Tissue Engineering: hMSC Differentiation and Seeding Concentration
VerifiedAdded on 2022/09/23
|10
|1850
|37
Report
AI Summary
This report investigates the effect of human mesenchymal stem cell (hMSC) seeding concentration on their differentiation, particularly into osteoblasts. The experiment involves culturing hMSCs at three different concentrations and assessing the differentiation rate after osteogenic induction, using osteoblast formation as an indicator. The methodology includes cell plating, washing, trypsinization, centrifugation, and incubation with HOB Growth Medium and Osteoblast Mineralization Medium. The expected conclusion is that higher seeding concentrations will lead to greater differentiation due to increased nutrient availability and growth factors. Silver plating is used to visualize microcalcification and assess differentiation. Potential pitfalls include the unknown contribution of other factors to differentiation and the inability to assess the effect of different assessment processes on seeding concentration. The report references studies on mesenchymal stem cells, osteoblast differentiation, and related topics.

Running head: TISSUE ENGINEERING
HUMAN STEM CELL DIFFERENTIATION- LAB report
Name of the Student:
Name of the University:
Author’s Note:
HUMAN STEM CELL DIFFERENTIATION- LAB report
Name of the Student:
Name of the University:
Author’s Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1TISSUE ENGINEERING
Table of Contents
Introduction................................................................................................................................2
Aim and Objectives....................................................................................................................3
Aim.........................................................................................................................................3
Objectives...............................................................................................................................3
Materials and Methodology.......................................................................................................4
Materials required..................................................................................................................4
Method...................................................................................................................................4
Expected Conclusion..................................................................................................................5
Potential Pitfalls.........................................................................................................................6
Bibliography...............................................................................................................................8
Table of Contents
Introduction................................................................................................................................2
Aim and Objectives....................................................................................................................3
Aim.........................................................................................................................................3
Objectives...............................................................................................................................3
Materials and Methodology.......................................................................................................4
Materials required..................................................................................................................4
Method...................................................................................................................................4
