Installation and Verification Protocol for Hot Detergent System
VerifiedAdded on 2020/04/15
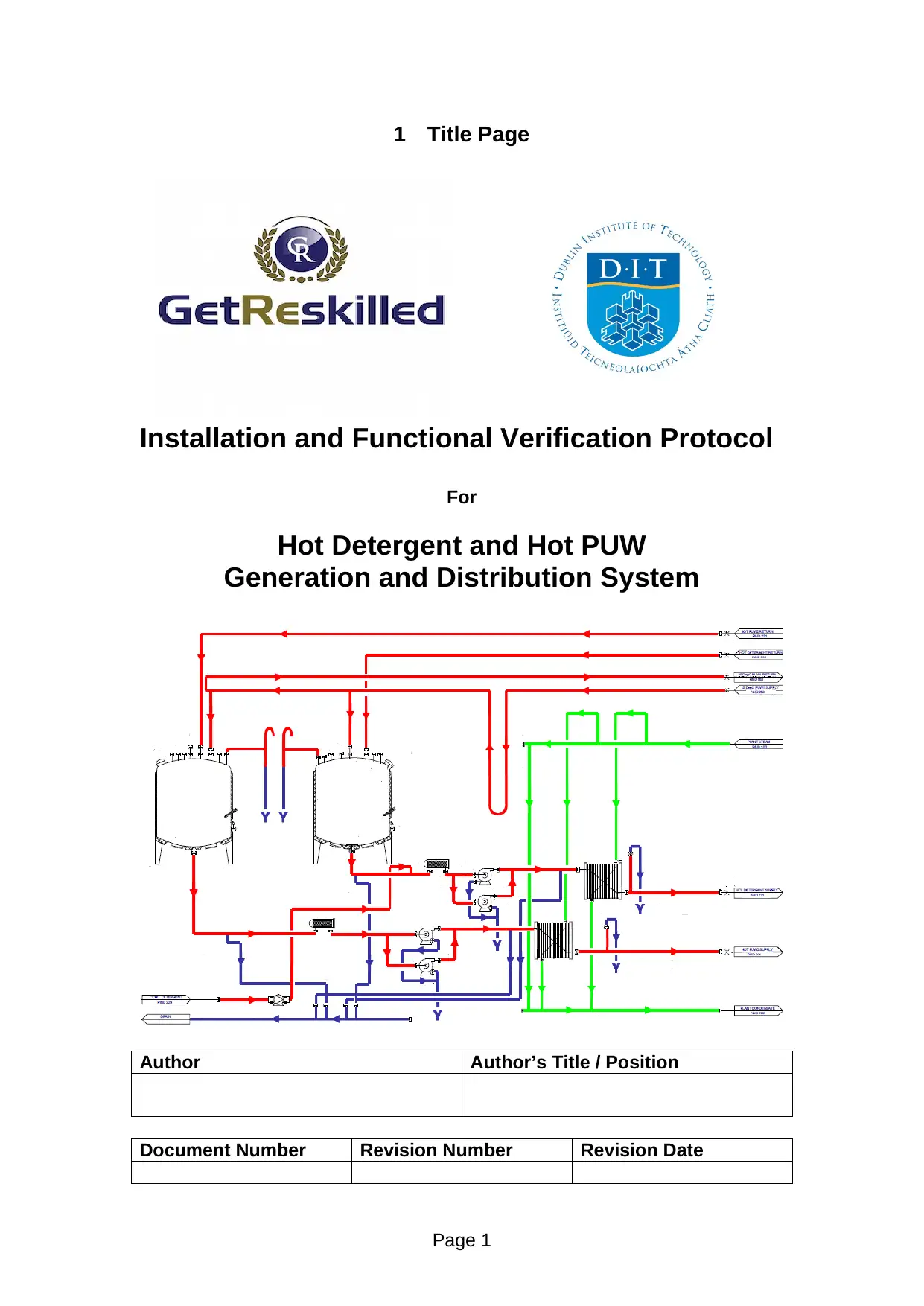
Installation and Functional Verification Protocol
For
Hot Detergent and Hot PUW
Generation and Distribution System
Author Author’s Title / Position
Document Number Revision Number Revision Date
Page 1
Paraphrase This Document

Prepared By: ________________________ Date: ____________
Validation Engineer
Approved By: ________________________ Date: ____________
Validation Manager
Your signature attests that you have reviewed this document
and it complies with relevant validation policies & procedures
Approved By: ________________________ Date: ____________
Engineering
Your signature attests that……(align with ‘Responsibilities’
Protocol Section-10)
Approved By: ________________________ Date: ____________
QA
Your signature attests that……(align with ‘Responsibilities’
Protocol Section-10)
Approved By: ________________________ Date: ____________
etc.
Your signature attests that……(align with ‘Responsibilities’
Protocol Section-10)
Page 3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Revision Date of Issue Reason for revision update
A 12 Jan 2012 Issued for review
0 14 Mar 2012 Issued for approval and use
Page 4
Paraphrase This Document

1 Title Page................................................................................................................1
2 Approvals Page......................................................................................................2
3 Document Revision History...................................................................................3
4 Table of Contents...................................................................................................4
5 Protocol Objective..................................................................................................6
6 System Description................................................................................................6
7 Scope......................................................................................................................7
7.1 System Impact Assessment.............................................................................7
7.2 Component Impact Assessment.......................................................................7
7.3 Summary Table - Operational Critical Components.......................................8
7.4 P&ID Scope.....................................................................................................9
8 Reference Documentation....................................................................................10
9 Definitions/ Glossary............................................................................................11
10 Responsibilities / Subject Matter Experts............................................................12
11 Installation Verification Testing Procedures........................................................13
11.1 P&ID Walkdown Testing Procedure.........................................................13
11.2 Equipment Installation Verification Procedure.........................................14
11.3 Instrument Installation Verification Procedure..........................................15
11.4 Piping Installation Verification Procedure.................................................16
11.5 Calibration Program Verification..............................................................17
11.6 Preventative Maintenance Program Verification.......................................17
11.7 Draft SOP Verification..............................................................................17
11.8 Test-Instrument Calibration Verification...................................................17
12 Progression Approval to Proceed to Functional Verification..............................18
12.1 Operational-Critical-Instrument Calibration Verification Form................19
13 Functional Verification Testing Procedures.........................................................20
13.1 Hot Detergent System – Detergent Dosing Control..................................21
13.2 Hot Detergent System – Temperature Control..........................................23
13.3 Hot Detergent System – Flow Control.......................................................23
13.4 Hot Detergent System – Level Control......................................................23
13.5 Hot PUW System – Temperature Control.................................................23
13.6 Hot PUW System – Flow Control.............................................................23
13.7 Hot PUW System – Level Control.............................................................23
13.8 Approved SOP Verification.......................................................................23
14 Protocol Quality Procedures.................................................................................23
14.1 Signature Log.............................................................................................23
14.2 Deviation Procedure...................................................................................24
14.3 Change Control Procedure.........................................................................24
15 IOQ Protocol Summary Report............................................................................24
16 List of Appendices...............................................................................................25
16.1 Appendix A - Protocol Signature Log.......................................................26
16.2 Appendix B - Installation Verification GMP checksheets.........................27
16.2.1 P&ID Walkdown Installation Verification............................................27
16.2.2 Equipment Installation Verification.......................................................28
16.2.3 Instrument Installation Verification.......................................................31
16.2.4 Piping Installation Verification..............................................................37
16.2.5 Calibration Program Verification...........................................................39
Page 5

