CAR T-Cell Therapy: Exploring the Advancement in Cancer Immunotherapy
VerifiedAdded on 2021/10/07
|9
|2683
|177
Report
AI Summary
This report delves into the innovative field of CAR T-cell therapy, a groundbreaking advancement in cancer treatment. It explains the therapy's mechanism, which involves modifying a patient's T-cells to target and destroy cancerous cells, primarily effective against leukemia and non-Hodgkin lymphoma. The paper details the process, from T-cell isolation and modification to infusion, highlighting the therapy's evolution, including the development of second-generation CARs and the FDA approval in 2017. The report also addresses the associated side effects, clinical challenges, and the high costs involved. It concludes by emphasizing the potential of CAR T-cell therapy as a live therapy that modifies the immune system to stop tumor growth, and the ongoing efforts to improve its efficacy and accessibility, including the efforts by the Canadian government to promote research and innovation in the development of therapeutic cell technologies.

Running head: THE NEW ERA OF CANCER TREATMENT
CAR T CELLS- A NEW ERA OF CANCER IMMUNOTHERAPY
Name of the Student:
Name of the University:
Author Note:
CAR T CELLS- A NEW ERA OF CANCER IMMUNOTHERAPY
Name of the Student:
Name of the University:
Author Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
CAR T CELLS- A NEW ERA OF CANCER IMMUNOTHERAPY
The field of science and technology is witnessing remarkable development in terms of
advancement and innovation. It is worth mentioning here that the human race has been
affected with a number of life-threatening diseases such as Cancer and autoimmune
disorders. Till the last decade, the human race struggled to fight against the quagmire but
there was no absolute treatment available for the life-threatening disease cancer. However,
recently there has been ground breaking inventions in the field of molecular biology that has
changed the fate of the scenario. Till date chemotherapy had been considered as the most
obvious treatment for cancer at initial or advanced stages but the medical cost associated with
the treatment procedure elevated the plight of the patients. Scientists have now come up with
the concept of CAR T CELLS that are thought to be extremely effective for treating cancer.
This paper intends to discuss the efficacy of CAR T CELLS in the treatment process of
cancer. It would also elaborate the properties of the therapy and talk about the associated
advantages that makes it a boon to the mankind.
The CAR T CELL therapy can be defined as a therapeutic procedure that involves the
redesigning of the T cells of the patient so that it targets the cancerous cells and destroys
them (Brentjens et al., 2013). It is more like an army training being imparted to the T cells of
the human body in order to prepare them to fight against the opponent party. The procedure
proceeds with the isolation of the T-cells from the human blood. After the procedure of
isolation, the gene that contains the receptor for binding the proteins expressed by the cancer
cells is modified within the laboratory. The receptor is known as the Cheminergic receptor
which is popularly known as the CAR. A large number of CAR T cells are thus grown in the
laboratory that is administered to the patient through the process of infusion (Davila et al.,
2014). The injected CAR T cells thus target the cancerous cells and destroy them so as to
CAR T CELLS- A NEW ERA OF CANCER IMMUNOTHERAPY
The field of science and technology is witnessing remarkable development in terms of
advancement and innovation. It is worth mentioning here that the human race has been
affected with a number of life-threatening diseases such as Cancer and autoimmune
disorders. Till the last decade, the human race struggled to fight against the quagmire but
there was no absolute treatment available for the life-threatening disease cancer. However,
recently there has been ground breaking inventions in the field of molecular biology that has
changed the fate of the scenario. Till date chemotherapy had been considered as the most
obvious treatment for cancer at initial or advanced stages but the medical cost associated with
the treatment procedure elevated the plight of the patients. Scientists have now come up with
the concept of CAR T CELLS that are thought to be extremely effective for treating cancer.
This paper intends to discuss the efficacy of CAR T CELLS in the treatment process of
cancer. It would also elaborate the properties of the therapy and talk about the associated
advantages that makes it a boon to the mankind.