Expected Conclusion..................................................................................................................5
Potential Pitfalls.........................................................................................................................6
Bibliography...............................................................................................................................8

2TISSUE ENGINEERING
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cell that is usually found in the
bone marrow that is used for repairing of the skeletal tissues that include bone, cartilage and
fats. It is composed of a small fraction of the cells in the bone marrow and the researchers
have been able to isolate MSCs and studied in an in-depth manner (Caplan, 2017). As per the
recent studies, it had been suggested that these cells are crucial for the creation of a niche
environment or a shelter for blood stem cells, which forms the large fraction of cell in the
bone marrow. Human mesenchymal stem cells (hMSCs) are non-haematopoietic and
multipotent stem cells that have the capacity to differentiate into mesodermal lineage known
as adipocytes osteocytes, chondrocytes and ectodermal and endodermal lineages. These cells
are not only present in the fetal tissue but are found in many adults tissues with some
exceptions (Chen et al., 2016). The most efficient population of MSCs have been reported
from the bone marrow. It can be stated that the ability of hMSC to differentiate into
osteoblasts was examined through the usage of osteogenic induction medium cultures. It can
be seen that these cells attached to the surface of the dish and thus, demonstrate fibroblast-
like spindle shapes that after the process of proliferation takes the shape of cubes. The
process of differentiation of the stem cells has been visualised in both in vivo and in vitro
experiment. The process was performed where the stem cells were kept in the media for a
long and replaced by induction of different by a special media (Rutkovskiy et al., 2016). The
osteoblast (HOB) is the specialised fibroblast that secretes as well as mineralise on the bone
matrix. Thus, they develop from the precursor of the mesenchymal cells. The extracellular
matrix of the mineralised cells is composed of type II collagen and a small amount of
osteocalcin, bone sialoprotein, matrix GLA protein, osteopontin, BMPs, inorganic mineral
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cell that is usually found in the
bone marrow that is used for repairing of the skeletal tissues that include bone, cartilage and
fats. It is composed of a small fraction of the cells in the bone marrow and the researchers
have been able to isolate MSCs and studied in an in-depth manner (Caplan, 2017). As per the
recent studies, it had been suggested that these cells are crucial for the creation of a niche
environment or a shelter for blood stem cells, which forms the large fraction of cell in the
bone marrow. Human mesenchymal stem cells (hMSCs) are non-haematopoietic and
multipotent stem cells that have the capacity to differentiate into mesodermal lineage known
as adipocytes osteocytes, chondrocytes and ectodermal and endodermal lineages. These cells
are not only present in the fetal tissue but are found in many adults tissues with some
exceptions (Chen et al., 2016). The most efficient population of MSCs have been reported
from the bone marrow. It can be stated that the ability of hMSC to differentiate into
osteoblasts was examined through the usage of osteogenic induction medium cultures. It can
be seen that these cells attached to the surface of the dish and thus, demonstrate fibroblast-
like spindle shapes that after the process of proliferation takes the shape of cubes. The
process of differentiation of the stem cells has been visualised in both in vivo and in vitro