16.2.7 Draft SOP Verification...........................................................................41
16.2.8 Test-Instrument Calibration Verification...............................................42
16.3 Appendix C: OQ GMP check-sheets.........................................................43
16.3.1 Hot Detergent System – Detergent Dosing Control...............................44
16.3.2 Hot Detergent System – Temperature Control.......................................47
16.3.3 Hot Detergent System – Flow Control...................................................48
16.3.4 Hot Detergent System –Level Control...................................................49
16.3.5 Hot PUW System – Temperature Control..............................................50
16.3.6 Hot PUW System – Flow Control..........................................................51
16.3.7 Hot PUW System – Level Control.........................................................52
16.3.8 Approved SOP Verification...................................................................53
16.4 Appendix D: Deviation log........................................................................54
16.5 Appendix E: Deviation Form.....................................................................55
16.6 Appendix F: Change Control Llog............................................................56
16.7 Appendix G: Change control Form............................................................57
16.8 Appendix H: Component Impact Assessment...........................................60
16.9 Appendix I: Traceability Matrix................................................................66
Page 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The objective of this protocol is to provide testing evidence that will prove that
the ‘Hot Detergent and Hot PUW Generation and Distribution’ system is fit for
its intended use. Testing will incorporate both installation verification and
functional verification test.
6 System Description
Prepare a summary ‘System Description’. Suggestions include drawing a
process flow / block diagram; listing critical operational features; and,
describing the major equipment components in the context of their installation
and functional features.
The system consists of a series of tanks and piping as well as heat exchanges that are
used to generate and transport hot water (steam) for the purposes of sterilization of
medical production systems to ensure the produced medicines are i8mpurity free and
safe from any contaminants. The system consists of a boiler that generates heat for
boiling water to a safe temperature. The pure steam is then transported through a
series of pipes and piping’s that are then used for tank sterilization. The pure hot
steam is also used in sterilizing piping systems, filters, and products that are contained
within sterilizers. Some tanks within the system are used as sterilizers for
pharmaceutical products. The system also moistens air in clean room systems while
the detergents are used for further after cleaning. Among the most crucial pipeline in
the system is 50-PUW1-S6-148-IH which carries out hot vapour, if this pipeline is not
been handled for example if the valve opening fails in this pipeline will ultimately
results in high pressure build-up leads to explosive incident. In addition to this
pipeline the another crucial line system is 80-PUW2-S6-155-IH an transport pipe of
hot raw reagent mixture to the consequent reactor system for yielding conversion of
hot detergent which are further connected and processed in the subsequent system for
the final product. The checks are performed so as to ensure the system operates safely
and as intended by enabling seamless controls and management of its functioning.
Page 7
Paraphrase This Document

7.1 System Impact Assessment
The ‘Hot Detergent and Hot PUW Generation and Distribution’ is considered a
‘direct impact’ system because:
The items to be sterilized are directly exposed to the pure steam where
sterilization is achieved by the direct thermal impact of the steam
The pure steam comes into direct contact with surfaces and medicines to
kill off any contaminants on the medicines
The temperature generated from the steam ‘directly’ impacts the on the
ionization extent
Page 8

The Component Impact Assessment will drive the scope of the installation and
functional verifications. Product contact plus operational critical components
must be included in the installation verifications. The operationally critical
components will drive the functionality testing.
Page 9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
#
*Operational
Critical
Component
**Additional
components in
the loop (if
applicable)
Rationale
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
Page 10
Paraphrase This Document