The CAR T CELL therapy can be defined as a therapeutic procedure that involves the
redesigning of the T cells of the patient so that it targets the cancerous cells and destroys
them (Brentjens et al., 2013). It is more like an army training being imparted to the T cells of
the human body in order to prepare them to fight against the opponent party. The procedure
proceeds with the isolation of the T-cells from the human blood. After the procedure of
isolation, the gene that contains the receptor for binding the proteins expressed by the cancer
cells is modified within the laboratory. The receptor is known as the Cheminergic receptor
which is popularly known as the CAR. A large number of CAR T cells are thus grown in the
laboratory that is administered to the patient through the process of infusion (Davila et al.,
2014). The injected CAR T cells thus target the cancerous cells and destroy them so as to
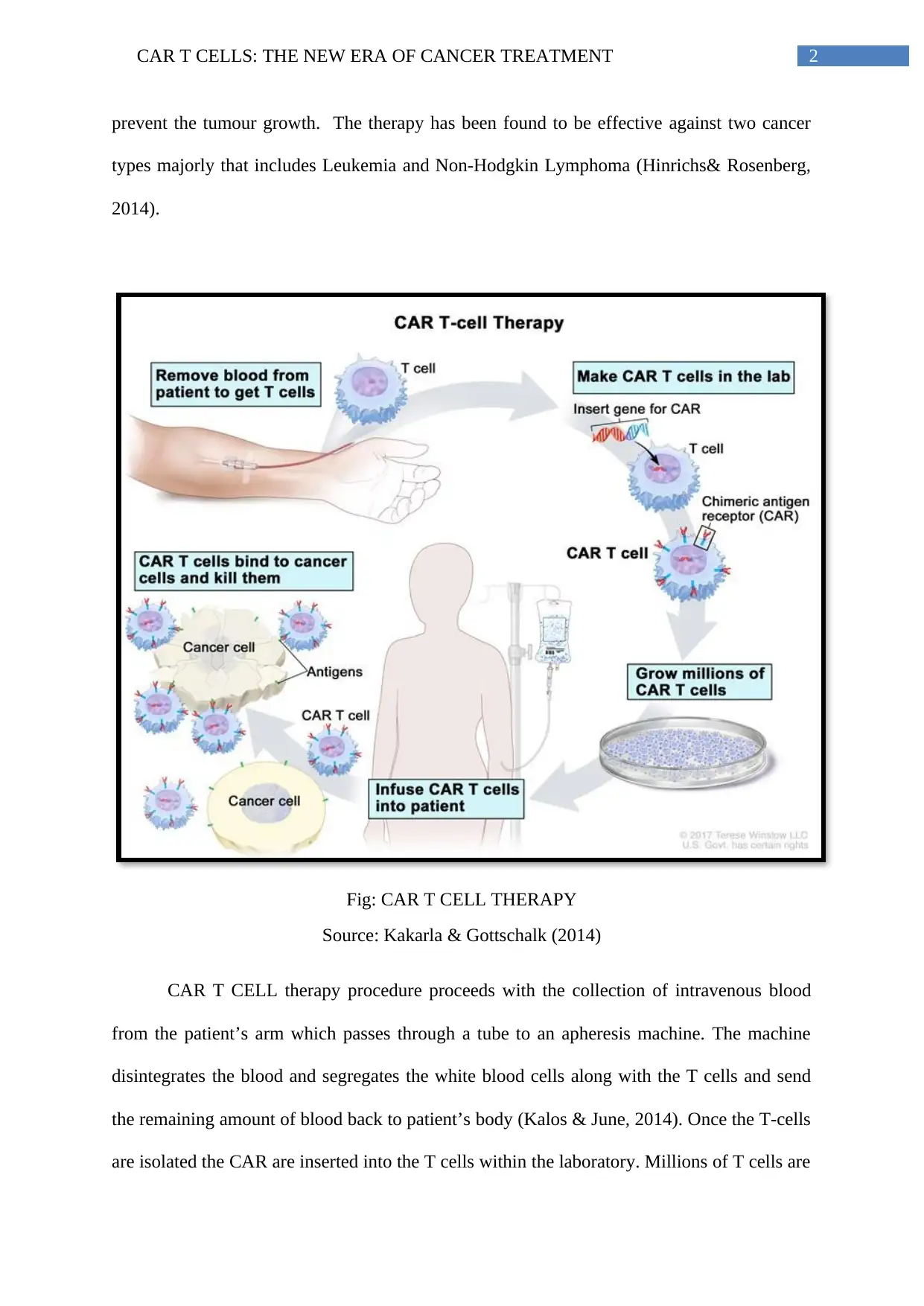
2CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
prevent the tumour growth. The therapy has been found to be effective against two cancer
types majorly that includes Leukemia and Non-Hodgkin Lymphoma (Hinrichs& Rosenberg,
2014).