experiment. The process was performed where the stem cells were kept in the media for a
long and replaced by induction of different by a special media (Rutkovskiy et al., 2016). The
osteoblast (HOB) is the specialised fibroblast that secretes as well as mineralise on the bone
matrix. Thus, they develop from the precursor of the mesenchymal cells. The extracellular
matrix of the mineralised cells is composed of type II collagen and a small amount of
osteocalcin, bone sialoprotein, matrix GLA protein, osteopontin, BMPs, inorganic mineral
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
3TISSUE ENGINEERING
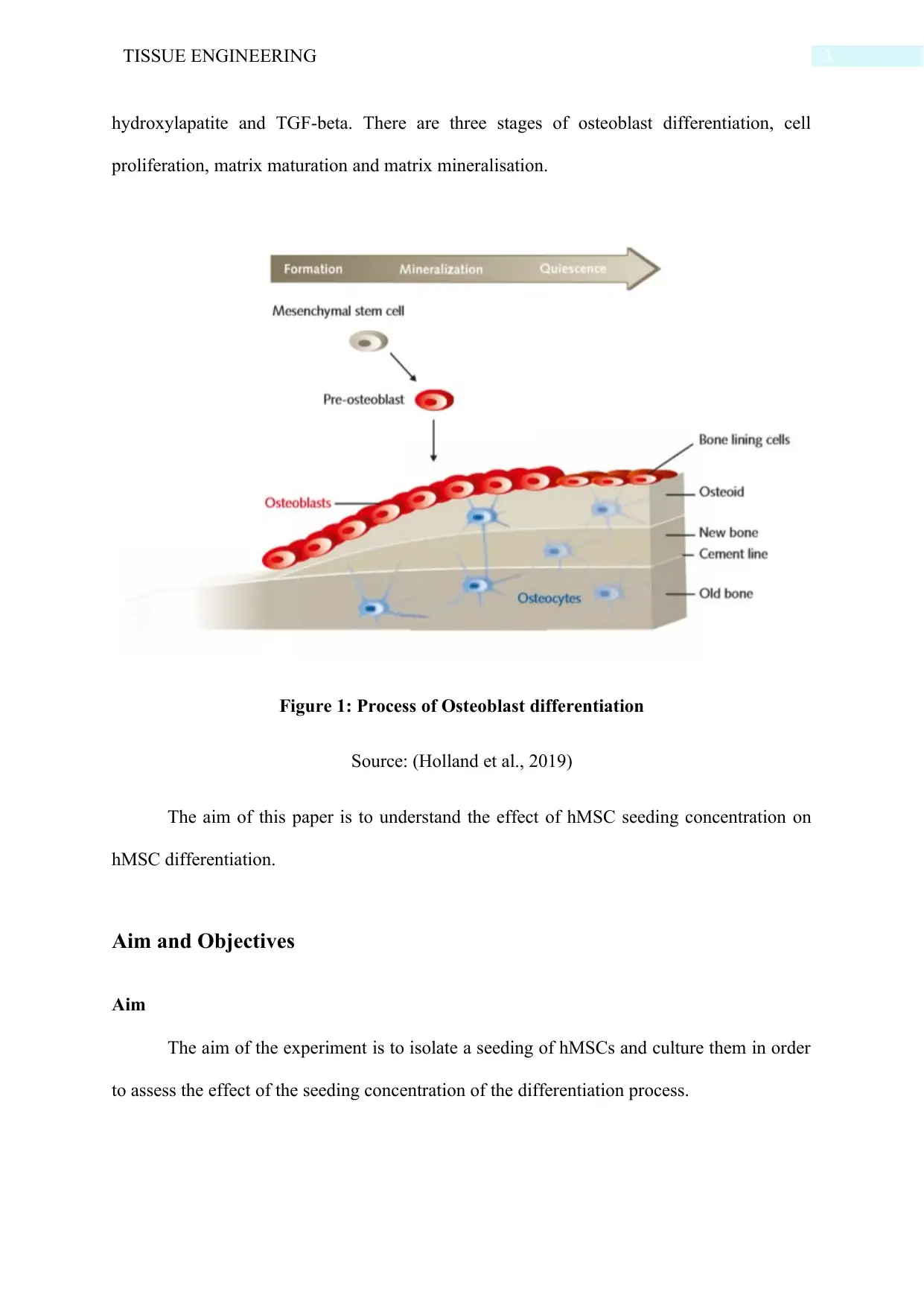
hydroxylapatite and TGF-beta. There are three stages of osteoblast differentiation, cell
proliferation, matrix maturation and matrix mineralisation.
Figure 1: Process of Osteoblast differentiation
Source: (Holland et al., 2019)
The aim of this paper is to understand the effect of hMSC seeding concentration on
hMSC differentiation.
Aim and Objectives
Aim
The aim of the experiment is to isolate a seeding of hMSCs and culture them in order
to assess the effect of the seeding concentration of the differentiation process.
hydroxylapatite and TGF-beta. There are three stages of osteoblast differentiation, cell
proliferation, matrix maturation and matrix mineralisation.
Figure 1: Process of Osteoblast differentiation
Source: (Holland et al., 2019)
The aim of this paper is to understand the effect of hMSC seeding concentration on
hMSC differentiation.
Aim and Objectives
Aim
The aim of the experiment is to isolate a seeding of hMSCs and culture them in order
to assess the effect of the seeding concentration of the differentiation process.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4TISSUE ENGINEERING
Objectives
The objectives of the experiment are:
To seed cells in three different concentration
To assess the cell seeding number and culture time
To evaluate the time of inducing differentiation
To check the differentiation rate after osteogenic induction
To use osteoblast formation for checking the differentiation rate
Materials and Methodology
Materials required
The same cell plate well apparatus of the experiment where differentiation of hMSCs
was done, Dulbecco’s phosphate-buffered saline trypsin/EDTA, three different plates for
three different concentration of cell seeding, HOB Growth Medium, Osteoblast
Mineralization Medium for Osteoblast Mineralization, laminar airflow for maintenance of
aseptic conditions.
Method
1. The same three cell plates were used that was used in the previous experiment for
subculturing the cells into three different concentrations.
2. The cell layers were washed with Dulbecco’s phosphate-buffered saline and the wash
solution was added to the side of the flask opposite to the attached cell layers. The flask was
rinsed by rocking back and forth several times. The wash solution was aseptically removed
and discarded.
3. Sufficient volume of Clonetics trypsin or EDTA solution was used for covering the cell
layer at approximately 0.05 ml/cm2. The flask was gently rocked in order to ensure that the
Objectives
The objectives of the experiment are:
To seed cells in three different concentration
To assess the cell seeding number and culture time
To evaluate the time of inducing differentiation
To check the differentiation rate after osteogenic induction
To use osteoblast formation for checking the differentiation rate
Materials and Methodology
Materials required
The same cell plate well apparatus of the experiment where differentiation of hMSCs
was done, Dulbecco’s phosphate-buffered saline trypsin/EDTA, three different plates for
three different concentration of cell seeding, HOB Growth Medium, Osteoblast
Mineralization Medium for Osteoblast Mineralization, laminar airflow for maintenance of
aseptic conditions.