In Protocol Section-7.4 include a copy/scan of the P&ID and either draw a
‘scope bubble’ or yellow-highlight the ‘in-scope’ components.
Page 11
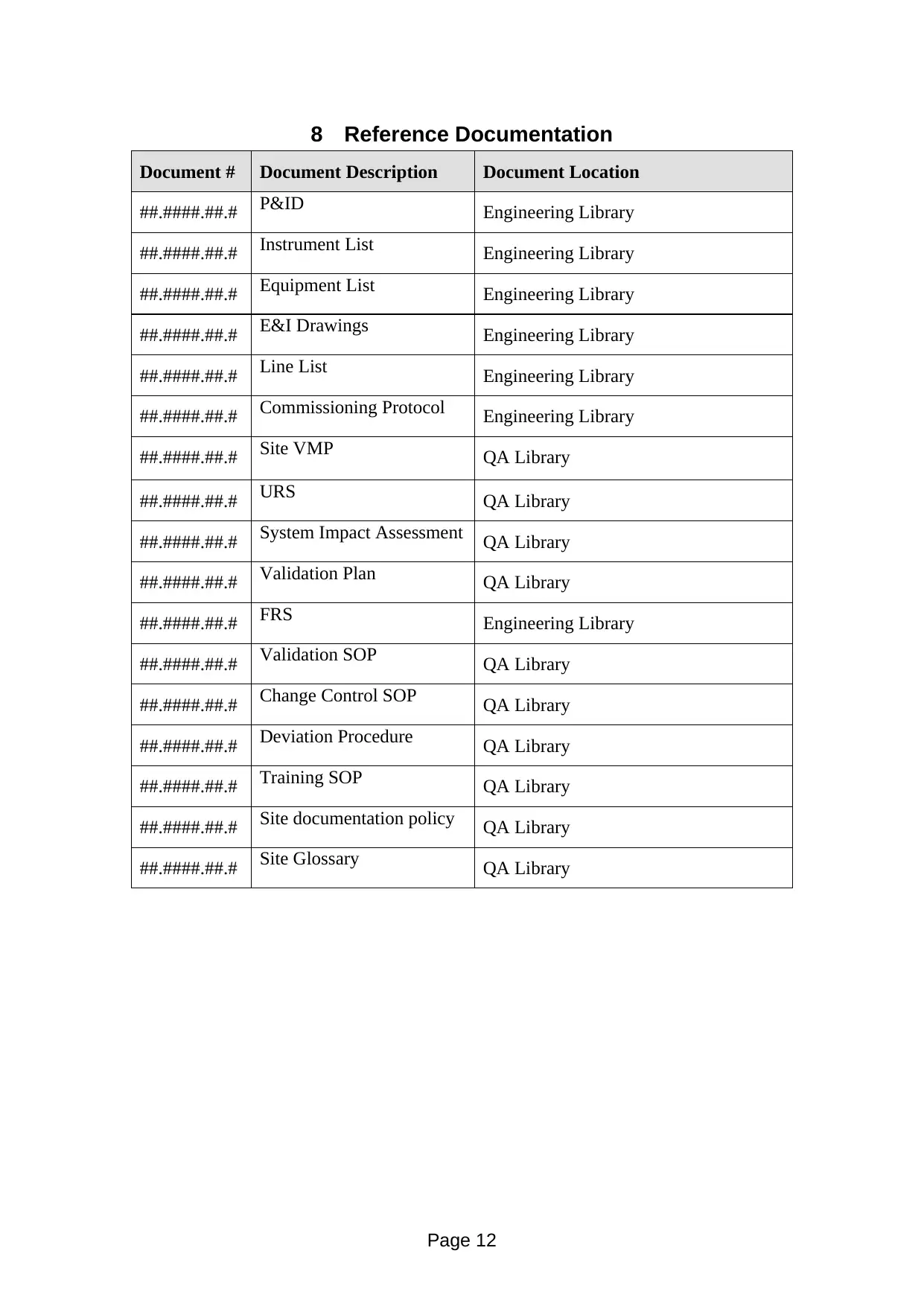
Document # Document Description Document Location
##.####.##.# P&ID Engineering Library
##.####.##.# Instrument List Engineering Library
##.####.##.# Equipment List Engineering Library
##.####.##.# E&I Drawings Engineering Library
##.####.##.# Line List Engineering Library
##.####.##.# Commissioning Protocol Engineering Library
##.####.##.# Site VMP QA Library
##.####.##.# URS QA Library
##.####.##.# System Impact Assessment QA Library
##.####.##.# Validation Plan QA Library
##.####.##.# FRS Engineering Library
##.####.##.# Validation SOP QA Library
##.####.##.# Change Control SOP QA Library
##.####.##.# Deviation Procedure QA Library
##.####.##.# Training SOP QA Library
##.####.##.# Site documentation policy QA Library
##.####.##.# Site Glossary QA Library
Page 12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
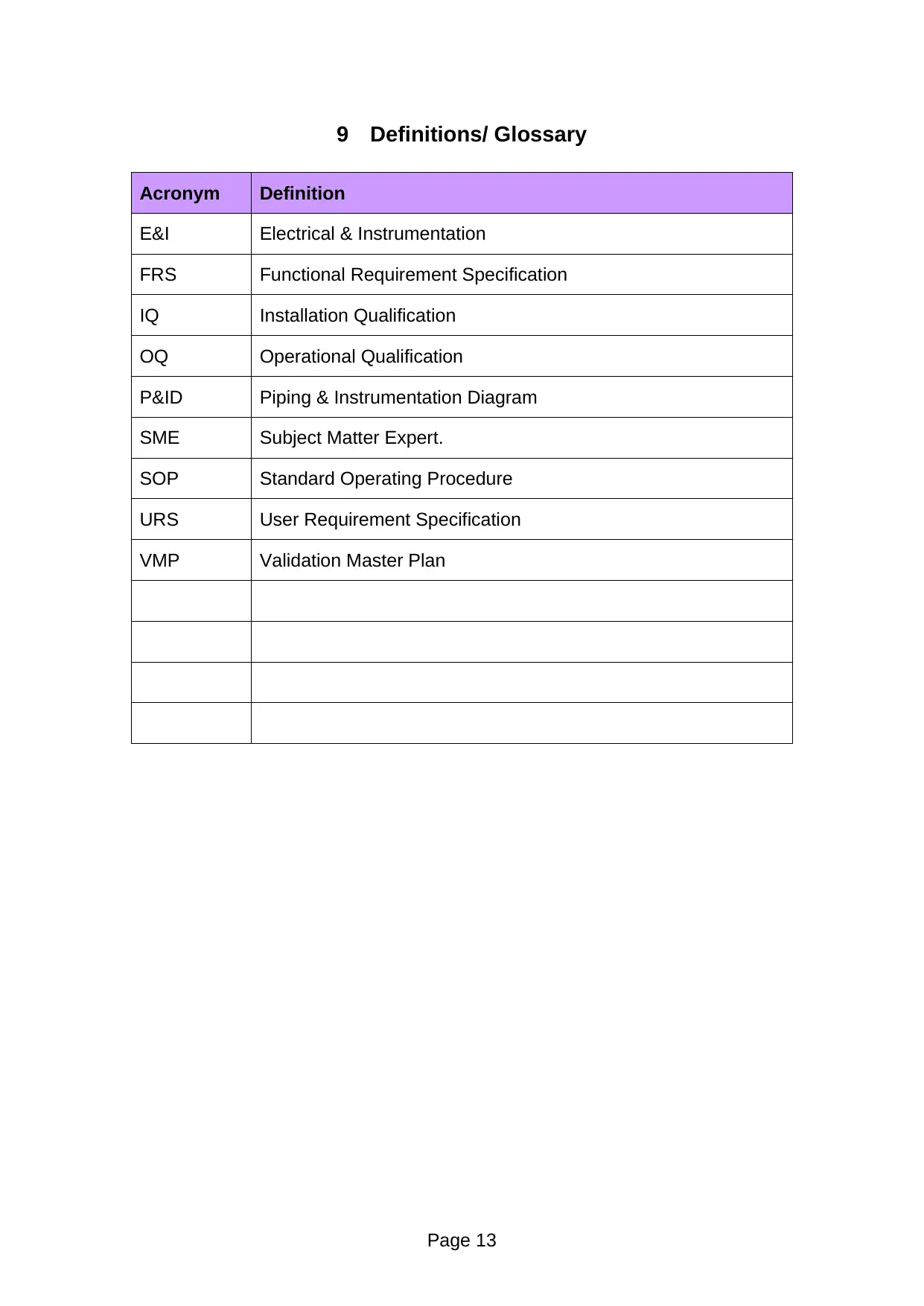
Acronym Definition
E&I Electrical & Instrumentation
FRS Functional Requirement Specification
IQ Installation Qualification
OQ Operational Qualification
P&ID Piping & Instrumentation Diagram
SME Subject Matter Expert.
SOP Standard Operating Procedure
URS User Requirement Specification
VMP Validation Master Plan
Page 13
Paraphrase This Document

1. Validation
Prepare Protocol
Departmental Review & Approval of Protocol
Execute Protocol, including dotting i’s & crossing t’s
Co-Ordinate input from other responsible departments
Document Deviations
Prepare Report
2. Quality Assurance
etc.
3. Quality control
etc.
4. Manufacturing
etc.
5. Engineering
etc.
6. Maintenance/ Calibrations
etc.
7. HR / Training
etc.
8. EHS
etc.
Page 14