Fig: CAR T CELL THERAPY
Source: Kakarla & Gottschalk (2014)
CAR T CELL therapy procedure proceeds with the collection of intravenous blood
from the patient’s arm which passes through a tube to an apheresis machine. The machine
disintegrates the blood and segregates the white blood cells along with the T cells and send
the remaining amount of blood back to patient’s body (Kalos & June, 2014). Once the T-cells
are isolated the CAR are inserted into the T cells within the laboratory. Millions of T cells are
prevent the tumour growth. The therapy has been found to be effective against two cancer
types majorly that includes Leukemia and Non-Hodgkin Lymphoma (Hinrichs& Rosenberg,
2014).
Fig: CAR T CELL THERAPY
Source: Kakarla & Gottschalk (2014)
CAR T CELL therapy procedure proceeds with the collection of intravenous blood
from the patient’s arm which passes through a tube to an apheresis machine. The machine
disintegrates the blood and segregates the white blood cells along with the T cells and send
the remaining amount of blood back to patient’s body (Kalos & June, 2014). Once the T-cells
are isolated the CAR are inserted into the T cells within the laboratory. Millions of T cells are
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
grown and transferred to the human body through the process of infusion. The CAR T cells
once infused inside the human body bind to the antigen on the cancerous cells and proceed
with the killing of the cells.
The T-cell lymphocytes play a central role in eliciting the T-cell mediated immune
response. The T cells mature from the thymus and possess a T-cell receptor on the surface of
the cells. A major proportion of the T-cells are termed as alpha beta T cells that form an
integral part of the adaptive immune response. The alpha beta cells rearrange their alpha beta
chains in accordance with the functional property (Kochenderfer et al., 2015). It should be
noted here that a group of specialized gamma delta T cells possess T cell receptors with
restricted diversity that can potentially present antigen to other T cells. On the other hand,
CARS can be considered as the recombinant constructs of T cell receptors (Koneru et al.,
2015). The recombinant receptor is then joined to a spacer peptide and a transmembrane
domain that is consecutively linked to the T-cell signalling g domains of the T-cell receptor
intracellularly. Hence in this regard, it can be said that the CAR T cells are modified forms of
T cells that possess the combination and specificity of an antibody with the memory and the
cytotoxic property of the T-cells (Qasim et al.,2017).
In the year 1992, Michael Sadelain was the first person to use biotechnological tools
in order to introduce the genes into the T cells so as to stimulate cancer fighter cells. The year
1993, witnessed the first generation of the CAR-S. The renowned immunologist ZeligEshhar
modified the T-cells with the initial chimeric molecule that formed a part of the antibody that
fused with the T-cell receptor. However, the clinical effectiveness of the first generation of
CAR was not of much significance. In the year 1994, the virus specific T-cells were used for
the first time in stem cell transplants. The year 1998, witnessed the second generation CARS
that showed a unique property which proved that introduction of a co-stimulatory molecule
can facilitate pertinent activeness of the engineered T-cells (Rosenberg & Restifo, 2015). In
grown and transferred to the human body through the process of infusion. The CAR T cells
once infused inside the human body bind to the antigen on the cancerous cells and proceed
with the killing of the cells.
The T-cell lymphocytes play a central role in eliciting the T-cell mediated immune
response. The T cells mature from the thymus and possess a T-cell receptor on the surface of
the cells. A major proportion of the T-cells are termed as alpha beta T cells that form an
integral part of the adaptive immune response. The alpha beta cells rearrange their alpha beta
chains in accordance with the functional property (Kochenderfer et al., 2015). It should be
noted here that a group of specialized gamma delta T cells possess T cell receptors with
restricted diversity that can potentially present antigen to other T cells. On the other hand,
CARS can be considered as the recombinant constructs of T cell receptors (Koneru et al.,
2015). The recombinant receptor is then joined to a spacer peptide and a transmembrane
domain that is consecutively linked to the T-cell signalling g domains of the T-cell receptor
intracellularly. Hence in this regard, it can be said that the CAR T cells are modified forms of
T cells that possess the combination and specificity of an antibody with the memory and the
cytotoxic property of the T-cells (Qasim et al.,2017).