Method
1. The same three cell plates were used that was used in the previous experiment for
subculturing the cells into three different concentrations.
2. The cell layers were washed with Dulbecco’s phosphate-buffered saline and the wash
solution was added to the side of the flask opposite to the attached cell layers. The flask was
rinsed by rocking back and forth several times. The wash solution was aseptically removed
and discarded.
3. Sufficient volume of Clonetics trypsin or EDTA solution was used for covering the cell
layer at approximately 0.05 ml/cm2. The flask was gently rocked in order to ensure that the

5TISSUE ENGINEERING
cells were entirely covered by the solution. It was incubated for 5 minutes at room
temperature and observed under a microscope.
4. In case, the cells are detached to less than 90%; it was incubated and observed after every
3 minutes.
5. After over 90% of the cells were detached and rounded, the flask was stood at a minimal
length of time to drain the cells. An equal volume of temperature equilibrated MSCGM was
added to each of the vessels and the solution was dispersed using a pipette over the surface of
the cell a number of times.
6. The cells were centrifuged at 600 x g for 5 minutes to remove trypsin and the cell pellets
were resuspended at the minimal volume of MSCGM and a sample was taken for counting.
7. The cells are counted using a hemacytometer and calculated. In order to achieve the
desired cells/ml, the suspension was diluted and cells were recounted.
8. In this manner, three different cell seeding concentration was obtained.
9. Plating of 3*104 HOB per well well on the collagen I coated 24 wells tissue culture plates
were made. This is done in duplication. The use of HOB Growth Medium was put in one
well, a negative control and Osteoblast Mineralization Medium was put in other wells.
10. The cells are incubated for 7 days and 14 days respectively and the change in the medium
after 7 and 14 days was observed.
11. The cells were observed using Silver plating kit that helps in the detection of
microcalcification at 7 and 14 days of the experiment.
cells were entirely covered by the solution. It was incubated for 5 minutes at room
temperature and observed under a microscope.
4. In case, the cells are detached to less than 90%; it was incubated and observed after every
3 minutes.
5. After over 90% of the cells were detached and rounded, the flask was stood at a minimal
length of time to drain the cells. An equal volume of temperature equilibrated MSCGM was
added to each of the vessels and the solution was dispersed using a pipette over the surface of
the cell a number of times.
6. The cells were centrifuged at 600 x g for 5 minutes to remove trypsin and the cell pellets
were resuspended at the minimal volume of MSCGM and a sample was taken for counting.
7. The cells are counted using a hemacytometer and calculated. In order to achieve the
desired cells/ml, the suspension was diluted and cells were recounted.
8. In this manner, three different cell seeding concentration was obtained.
9. Plating of 3*104 HOB per well well on the collagen I coated 24 wells tissue culture plates
were made. This is done in duplication. The use of HOB Growth Medium was put in one
well, a negative control and Osteoblast Mineralization Medium was put in other wells.
10. The cells are incubated for 7 days and 14 days respectively and the change in the medium
after 7 and 14 days was observed.