11.1 P&ID Walkdown Testing Procedure
1 Description of Test
The objective of the test is to verify all direct impact components are installed
and orientated correctly as per the functional illustration on the P&ID.
2 Linkage to Requirements Challenged
Confirm correct installation and orientation of direct impact components as per
the P&ID schematic illustration.
3 Acceptance Criteria
All direct impact components are functionally installed and orientated correctly
as per the P&ID specification.
4 Prerequisites and/or Assumptions
1. All commissioning activities are complete and commissioning punch-list
items are closed out.
2. All testers shall be trained and educated in the test method listed in
section-5 below: it is crucial to the success of the testing effort that the
testing process be well understood by all participants.
5 Test Method
1. On a blank copy of the P&ID draw in the direct-impact system boundary in
black-ink
2. Walk down the P&ID and using a yellow-highlighter confirm each
component is correctly installed and orientated as represented on the
P&ID
3. Confirm all component tag numbers are correct
4. Confirm all lines are correctly labelled
5. Confirm all lines where applicable are correctly insulated
6. Ensure all flow direction components are orientated correctly
7. Ensure all instruments indicators are orientated correctly so as operators
can readily view them in the field
8. Ensure all commissioning filters are installed in filter-housings
9. On the P&ID any additions shall be drawn in using blue-ink, any deletions
shall be crossed-out using red ink, comments shall be inserted using
green-ink, and sign and date any entries on the P&ID using black-ink
6 Expected Results and Actual results
1. In the IQ GMP test-sheet(s) overleaf, reach a conclusion as to whether
each test step has been successfully completed by transcribing bold text
from the ‘Expected Results’ column into the corresponding ‘Actual Results’
field.
2. Assess each step listed and determine whether the step has passed or
failed.
3. The person performing the test should be identified and the date the
testing was performed should be recorded.
Page 15
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

performing the test) shall review the script post execution.
5. Quality Assurance (QA) shall approve the completed test.
11.2 Equipment Installation Verification Procedure
1 Description of Test
The objective of the test is twofold:
To verify that all direct impact equipment components are of the correct
specification as per the detailed mechanical data sheets
To verify that all direct impact equipment components are installed and
configured correctly as per the functional illustration on the P&ID.
2 Linkage to Requirements Challenged
Confirm correct installation and primary specifications of direct impact
equipment components as detailed in the P&ID and URS.
3 Acceptance Criteria
All direct impact components are of the correct specification, are functionally
installed and configured as per the P&ID, the vendor handover documentation
is complete, and appropriate material certification is available.
4 Prerequisites and/or Assumptions
1. All commissioning activities are complete and commissioning punch-list
items are closed out.
2. All testers shall be trained and educated in the test method listed in
section-5 below: it is crucial to the success of the testing effort that the
testing process be well understood by all participants.
5 Test Method
1. On the equipment IQ GMP test sheets confirm the equipment description
and critical design features. Inspect the installed equipment item in the
field and while referencing the vendor’s documentation package verify
specification conformance.
2. Confirm that the vendor handover package is in place and contains correct
material certificates.
3. In the field visually confirm the following:
Equipment item is installed as per P&ID, is securely fitted and is free
from damage
Nameplate is securely fitted, clearly legible and its details conform to
the design specification
All process and utility connections, and instrumentation are connected
up to the correct nozzles/flanges, as per P&ID
6 Expected Results and Actual results
1. In the equipment IQ verification forms, reach a conclusion as to whether
each test step has been successfully completed by transcribing bold text
from the ‘Expected Results’ column into the corresponding ‘Actual Results’
field where appropriate.
Page 16
Paraphrase This Document

failed.
3. The person performing the test should be identified and the date the
testing was performed should be recorded.
4. At the bottom of the test script a validation peer (other than the person
performing the test) shall review the script post execution.
5. Quality Assurance (QA) shall approve the completed test.
11.3 Instrument Installation Verification Procedure
1 Description of Test
The objective of the test is twofold:
To verify that all direct impact instruments are of the correct specification
as per the detailed instrument data sheets
To verify that all direct impact instruments components are installed and
configured correctly as per the functional illustration on the P&ID.
2 Linkage to Requirements Challenged
URS refers to major P&ID components. The system P&ID details all
necessary process instrumentation: confirm correct installation and
specification of direct impact instrument components.
3 Acceptance Criteria
All direct impact instruments are of the correct specification, are functionally
installed and configured as per the P&ID, the vendor handover documentation
is complete, and appropriate material, calibration and loop-check certification
is available.
4 Prerequisites and/or Assumptions
1. All commissioning activities are complete and commissioning punch-list
items are closed out.
2. All testers shall be trained and educated in the test method listed in
section-5 below: it is crucial to the success of the testing effort that the
testing process be well understood by all participants.
5 Test Method
1. On Part-1 of the instrument GMP test sheets confirm the instrument
description and critical design features. Inspect the installed instrument
item in the field and while referencing the vendor’s documentation
package verify conformance:
Manufacturer
Calibrated Range
MOC
Loop Checked
2. On Part-2 of the instrument GMP test sheets confirm that the appropriate
vendor documentation is available in accordance with the following:
Vendor handover package is in place
Material Certification is available for wetted parts
Calibration Certification is available
Loop Checksheet is available (Yes/No)
Page 17

confirm, the following:
Instrument item is installed as per P&ID, is securely fitted and is free
from damage
Instrument and cabling is correctly tagged and details conform to the
design specification
Instrument orientated correctly and is accessible for calibration and
maintenance
6 Expected Results and Actual results
1. In the instrument verification forms, reach a conclusion as to whether each
test step has been successfully completed by transcribing bold text from
the ‘Expected Results’ column into the corresponding ‘Actual Results’ field
where appropriate.
2. Assess each step listed and determine whether the step has passed or
failed.
3. The person performing the test should be identified and the date the
testing was performed should be recorded.
4. At the bottom of the test script a validation peer (other than the person
performing the test) shall review the script post execution.
5. Quality Assurance (QA) shall approve the completed test.
11.4 Piping Installation Verification Procedure
1 Description of Test
The objective of the test is to verify all direct impact pipes are installed and
orientated correctly as per the functional illustration on the P&ID and piping
Isometrics.
2 Linkage to Requirements Challenged
Confirm correct installation and orientation of direct impact piping as per the
P&ID schematic illustration and piping isometrics.
3 Acceptance Criteria
All direct impact components are functionally installed and orientated correctly
as per the P&ID’s and isometrics’ specifications.
4 Prerequisites and/or Assumptions
1. All commissioning activities are complete and commissioning punch-list
items are closed out.
2. All testers shall be trained and educated in the test method listed in
section-5 below: it is crucial to the success of the testing effort that the
testing process be well understood by all participants.
5 Test Method
1. Pre-populate the piping verification with the Tag # and material-of-
construction (MOC) details.
2. In the field enter the details of the weld log for every each pipe Tag #.
Page 18
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