In the year 1992, Michael Sadelain was the first person to use biotechnological tools
in order to introduce the genes into the T cells so as to stimulate cancer fighter cells. The year
1993, witnessed the first generation of the CAR-S. The renowned immunologist ZeligEshhar
modified the T-cells with the initial chimeric molecule that formed a part of the antibody that
fused with the T-cell receptor. However, the clinical effectiveness of the first generation of
CAR was not of much significance. In the year 1994, the virus specific T-cells were used for
the first time in stem cell transplants. The year 1998, witnessed the second generation CARS
that showed a unique property which proved that introduction of a co-stimulatory molecule
can facilitate pertinent activeness of the engineered T-cells (Rosenberg & Restifo, 2015). In
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
the year 2002, the second generation CAR T cells were successfully used against a Prostate
cancer antigen. In the year 2003, Dr Sadelain and his team worked to design CARS against
CD19 (Rosenberg &Restifo, 2015). In the year, 2009, published the protocol of the
manufacturing process of CD19 CAR cells which was found to be effective in treating
Leukemia in patients with recurrent cancer symptoms after chemotherapy (Qasim et al.,
2017) .In the year 2014, the CAR T therapy was designated as the major breakthrough
therapy in the history of molecular biology. Mesothilin directed CARS were developed that
helped in the identification of solid tumours also known as Mesothilin (Qasim et al., 2017). In
the year 2015, armored CARS were developed as a potential therapy for ovarian cancer. The
first half of the year 2017, witnessed another revolution in the field of Molecular Biology that
popularised the concept of CRISPR technology to place CAR at a specific location so as to
improvise the functioning of T-cells. CAR T therapy was approved by the FDA in 2017 and
was designated as the standard treatment therapy for lymphoblastic lymphoma in children
and adults (Qasim et al., 2017).
The procedure of administering CAR T cell therapy is invasive. The procedure takes
about a few weeks. The initial step includes performing leukapheresis. The patient is either
made to sit comfortably or lie down and two IV needles are then inserted. One carries the
blood back to the body and the other removes the blood from the body. A central intravenous
catheter is sometimes inserted that comprises of both the intravenous needles. The patient is
supposed to remain still for a time period of approximately 2 hours. During the procedure, the
patient might feel numbness in the muscles which happens because of reduced calcium
levels. This is treated by administering calcium through the IV catheter (Turtle et al., 2016).
Finally the WBC is extracted from the patient’s blood sample and sent to the laboratory for
the further steps. At present, three CAR T therapies have been approved in the United States
of America, that include the treatment of acute lymphoblastic Leukemia in children and
the year 2002, the second generation CAR T cells were successfully used against a Prostate
cancer antigen. In the year 2003, Dr Sadelain and his team worked to design CARS against
CD19 (Rosenberg &Restifo, 2015). In the year, 2009, published the protocol of the
manufacturing process of CD19 CAR cells which was found to be effective in treating
Leukemia in patients with recurrent cancer symptoms after chemotherapy (Qasim et al.,
2017) .In the year 2014, the CAR T therapy was designated as the major breakthrough
therapy in the history of molecular biology. Mesothilin directed CARS were developed that
helped in the identification of solid tumours also known as Mesothilin (Qasim et al., 2017). In
the year 2015, armored CARS were developed as a potential therapy for ovarian cancer. The
first half of the year 2017, witnessed another revolution in the field of Molecular Biology that
popularised the concept of CRISPR technology to place CAR at a specific location so as to
improvise the functioning of T-cells. CAR T therapy was approved by the FDA in 2017 and
was designated as the standard treatment therapy for lymphoblastic lymphoma in children
and adults (Qasim et al., 2017).
The procedure of administering CAR T cell therapy is invasive. The procedure takes
about a few weeks. The initial step includes performing leukapheresis. The patient is either
made to sit comfortably or lie down and two IV needles are then inserted. One carries the
blood back to the body and the other removes the blood from the body. A central intravenous
catheter is sometimes inserted that comprises of both the intravenous needles. The patient is
supposed to remain still for a time period of approximately 2 hours. During the procedure, the
patient might feel numbness in the muscles which happens because of reduced calcium
levels. This is treated by administering calcium through the IV catheter (Turtle et al., 2016).