11. The cells were observed using Silver plating kit that helps in the detection of
microcalcification at 7 and 14 days of the experiment.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6TISSUE ENGINEERING
Expected Conclusion
According to the study, osteoblast is bone-building cells that originate from
mesenchymal and thus, differentiate from mesenchyme progeny directly or through
osteochondro progenitor (Khamees et al., 2020). The osteoblast can differentiate into
osteocytes that are stellate cells present in the narrow interconnecting passages in the bone
matrix. The key molecular switch that can be used for converted mesenchymal progenitor to
osteoblast lineage is the transcription factor CBA/runx2 that has a various upstream regulator
and targets a variety of cells. The process of osteoblast differentiation is subjected to
regulation of the physical stimuli and thus, this ensures that the bone formation occurs in a
proper way with structural and dynamic support to the entire body. The physical stimuli that
are found have an influence on the process include compression, stress, micro and macro
gravity, stretch and ultrasound (Persson et al., 2018). From the experiment, it can be expected
that on the seventh day, the cells on different plates having different concentration may show
results that indicate that higher the concentration, more the differentiation. This is because
when the concentration of cell seeding is more, the osteoblast will be able to get more
nutrient and growth factors from the mesenchymal progenitor to proliferate. On the other
hand, the low concentration of cell seeding will show less osteoblast differentiation because
of lack of mesenchymal progenitors. The observation will be done using the silver plating
where the silver ions in the silver nitrate solution will react with the phosphate, carbonate
ions of the calcium in the matrix, and thus, displace them. The silver ions will be reduced to
metallic silver on exposure to light and thus, can be evaluated by microscopy. After 14 days
of the experiment, when we would observe the plates under a microscope, the rate of
differentiating had increased in all the plates irrespective of the concentration. Thus, it can be
stated that with time, the rate of differentiation increase. On further evaluation of each plate,
it would be observed that low concentration of cells has low differentiation or silver
Expected Conclusion
According to the study, osteoblast is bone-building cells that originate from
mesenchymal and thus, differentiate from mesenchyme progeny directly or through
osteochondro progenitor (Khamees et al., 2020). The osteoblast can differentiate into
osteocytes that are stellate cells present in the narrow interconnecting passages in the bone
matrix. The key molecular switch that can be used for converted mesenchymal progenitor to
osteoblast lineage is the transcription factor CBA/runx2 that has a various upstream regulator
and targets a variety of cells. The process of osteoblast differentiation is subjected to
regulation of the physical stimuli and thus, this ensures that the bone formation occurs in a
proper way with structural and dynamic support to the entire body. The physical stimuli that
are found have an influence on the process include compression, stress, micro and macro
gravity, stretch and ultrasound (Persson et al., 2018). From the experiment, it can be expected
that on the seventh day, the cells on different plates having different concentration may show
results that indicate that higher the concentration, more the differentiation. This is because
when the concentration of cell seeding is more, the osteoblast will be able to get more
nutrient and growth factors from the mesenchymal progenitor to proliferate. On the other
hand, the low concentration of cell seeding will show less osteoblast differentiation because
of lack of mesenchymal progenitors. The observation will be done using the silver plating
where the silver ions in the silver nitrate solution will react with the phosphate, carbonate
ions of the calcium in the matrix, and thus, displace them. The silver ions will be reduced to
metallic silver on exposure to light and thus, can be evaluated by microscopy. After 14 days
of the experiment, when we would observe the plates under a microscope, the rate of
differentiating had increased in all the plates irrespective of the concentration. Thus, it can be
stated that with time, the rate of differentiation increase. On further evaluation of each plate,
it would be observed that low concentration of cells has low differentiation or silver
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7TISSUE ENGINEERING
deposition as compared to high concentration plates. This clearly indicates the fact that there
is an increase in hMSC differentiation with an increase in hMSC seeding concentration.
Potential Pitfalls
From the expected conclusion, it can be seen that with an increase in hMSC seeding
concentration, the rate of hMSC differentiation was found to increase by using the process of
osteoblast Mineralization; however, the factors that had to contribute to it was not known.
Moreover, it can be seen that there are different types of process used for assessing the
differentiation rate such as Alkaline Phosphate, osteoblast formation, or mineralization,
however; the effect of the different process on the seeding concentration could not be
assessed in the experiment.
deposition as compared to high concentration plates. This clearly indicates the fact that there
is an increase in hMSC differentiation with an increase in hMSC seeding concentration.
Potential Pitfalls
From the expected conclusion, it can be seen that with an increase in hMSC seeding
concentration, the rate of hMSC differentiation was found to increase by using the process of
osteoblast Mineralization; however, the factors that had to contribute to it was not known.