construction certificates and weld inspection certificates.
4. In the field record the details of the Pressure Test Pack for every each pipe
Tag #.
5. Confirm the Pressure Test is complete verifying the absence of leaks
6. In the field enter the details of the Construction Dossier for every each pipe
Tag #.
7. Confirm all slopes have been checked and verified as correct from the
Construction Dossier.
6 Expected Results and Actual results
1. In the IQ GMP test-sheet(s) overleaf, reach a conclusion as to whether
each test step has been successfully completed by transcribing bold text
from the ‘Expected Results’ column into the corresponding ‘Actual Results’
field.
2. Assess each step listed and determine whether the step has passed or
failed.
3. The person performing the test should be identified and the date the
testing was performed should be recorded.
4. At the bottom of the test script a validation peer (other than the person
performing the test) shall review the script post execution.
5. Quality Assurance (QA) shall approve the completed test.
11.5 Calibration Program Verification
During installation verification we need to verify that instruments are in the
calibration system. Here summarize a ‘Calibration Program Verification’
procedure that compliments the form in Protocol Section-16.2.5.
11.6 Preventative Maintenance Program Verification
During IQ, we need to verify that instruments are in the Preventative
Maintenance system. Here summarize a ‘Preventative Maintenance Program
Verification’ procedure that compliments the form in Protocol Section-16.2.6.
11.7 Draft SOP Verification
Prior to proceeding to functional verification we need to ensure the required
SOPs are in-draft. Propose a list of necessary standard operating procedures
for the ‘Hot Detergent and Hot PUW Generation and Distribution’ system and
enter the equivalent details into the form in Protocol Section-16.2.7. Ensure
the details in the list of draft SOPs aligns with Protocol Section-13.8 ‘Approved
SOP Verification’ and its associated form in Protocol Section-16.3.8.
11.8 Test-Instrument Calibration Verification
Suggest a list of test instruments that may be required for the testing phase.
Enter the equivalent details into the form in Protocol Section-16.2.8
Page 19
Paraphrase This Document

Verification
The signatories below verify the following:
That the installation verification testing phase is sufficiently complete and
permission is given to progress to the functional verification testing phase.
All critical deviations from the installation verification testing phase have
been closed out, or a change control initiated in its place.
All critical items are installed.
All operational critical instruments will remain in a calibrated state during
the functional verification testing phase.
Prepared By: ________________________ Date: ____________
Validation Engineer
Approved By: ________________________ Date: ____________
Validation Manager
Your signature attests that you have reviewed this document
and it complies with relevant validation policies & procedures
Approved By: ________________________ Date: ____________
Engineering
Your signature attests that……(align with ‘Responsibilities’
Protocol Section-2)
Approved By: ________________________ Date: ____________
QA
Your signature attests that……(align with ‘Responsibilities’
Protocol Section-2)
Approved By: ________________________ Date: ____________
etc.
Your signature attests that……(align with ‘Responsibilities’
Protocol Section-2)
Page 20
Form
Page _ __ of _ __
Operational-Critical-Instrument Calibration Verification Form
Critical instruments associated with the system will
remain in a calibrated state during the anticipated OQ
execution timeframe? (Yes/No)
Verified By / Date
Comments:
Reviewed By:
Validation
Date:
Approved By:
(QA)
Date:
Page 21
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

13.3.1 Hot Detergent System – Detergent Dosing Control
13.3.2 Hot Detergent System – Temperature Control
13.3.3 Hot Detergent System – Flow Control
13.3.4 Hot Detergent System – Level Control
13.3.5 Hot PUW System – Temperature Control
13.3.6 Hot PUW System – Flow Control
13.3.7 Hot PUW System – Level Control
Page 22
Paraphrase This Document

1 Short description of the test
At 80°C±5°C, 2% ± 0.05% detergent concentration is equivalent to 500
μScm-1 ± 0.25 μScm-1. The objective of this test is to confirm that the
supply detergent concentration, at a temperature of 80°C±5°C, is
maintained at 500 μScm-1 ± 0.25 μScm-1, before, during and after
maximum instantaneous detergent drawdown when the two biggest
users (reactors RE-119 and RE-140) are being be charged
simultaneously.
2 Linkage to the requirements challenged
URS Section 3.5:
1. The system shall have the facility to automatically dose STERIS
100 detergent to the required concentration (2% ± 0.05%)
2. Online conductivity measurement shall be provided to verify the
detergent concentration
Operationally Critical Component(s):
Conductivity transmitter CT-001
Temperature transmitter TT-002
3 Acceptance criteria for the test
At 80°C±5°C, the online conductivity measurement CT-001 read 500
μScm-1 ± 0.25 μScm-1 before, during and after the maximum
instantaneous detergent drawdown.
4 Prerequisites and or assumptions
3. All commissioning activities are complete and commissioning punch-list
items are closed out.
4. All testers shall be trained and educated in the test method listed in
section-5 below: it is crucial to the success of the testing effort that the
testing process be well understood by all participants.
5. Conductivity transmitter CT-001 is currently within calibration
6. Temperature transmitter TT-002 is currently within calibration
5 Specific Test Steps
1. Start up the WIP skid and after 1-hour confirm that both 80°C
detergent and 80°C hot process water are recirculating.
2. At time = 0 (t0-min) confirm the online conductivity measurement CT-
001 is reading 500 μScm-1 ± 0.25 μScm-1 equivalent to a detergent
concentration of 2% ± 0.05% STERIS 100.
3. Initiate a simultaneous maximum instantaneous detergent
drawdown into the two biggest users, reactor RE-119 and reactor
RE-140, and charge 11,000 litres into both vessels.
4. After the charge stop the detergent drawdown into both reactor RE-
119 and reactor RE-140 (approximately 30-minutes; 22,000 kg/hr
±10% ≈ 11m3/30min = 11,000litres/30min ≈ 5,500
litres/vessel/30min).
Page 23

mode for a further 30-minutes with no further drawdown.
6. In the table provided confirm that at 80°C±5°C the online
conductivity measurement CT-001 read 500 μScm-1 ± 0.25 by
recording the value at successive 5-minute intervals. Be sure that
the time period when the values are recorded correspond to the
following:
Before maximum instantaneous detergent drawdown (t0-min)
During the instantaneous detergent drawdown, up to the full
5,500 litre charges
The 30-minutes following the instantaneous drawdown
7. In the same table from the readings of temperature transmitter TT-
002 record the associated temperature values of the detergent loop
and confirm consistent values of 80°C±5°C.
8. In the same table also record the values for level in reactor RE-119
and reactor RE-140 from level-transmitters LT-119 and LT-140.
9. Print out the associated trends for conductivity transmitter CT-001,
temperature transmitter TT-002, and level-transmitters LT-119 and
LT-140 during the test period and append to this test section.
6 Expected results and Actual results
1. In the check-sheets in Appendix-C1, reach a conclusion as to
whether each test step has been successfully completed by
transcribing bold text from the ‘Expected Results’ column into the
corresponding ‘Actual Results’ field.
2. Assess each step listed and determine whether the step has
passed or failed.
3. The person performing the test should be identified and the date the
testing was performed should be recorded.
4. At the bottom of the test script a validation peer (other than the
person performing the test) shall review the script post execution.
5. Quality Assurance (QA) shall approve the completed test.
Page 24
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

