parental
sex gene on the overall F2 phenotype could have been influenced through the subjection of
environmental factors limiting its expression. Female offspring g carrying the mutant white
eye allele but do not offer phenotypically thus indicating recessive gene (Kim, Kim & Yim,
2013).
Assessing parental mutation allows for crossing for the desired trait. The phenotypes
occurrence was attributed to overexpression and underexpression of the parental genotypes
genes leading to alteration in the crossing process. Further environmental changes could have
an effect on the overall progeny mutation process (Reame, Knecht & Chovnk, 1991). In the
reciprocal process, the male offspring which are white eyed often tend to give red-eyed
female offspring. The presence of recessive genes has led to the development of cinnabar
and orange double mutants which have been overexpressed while recessive genes are
associated with lower expressions of white apricot and wild type females and males
respectively (Ziegler, 2003).

Thus the results obtained in this study during the crossing the white females with the
cinnabar males prediction on F1 generation did not offer purely wild types mutations.
Further, the F2 generation results yielded fewer white apricots and more double mutants than
expected. Thus the ire is needed to improve on the overall reciprocal process and subsection
of temperature as it signifies alterations on the gene mutations in cross over effects of
phenotypes.
cinnabar males prediction on F1 generation did not offer purely wild types mutations.
Further, the F2 generation results yielded fewer white apricots and more double mutants than
expected. Thus the ire is needed to improve on the overall reciprocal process and subsection
of temperature as it signifies alterations on the gene mutations in cross over effects of
phenotypes.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References
Dickinson, W. J., & Sullivan, D. T. (2013). Gene-enzyme systems in Drosophila (Vol. 6).
Springer Science & Business Media.
Hadorn, E., & Mitchell, H. K. (1951). Properties of mutants of Drosophila melanogaster and
changes during development as revealed by paper chromatography. Proceedings of
the National Academy of Sciences of the United States of America, 37(10), 650.
Harnly, M. H., & Ephrussi, B. (1937). Development of eye colors in Drosophila: time of
action of body fluid on cinnabar. Genetics, 22(3), 393.
Kamleh, M. A., Hobani, Y., Dow, J. A., Zheng, L., & Watson, D. G. (2009). Towards a
platform for the metabonomic profiling of different strains of Drosophila
melanogaster using liquid chromatography–Fourier transform mass spectrometry. The
FEBS journal, 276(22), 6798-6809.
Kim, H., Kim, K., & Yim, J. (2013). Biosynthesis of drosopterins, the red eye pigments of
Drosophila melanogaster. IUBMB life, 65(4), 334-340.
Kinjo, Hirotoshi, Yasuhisa Kunimi, and Madoka Nakai (2014). "Effects of temperature on
the reproduction and development of Drosophila suzukii (Diptera: Drosophilidae)."
Applied entomology and zoology 49(.2): 297-304.
Li, C., Wang, Z., & Zhang, J. (2013). Toward Genome-Wide Identification of Bateson–
Dobzhansky–Muller Incompatibilities in Yeast: A Simulation Study. Genome biology
and evolution, 5(7), 1261-1272.
Lin, S., Owald, D., Chandra, V., Talbot, C., Huetteroth, W., & Waddell, S. (2014). Neural
correlates of water reward in thirsty Drosophila. Nature neuroscience, 17(11), 1536.
Lőrincz, P., Takáts, S., Kárpáti, M., & Juhász, G. (2016). iFly: The eye of the fruit fly as a
model to study autophagy and related trafficking pathways. Experimental eye
research, 144, 90-98.
Mertens, T.R. and R.L. Hammersmith. (2007). Genetics laboratory investigations. Pearson-
Prentice Hall. Upper Saddle River,66(2)211-217.
Dickinson, W. J., & Sullivan, D. T. (2013). Gene-enzyme systems in Drosophila (Vol. 6).
Springer Science & Business Media.
Hadorn, E., & Mitchell, H. K. (1951). Properties of mutants of Drosophila melanogaster and
changes during development as revealed by paper chromatography. Proceedings of
the National Academy of Sciences of the United States of America, 37(10), 650.