Finally the WBC is extracted from the patient’s blood sample and sent to the laboratory for
the further steps. At present, three CAR T therapies have been approved in the United States
of America, that include the treatment of acute lymphoblastic Leukemia in children and

5CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
adults, B-cell lymphoma and non-Hodgkin’s lymphoma. The CAR T therapy has shown
positive results in the clinical trials of the mentioned types of cancer (Turtle et al., 2016). It
should be noted here that the CAR T therapy has been associated with a number of severe
side-effects pertaining to the fact the CAR T cells multiply inside the human body to
effectively fight against cancer. Some of the serious side-effects include, high temperature
and low blood pressure. Further, other invasive side-effects include, neurotoxicity and
changes in the brain functioning (Carpenter et al., 2013). The changes in the brain
functioning is characterized by, confusion, seizures and serious headaches. Serious infections,
lower RBC count and a weakened immune response have also been attributes as major side-
effects which cause life-threatening risks.
Some of the most prevalent clinical challenges associated with CAR T therapy
includes proper management of the side effects and stringent monitoring so as to avoid the
worsening of the side-effects. Other clinical challenges also include, promoting continuity in
patients undergoing CAR T cell therapy. Studies reveal that on many occasions the CART
cells end up killing off target healthy B cells in patients with Leukemia (The Alliance of
Advanced BioMedical Engineering, 2018). This leads to the creation of adverse situation that
might even lead to fatal death. In addition to this, it should further be noted that high medical
cost expenditure involved in availing the therapy has served to be the main reason why the
therapy has not emerged out to be a popular one. Despite the enhance effectiveness of the
therapy, it is still not popular among a major proportion of the global population (Carpenter et
al., 2013). Hence, there is a need to adapt measures to spread awareness and popularise the
therapeutic process. The two approve CAR T therapy are associated with a heavy financial
expenditure of almost $400,000 dollars each. Studies have revealed that the effective
intervention for the management of side-effects include, administration of tocilizumab which
involves expenditure up to $2500 per dose (The Alliance of Advanced BioMedical
adults, B-cell lymphoma and non-Hodgkin’s lymphoma. The CAR T therapy has shown
positive results in the clinical trials of the mentioned types of cancer (Turtle et al., 2016). It
should be noted here that the CAR T therapy has been associated with a number of severe
side-effects pertaining to the fact the CAR T cells multiply inside the human body to
effectively fight against cancer. Some of the serious side-effects include, high temperature
and low blood pressure. Further, other invasive side-effects include, neurotoxicity and
changes in the brain functioning (Carpenter et al., 2013). The changes in the brain
functioning is characterized by, confusion, seizures and serious headaches. Serious infections,
lower RBC count and a weakened immune response have also been attributes as major side-
effects which cause life-threatening risks.
Some of the most prevalent clinical challenges associated with CAR T therapy
includes proper management of the side effects and stringent monitoring so as to avoid the
worsening of the side-effects. Other clinical challenges also include, promoting continuity in
patients undergoing CAR T cell therapy. Studies reveal that on many occasions the CART
cells end up killing off target healthy B cells in patients with Leukemia (The Alliance of
Advanced BioMedical Engineering, 2018). This leads to the creation of adverse situation that
might even lead to fatal death. In addition to this, it should further be noted that high medical
cost expenditure involved in availing the therapy has served to be the main reason why the
therapy has not emerged out to be a popular one. Despite the enhance effectiveness of the
therapy, it is still not popular among a major proportion of the global population (Carpenter et
al., 2013). Hence, there is a need to adapt measures to spread awareness and popularise the
therapeutic process. The two approve CAR T therapy are associated with a heavy financial
expenditure of almost $400,000 dollars each. Studies have revealed that the effective
intervention for the management of side-effects include, administration of tocilizumab which
involves expenditure up to $2500 per dose (The Alliance of Advanced BioMedical
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
Engineering, 2018). In order to make the treatment intervention feasible the provider of the
Medicare Advantage plan aimed at launching a coverage policy for CAR-T therapy so as to
ensure adequate access to treatment opportunities for all. The law intends to help patients
who are 65 years and above and intends to impart treatment at a feasible rate (The Alliance of
Advanced BioMedical Engineering, 2018).
Hence, it can be hoped that with the passing years the technological innovation would
revolutionise the CAR T therapeutic intervention and completely cure cancer of all types.