13.3 Hot Detergent System – Flow Control
13.4 Hot Detergent System – Level Control
13.5 Hot PUW System – Temperature Control
13.6 Hot PUW System – Flow Control
13.7 Hot PUW System – Level Control
13.8 Approved SOP Verification
In Protocol Section-13.8 ‘Approved SOP Verification’ ensure this list of
approved SOPs aligns with Protocol Section-11.7 and its associated form in
Protocol Section-16.2.7. Enter the equivalent details in the form collated in
Protocol Section-16.3.8.
14 Protocol Quality Procedures
14.1 Signature Log
Summarize ‘Signature-Log’ procedure that compliments the log in Protocol
Section-16.1.
Page 25
Paraphrase This Document

Summarize a protocol deviation procedure that compliments the Deviation log
in Protocol Section-16.4 and the Deviation Form in Protocol Section-16.5.
14.3 Change Control Procedure
Summarize a Change Control deviation procedure that compliments the
Change Control log in Protocol Section-16.6 and the Change Control Form in
Protocol Section-16.7.
15 IOQ Protocol Summary Report
Following field execution of this validation protocol a validation report that
cross-references the validation protocol will be prepared, summarising the
results obtained, commenting on any deviations observed, and drawing the
appropriate conclusions, including recommending changes to correct
deficiencies.
Page 26