Harnly, M. H., & Ephrussi, B. (1937). Development of eye colors in Drosophila: time of
action of body fluid on cinnabar. Genetics, 22(3), 393.
Kamleh, M. A., Hobani, Y., Dow, J. A., Zheng, L., & Watson, D. G. (2009). Towards a
platform for the metabonomic profiling of different strains of Drosophila
melanogaster using liquid chromatography–Fourier transform mass spectrometry. The
FEBS journal, 276(22), 6798-6809.
Kim, H., Kim, K., & Yim, J. (2013). Biosynthesis of drosopterins, the red eye pigments of
Drosophila melanogaster. IUBMB life, 65(4), 334-340.
Kinjo, Hirotoshi, Yasuhisa Kunimi, and Madoka Nakai (2014). "Effects of temperature on
the reproduction and development of Drosophila suzukii (Diptera: Drosophilidae)."
Applied entomology and zoology 49(.2): 297-304.
Li, C., Wang, Z., & Zhang, J. (2013). Toward Genome-Wide Identification of Bateson–
Dobzhansky–Muller Incompatibilities in Yeast: A Simulation Study. Genome biology
and evolution, 5(7), 1261-1272.
Lin, S., Owald, D., Chandra, V., Talbot, C., Huetteroth, W., & Waddell, S. (2014). Neural
correlates of water reward in thirsty Drosophila. Nature neuroscience, 17(11), 1536.
Lőrincz, P., Takáts, S., Kárpáti, M., & Juhász, G. (2016). iFly: The eye of the fruit fly as a
model to study autophagy and related trafficking pathways. Experimental eye
research, 144, 90-98.
Mertens, T.R. and R.L. Hammersmith. (2007). Genetics laboratory investigations. Pearson-
Prentice Hall. Upper Saddle River,66(2)211-217.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Nassau, K. (1983). The physics and chemistry of color. John Wiley & Sons, Inc.pp. 111,
287.
Reaume, A. G., Knecht, D. A., & Chovnick, A. (1991). The rosy locus in Drosophila
melanogaster: xanthine dehydrogenase and eye pigments. Genetics, 129(4), 1099-
1109.
Sharpe, D. (2015). Your chi-square test is statistically significant: now what?. Practical
Assessment, Research & Evaluation, 20.
Tatar, M., Post, S., & Yu, K. (2014). Nutrient control of Drosophila longevity. Trends in
Endocrinology & Metabolism, 25(10), 509-517.
Yamamoto, S., Jaiswal, M., Charng, W. L., Gambin, T., Karaca, E., Mirzaa, G., ... & Zhang,
K. (2014). A drosophila genetic resource of mutants to study mechanisms underlying
human genetic diseases. Cell, 159(1), 200-214.
Ziegler, I. (2003). The pteridine pathway in zebrafish: regulation and specification during the
determination of neural crest cell‐fate. Pigment cell research, 16(3), 172-182.
287.
Reaume, A. G., Knecht, D. A., & Chovnick, A. (1991). The rosy locus in Drosophila
melanogaster: xanthine dehydrogenase and eye pigments. Genetics, 129(4), 1099-
1109.
Sharpe, D. (2015). Your chi-square test is statistically significant: now what?. Practical
Assessment, Research & Evaluation, 20.
Tatar, M., Post, S., & Yu, K. (2014). Nutrient control of Drosophila longevity. Trends in
Endocrinology & Metabolism, 25(10), 509-517.
Yamamoto, S., Jaiswal, M., Charng, W. L., Gambin, T., Karaca, E., Mirzaa, G., ... & Zhang,
K. (2014). A drosophila genetic resource of mutants to study mechanisms underlying
human genetic diseases. Cell, 159(1), 200-214.
Ziegler, I. (2003). The pteridine pathway in zebrafish: regulation and specification during the
determination of neural crest cell‐fate. Pigment cell research, 16(3), 172-182.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