Further, researchers are also trying to develop methodologies by virtue of which the side-
effects can be treated automatically and the CAR T cells could automatically deactivate itself
once it kills the cancerous cells. With respect to the medical scenario in Canada, it can be
said that the CAR T therapy is gaining wide popularity. The government of Canada has
collaborated with GE life sciences to develop an innovative research hub that would
primarily focus on developing CAR T therapies and other associated cellular therapies. The
Canadian government has extended a $ 20 million grant from Canada’s Federal Economic
Development Agency for Southern Ontario in order to encourage research and innovation in
the development of therapeutic cell technologies (The Alliance of Advanced BioMedical
Engineering, 2018).
Therefore, to conclude it can be said that CAR T therapy has emerged out to be a live
therapy that modified the immune system so as to stop tumour growth. At this phase, the
therapy has been found effective in treating a limited number of cancerous disorders,
however the research is area is promising and soon it would serve as an ultimate respite for
all types of cancer.
Engineering, 2018). In order to make the treatment intervention feasible the provider of the
Medicare Advantage plan aimed at launching a coverage policy for CAR-T therapy so as to
ensure adequate access to treatment opportunities for all. The law intends to help patients
who are 65 years and above and intends to impart treatment at a feasible rate (The Alliance of
Advanced BioMedical Engineering, 2018).
Hence, it can be hoped that with the passing years the technological innovation would
revolutionise the CAR T therapeutic intervention and completely cure cancer of all types.
Further, researchers are also trying to develop methodologies by virtue of which the side-
effects can be treated automatically and the CAR T cells could automatically deactivate itself
once it kills the cancerous cells. With respect to the medical scenario in Canada, it can be
said that the CAR T therapy is gaining wide popularity. The government of Canada has
collaborated with GE life sciences to develop an innovative research hub that would
primarily focus on developing CAR T therapies and other associated cellular therapies. The
Canadian government has extended a $ 20 million grant from Canada’s Federal Economic
Development Agency for Southern Ontario in order to encourage research and innovation in
the development of therapeutic cell technologies (The Alliance of Advanced BioMedical
Engineering, 2018).
Therefore, to conclude it can be said that CAR T therapy has emerged out to be a live
therapy that modified the immune system so as to stop tumour growth. At this phase, the
therapy has been found effective in treating a limited number of cancerous disorders,
however the research is area is promising and soon it would serve as an ultimate respite for
all types of cancer.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
References:
Brentjens, R. J., Davila, M. L., Riviere, I., Park, J., Wang, X., Cowell, L. G., ...&Borquez-
Ojeda, O. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults
with chemotherapy-refractory acute lymphoblastic leukemia. Science translational
medicine, 5(177), 177ra38-177ra38.
Carpenter, R. O., Evbuomwan, M. O., Pittaluga, S., Rose, J. J., Raffeld, M., Yang,
S., ...&Kochenderfer, J. N. (2013). B-cell maturation antigen is a promising target for
adoptive T-cell therapy of multiple myeloma. Clinical cancer research.
Davila, M. L., Riviere, I., Wang, X., Bartido, S., Park, J., Curran, K., ...& Qu, J. (2014).
Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute
lymphoblastic leukemia. Science translational medicine, 6(224), 224ra25-224ra25.
Hinrichs, C. S., & Rosenberg, S. A. (2014). Exploiting the curative potential of adoptive T‐
cell therapy for cancer. Immunological reviews, 257(1), 56-71.
Kakarla, S., & Gottschalk, S. (2014). CAR T cells for solid tumors: armed and ready to
go?. Cancer journal (Sudbury, Mass.), 20(2), 151.
Kalos, M., & June, C. H. (2013). Adoptive T cell transfer for cancer immunotherapy in the
era of synthetic biology. Immunity, 39(1), 49-60.
Kochenderfer, J. N., Dudley, M. E., Kassim, S. H., Somerville, R. P., Carpenter, R. O.,
Stetler-Stevenson, M., ... &Raffeld, M. (2015). Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively treated with
autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of
Clinical Oncology, 33(6), 540.
References:
Brentjens, R. J., Davila, M. L., Riviere, I., Park, J., Wang, X., Cowell, L. G., ...&Borquez-
Ojeda, O. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults
with chemotherapy-refractory acute lymphoblastic leukemia. Science translational
medicine, 5(177), 177ra38-177ra38.