Appendix A: Signature Log
Appendix B: Installation Verification GMP checksheets
P&ID Walkdown Installation Verification
Equipment Installation Verification
Instrument Installation Verification
Piping Installation Verification
Calibration Program Verification
Preventative Maintenance Program Verification
Draft SOP Verification
Test-Instrument Calibration Verification
Appendix C: Functional Verification check-sheets
Hot Detergent System – Detergent Dosing Control
Hot Detergent System – Temperature Control
Hot Detergent System – Flow Control
Hot Detergent System – Level Control
Hot PUW System – Temperature Control
Hot PUW System – Flow Control
Hot PUW System – Level Control
Appendix D: Deviation log
Appendix E: Deviation Form
Appendix F: Change control log
Appendix G: Change control Form
Appendix H: Component Impact Assessment
Appendix I: Traceability Matrix
Page 27
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Page __ of __
Signature Log
Printed Name Signature Initials Date Company/
Title/
Department
GMP Trained
(Yes / No)
Comments:
Reviewed By:
Validation
Date:
Approved By:
(QA)
Date:
Page 28
Paraphrase This Document
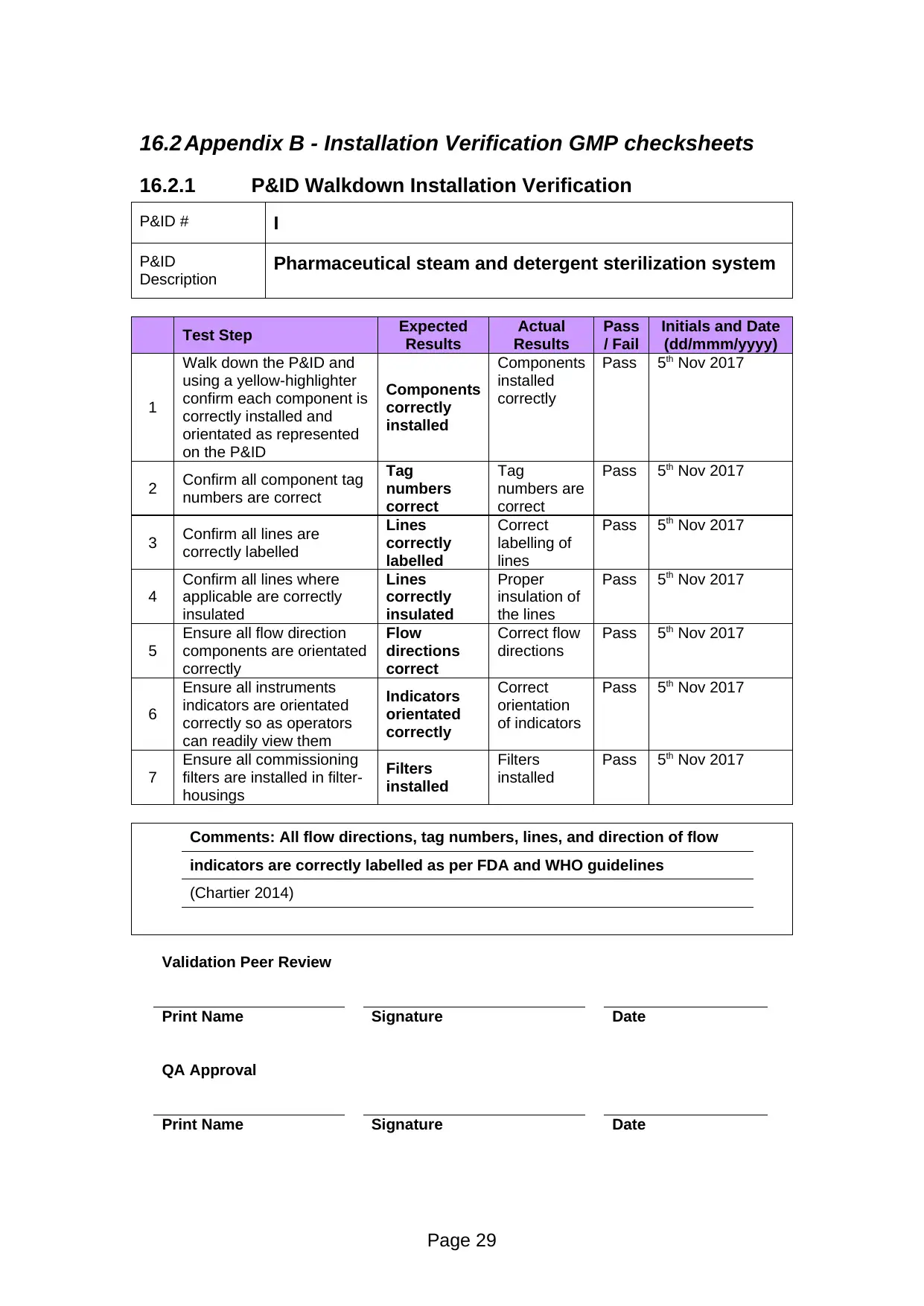
16.2.1 P&ID Walkdown Installation Verification
P&ID # I
P&ID
Description Pharmaceutical steam and detergent sterilization system
Test Step Expected
Results
Actual
Results
Pass
/ Fail
Initials and Date
(dd/mmm/yyyy)
1
Walk down the P&ID and
using a yellow-highlighter
confirm each component is
correctly installed and
orientated as represented
on the P&ID
Components
correctly
installed
Components
installed
correctly
Pass 5th Nov 2017
2 Confirm all component tag
numbers are correct
Tag
numbers
correct
Tag
numbers are
correct
Pass 5th Nov 2017
3 Confirm all lines are
correctly labelled
Lines
correctly
labelled
Correct
labelling of
lines
Pass 5th Nov 2017
4
Confirm all lines where
applicable are correctly
insulated
Lines
correctly
insulated
Proper
insulation of
the lines
Pass 5th Nov 2017
5
Ensure all flow direction
components are orientated
correctly
Flow
directions
correct
Correct flow
directions
Pass 5th Nov 2017
6
Ensure all instruments
indicators are orientated
correctly so as operators
can readily view them
Indicators
orientated
correctly
Correct
orientation
of indicators
Pass 5th Nov 2017
7
Ensure all commissioning
filters are installed in filter-
housings
Filters
installed
Filters
installed
Pass 5th Nov 2017
Comments: All flow directions, tag numbers, lines, and direction of flow
indicators are correctly labelled as per FDA and WHO guidelines
(Chartier 2014)
Validation Peer Review
Print Name Signature Date
QA Approval
Print Name Signature Date
Page 29
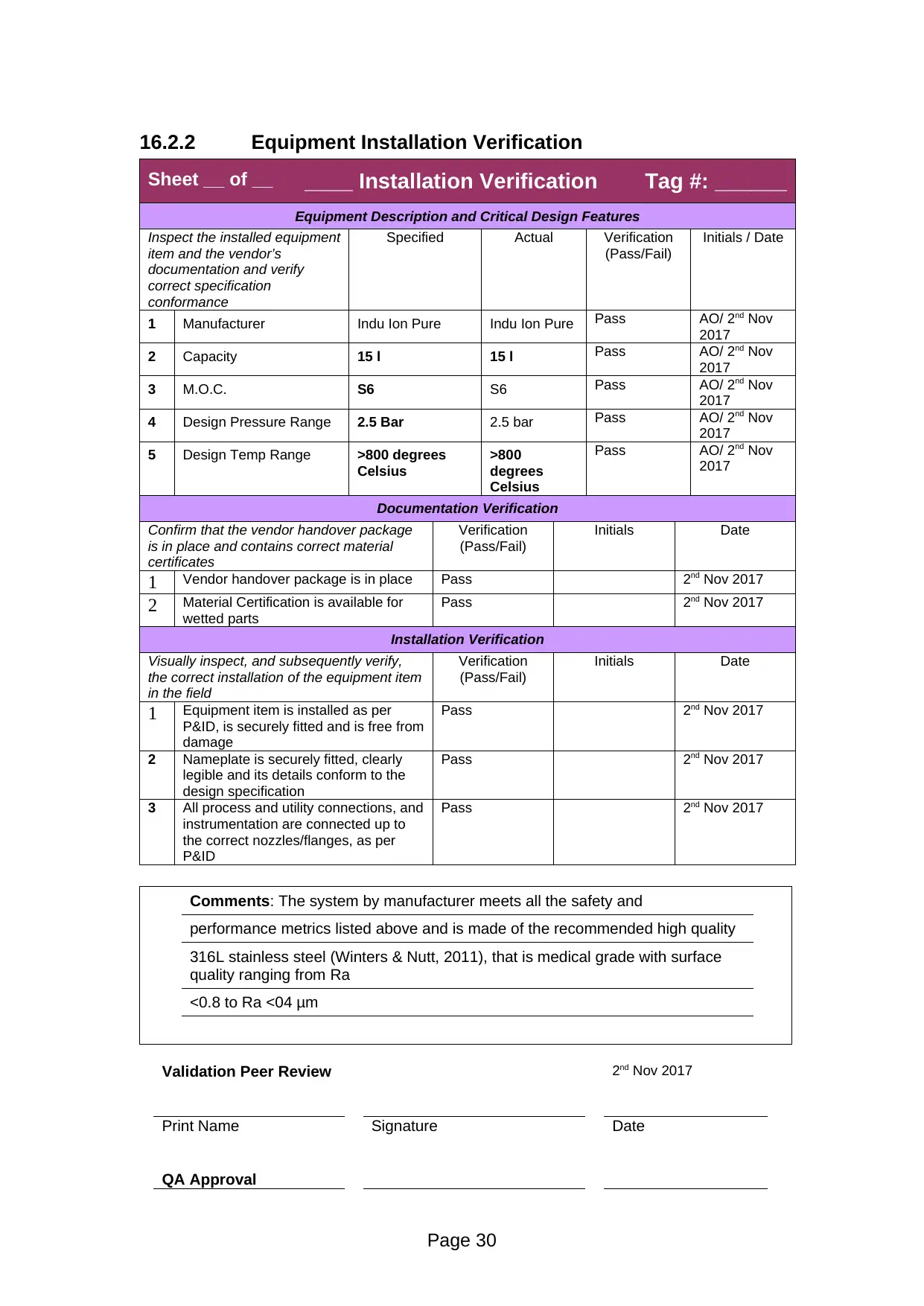
Sheet __ of __ ____ Installation Verification Tag #: ______
Equipment Description and Critical Design Features
Inspect the installed equipment
item and the vendor’s
documentation and verify
correct specification
conformance
Specified Actual Verification
(Pass/Fail)
Initials / Date
1 Manufacturer Indu Ion Pure Indu Ion Pure Pass AO/ 2nd Nov
2017
2 Capacity 15 l 15 l Pass AO/ 2nd Nov
2017
3 M.O.C. S6 S6 Pass AO/ 2nd Nov
2017
4 Design Pressure Range 2.5 Bar 2.5 bar Pass AO/ 2nd Nov
