Role of Hsp70 Chaperones in Mitochondrial β-barrel Protein Biogenesis
VerifiedAdded on 2022/10/04
|6
|330
|18
Report
AI Summary
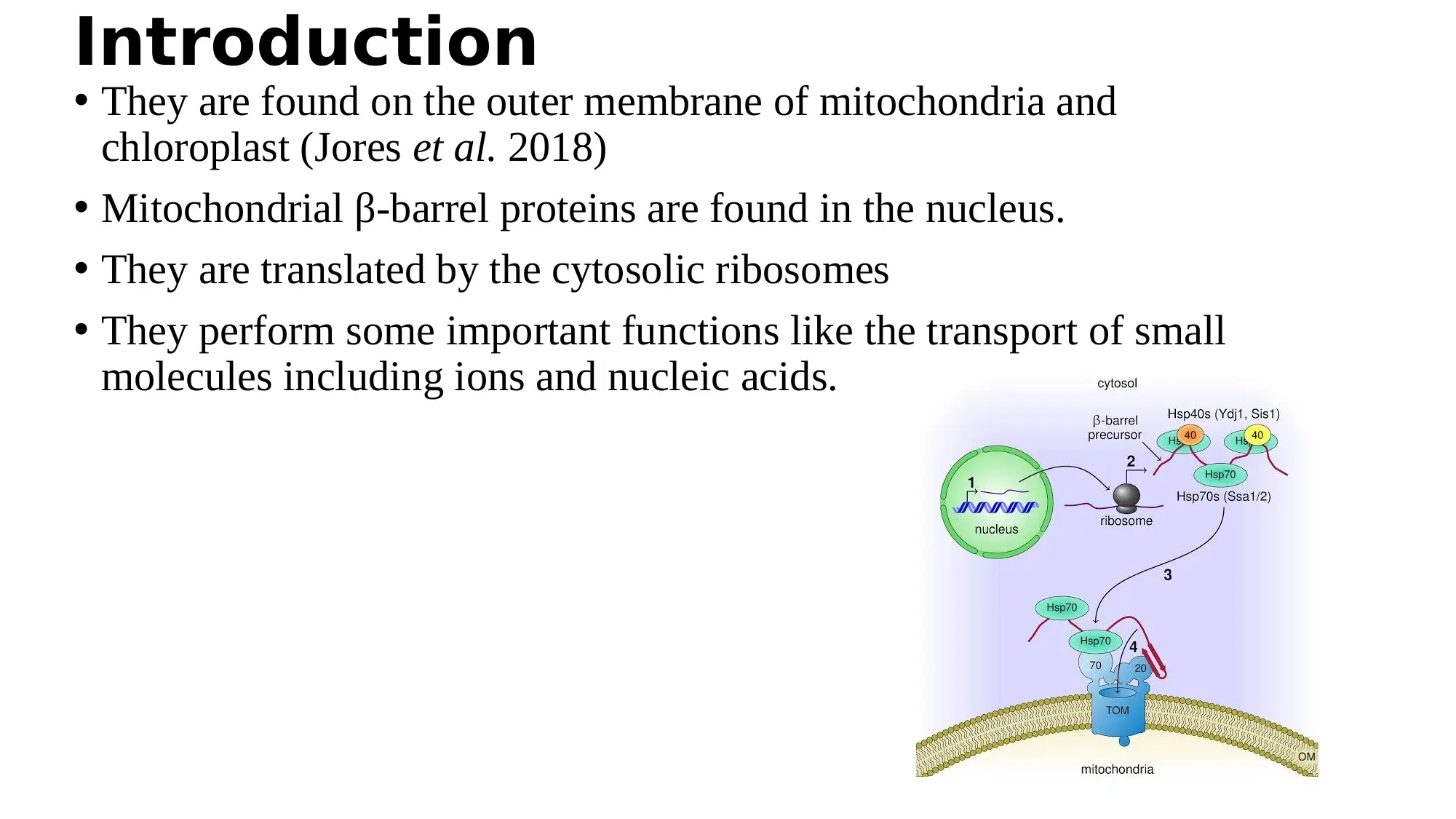
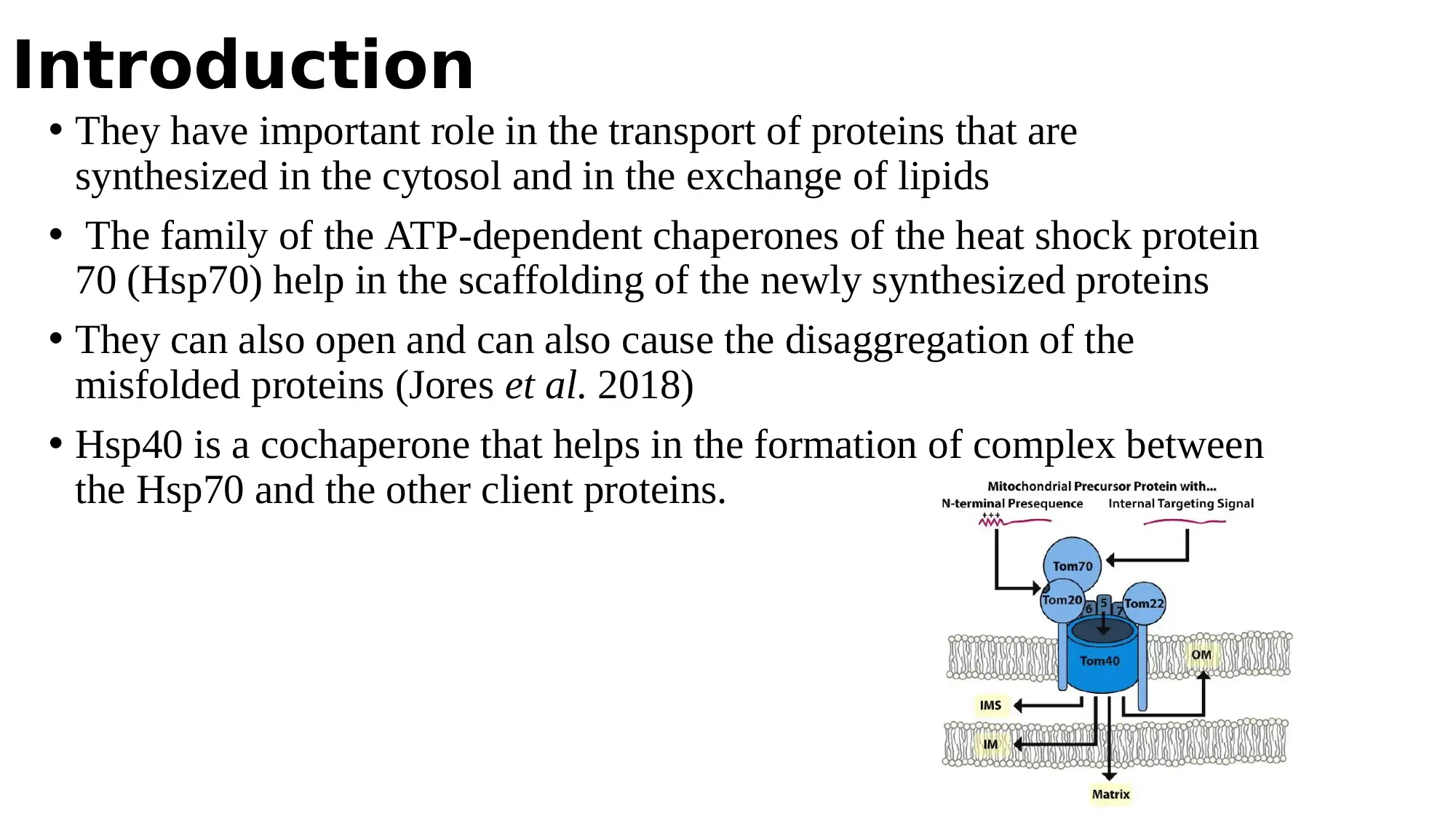
This report examines the role of cytosolic Hsp70 chaperones and their Hsp40 cochaperones in the biogenesis of mitochondrial β-barrel proteins. The study, based on the work of Jores et al. (2018), investigates how these chaperones interact with newly synthesized β-barrel proteins, which are encoded in the nucleus, translated by cytosolic ribosomes, and then imported into the mitochondria. The report details the experimental methods used, including the use of yeast strains, in vitro translation and import of radiolabeled proteins, pull-down assays, and UV-induced cross-linking. The findings demonstrate the importance of Hsp70 chaperones in facilitating the import of β-barrel proteins into mitochondria, as inhibiting their activity or depleting the related cochaperones resulted in reduced import. The interactions between β-barrel proteins and Hsp70 chaperones were also found to be conserved in mammalian cells, highlighting a novel mechanism in the biogenesis of these proteins. The study provides a comprehensive overview of the role of cytosolic chaperones in the import and proper folding of mitochondrial proteins.
1 out of 6




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)