Carpenter, R. O., Evbuomwan, M. O., Pittaluga, S., Rose, J. J., Raffeld, M., Yang,
S., ...&Kochenderfer, J. N. (2013). B-cell maturation antigen is a promising target for
adoptive T-cell therapy of multiple myeloma. Clinical cancer research.
Davila, M. L., Riviere, I., Wang, X., Bartido, S., Park, J., Curran, K., ...& Qu, J. (2014).
Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute
lymphoblastic leukemia. Science translational medicine, 6(224), 224ra25-224ra25.
Hinrichs, C. S., & Rosenberg, S. A. (2014). Exploiting the curative potential of adoptive T‐
cell therapy for cancer. Immunological reviews, 257(1), 56-71.
Kakarla, S., & Gottschalk, S. (2014). CAR T cells for solid tumors: armed and ready to
go?. Cancer journal (Sudbury, Mass.), 20(2), 151.
Kalos, M., & June, C. H. (2013). Adoptive T cell transfer for cancer immunotherapy in the
era of synthetic biology. Immunity, 39(1), 49-60.
Kochenderfer, J. N., Dudley, M. E., Kassim, S. H., Somerville, R. P., Carpenter, R. O.,
Stetler-Stevenson, M., ... &Raffeld, M. (2015). Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively treated with
autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of
Clinical Oncology, 33(6), 540.

8CAR T CELLS: THE NEW ERA OF CANCER TREATMENT
Koneru, M., O’Cearbhaill, R., Pendharkar, S., Spriggs, D. R., &Brentjens, R. J. (2015). A
phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16 ecto
directed chimeric antigen receptors for recurrent ovarian cancer. Journal of
translational medicine, 13(1), 102.
Qasim, W., Zhan, H., Samarasinghe, S., Adams, S., Amrolia, P., Stafford, S., ...&Ghorashian,
S. (2017). Molecular remission of infant B-ALL after infusion of universal TALEN
gene-edited CAR T cells. Science translational medicine, 9(374), eaaj2013.
Rosenberg, S. A., &Restifo, N. P. (2015). Adoptive cell transfer as personalized
immunotherapy for human cancer. Science, 348(6230), 62-68.
The Alliance of Advanced BioMedical Engineering (2018). Canada Joins the CAR T-
Cell Club. [online] The Alliance of Advanced BioMedical Engineering. Available
at: https://aabme.asme.org/posts/canada-joins-the-car-t-club [Accessed 5 Nov. 2018].
Turtle, C. J., Hanafi, L. A., Berger, C., Gooley, T. A., Cherian, S., Hudecek, M., ...&
Robinson, E. (2016). CD19 CAR–T cells of defined CD4+: CD8+ composition in
adult B cell ALL patients. The Journal of clinical investigation, 126(6), 2123-2138.
Koneru, M., O’Cearbhaill, R., Pendharkar, S., Spriggs, D. R., &Brentjens, R. J. (2015). A
phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16 ecto
directed chimeric antigen receptors for recurrent ovarian cancer. Journal of
translational medicine, 13(1), 102.
Qasim, W., Zhan, H., Samarasinghe, S., Adams, S., Amrolia, P., Stafford, S., ...&Ghorashian,
S. (2017). Molecular remission of infant B-ALL after infusion of universal TALEN
gene-edited CAR T cells. Science translational medicine, 9(374), eaaj2013.
Rosenberg, S. A., &Restifo, N. P. (2015). Adoptive cell transfer as personalized
immunotherapy for human cancer. Science, 348(6230), 62-68.
The Alliance of Advanced BioMedical Engineering (2018). Canada Joins the CAR T-
Cell Club. [online] The Alliance of Advanced BioMedical Engineering. Available
at: https://aabme.asme.org/posts/canada-joins-the-car-t-club [Accessed 5 Nov. 2018].
Turtle, C. J., Hanafi, L. A., Berger, C., Gooley, T. A., Cherian, S., Hudecek, M., ...&
Robinson, E. (2016). CD19 CAR–T cells of defined CD4+: CD8+ composition in
adult B cell ALL patients. The Journal of clinical investigation, 126(6), 2123-2138.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