Moreover, it can be seen that there are different types of process used for assessing the
differentiation rate such as Alkaline Phosphate, osteoblast formation, or mineralization,
however; the effect of the different process on the seeding concentration could not be
assessed in the experiment.

8TISSUE ENGINEERING
Bibliography
Caplan, A. I. (2017). Mesenchymal stem cells: time to change the name!. Stem cells
translational medicine, 6(6), 1445-1451. http://dx.doi.org/10.1002/sctm.17-0051
Chen, Q., Shou, P., Zheng, C., Jiang, M., Cao, G., Yang, Q., ... & Xu, C. (2016). Fate
decision of mesenchymal stem cells: adipocytes or osteoblasts?. Cell Death &
Differentiation, 23(7), 1128-1139. https://doi.org/10.1038/cdd.2015.168
Holland, R., Bain, C., & Utreja, A. (2019). Osteoblast differentiation during orthodontic tooth
movement. Orthodontics & craniofacial research, 22(3), 177-182.
https://doi.org/10.1111/ocr.12308
Khamees, N., Hill, D. J., & Kafienah, W. (2020). Mechanisms of interaction of
staphylococcus aureus with human mesenchymal stem cells and their differentiated
phenotypes. bioRxiv. https://doi.org/2020/01/09/2020.01.09.900373
Persson, M., Lehenkari, P. P., Berglin, L., Turunen, S., Finnilä, M. A., Risteli, J., ... &
Tuukkanen, J. (2018). Osteogenic differentiation of human mesenchymal stem cells in
a 3D woven scaffold. Scientific reports, 8(1), 1-12. https://doi.org/10.1038/s41598-
018-28699-x
Ridge, S. M., Sullivan, F. J., & Glynn, S. A. (2017). Mesenchymal stem cells: key players in
cancer progression. Molecular cancer, 16(1), 31. https://doi.org/10.1186/s12943-017-
0597-8
Rutkovskiy, A., Stensløkken, K. O., & Vaage, I. J. (2016). Osteoblast differentiation at a
glance. Medical science monitor basic research, 22, 95.
https://doi.org/10.12659/MSMBR.901142
Bibliography
Caplan, A. I. (2017). Mesenchymal stem cells: time to change the name!. Stem cells
translational medicine, 6(6), 1445-1451. http://dx.doi.org/10.1002/sctm.17-0051
Chen, Q., Shou, P., Zheng, C., Jiang, M., Cao, G., Yang, Q., ... & Xu, C. (2016). Fate
decision of mesenchymal stem cells: adipocytes or osteoblasts?. Cell Death &
Differentiation, 23(7), 1128-1139. https://doi.org/10.1038/cdd.2015.168
Holland, R., Bain, C., & Utreja, A. (2019). Osteoblast differentiation during orthodontic tooth
movement. Orthodontics & craniofacial research, 22(3), 177-182.
https://doi.org/10.1111/ocr.12308
Khamees, N., Hill, D. J., & Kafienah, W. (2020). Mechanisms of interaction of
staphylococcus aureus with human mesenchymal stem cells and their differentiated
phenotypes. bioRxiv. https://doi.org/2020/01/09/2020.01.09.900373
Persson, M., Lehenkari, P. P., Berglin, L., Turunen, S., Finnilä, M. A., Risteli, J., ... &
Tuukkanen, J. (2018). Osteogenic differentiation of human mesenchymal stem cells in
a 3D woven scaffold. Scientific reports, 8(1), 1-12. https://doi.org/10.1038/s41598-
018-28699-x
Ridge, S. M., Sullivan, F. J., & Glynn, S. A. (2017). Mesenchymal stem cells: key players in
cancer progression. Molecular cancer, 16(1), 31. https://doi.org/10.1186/s12943-017-
0597-8
Rutkovskiy, A., Stensløkken, K. O., & Vaage, I. J. (2016). Osteoblast differentiation at a
glance. Medical science monitor basic research, 22, 95.
https://doi.org/10.12659/MSMBR.901142
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9TISSUE ENGINEERING
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