2017
5 Design Temp Range >800 degrees
Celsius
>800
degrees
Celsius
Pass AO/ 2nd Nov
2017
Documentation Verification
Confirm that the vendor handover package
is in place and contains correct material
certificates
Verification
(Pass/Fail)
Initials Date
1 Vendor handover package is in place Pass 2nd Nov 2017
2 Material Certification is available for
wetted parts
Pass 2nd Nov 2017
Installation Verification
Visually inspect, and subsequently verify,
the correct installation of the equipment item
in the field
Verification
(Pass/Fail)
Initials Date
1 Equipment item is installed as per
P&ID, is securely fitted and is free from
damage
Pass 2nd Nov 2017
2 Nameplate is securely fitted, clearly
legible and its details conform to the
design specification
Pass 2nd Nov 2017
3 All process and utility connections, and
instrumentation are connected up to
the correct nozzles/flanges, as per
P&ID
Pass 2nd Nov 2017
Comments: The system by manufacturer meets all the safety and
performance metrics listed above and is made of the recommended high quality
316L stainless steel (Winters & Nutt, 2011), that is medical grade with surface
quality ranging from Ra
<0.8 to Ra <04 μm
Validation Peer Review 2nd Nov 2017
Print Name Signature Date
QA Approval
Page 30
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Page 31
Paraphrase This Document
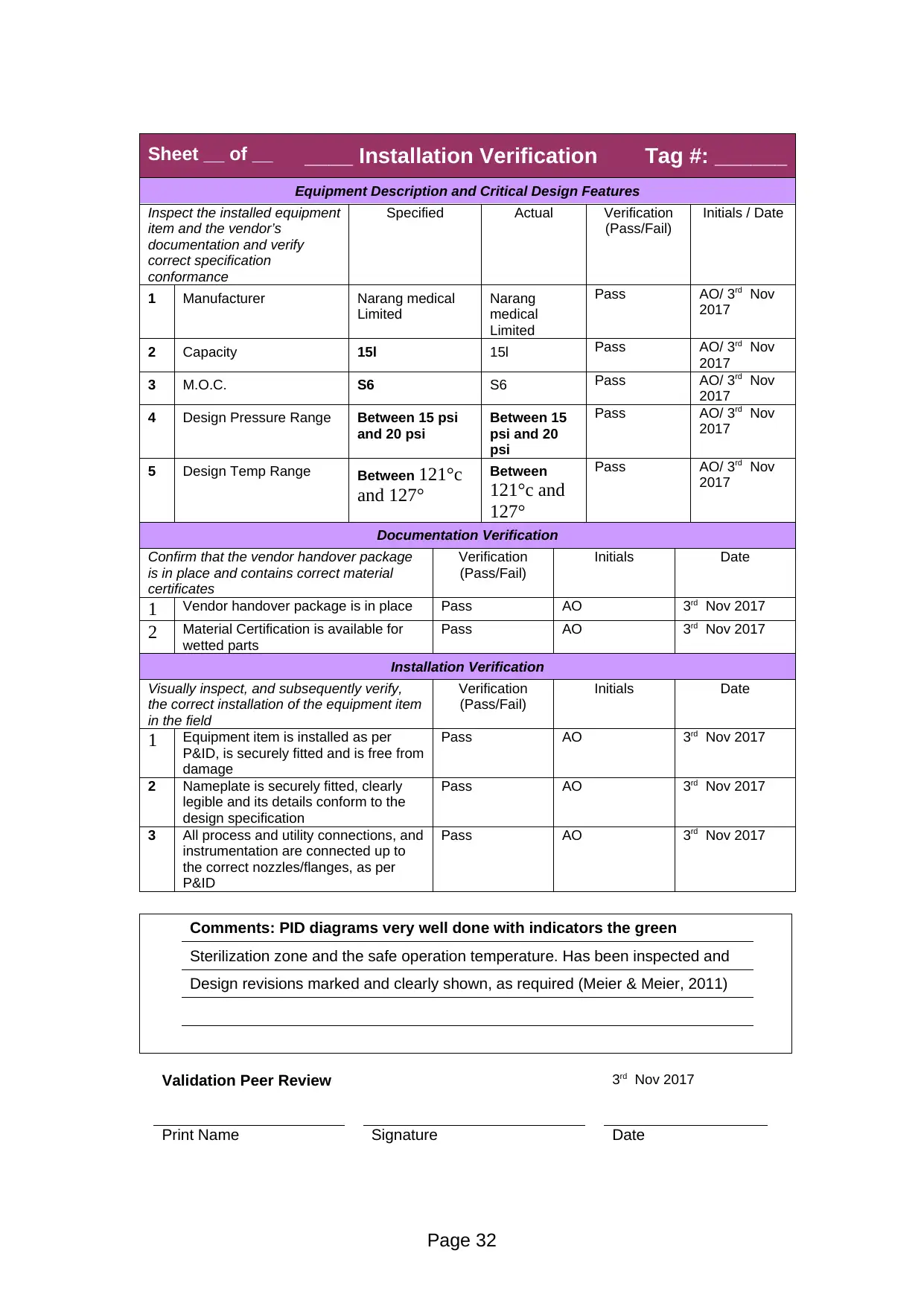
Equipment Description and Critical Design Features
Inspect the installed equipment
item and the vendor’s
documentation and verify
correct specification
conformance
Specified Actual Verification
(Pass/Fail)
Initials / Date
1 Manufacturer Narang medical
Limited
Narang
medical
Limited
Pass AO/ 3rd Nov
2017
2 Capacity 15l 15l Pass AO/ 3rd Nov
2017
3 M.O.C. S6 S6 Pass AO/ 3rd Nov
2017
4 Design Pressure Range Between 15 psi
and 20 psi
Between 15
psi and 20
psi
Pass AO/ 3rd Nov
2017
5 Design Temp Range Between 121°c
and 127°
Between
121°c and
127°
Pass AO/ 3rd Nov
2017
Documentation Verification
Confirm that the vendor handover package
is in place and contains correct material
certificates
Verification
(Pass/Fail)
Initials Date
1 Vendor handover package is in place Pass AO 3rd Nov 2017
2 Material Certification is available for
wetted parts
Pass AO 3rd Nov 2017
Installation Verification
Visually inspect, and subsequently verify,
the correct installation of the equipment item
in the field
Verification
(Pass/Fail)
Initials Date
1 Equipment item is installed as per
P&ID, is securely fitted and is free from
damage
Pass AO 3rd Nov 2017
2 Nameplate is securely fitted, clearly
legible and its details conform to the
design specification
Pass AO 3rd Nov 2017
3 All process and utility connections, and
instrumentation are connected up to
the correct nozzles/flanges, as per
P&ID
Pass AO 3rd Nov 2017
Comments: PID diagrams very well done with indicators the green
Sterilization zone and the safe operation temperature. Has been inspected and
Design revisions marked and clearly shown, as required (Meier & Meier, 2011)
Validation Peer Review 3rd Nov 2017
Print Name Signature Date
Page 32

Print Name Signature Date
Page 33
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
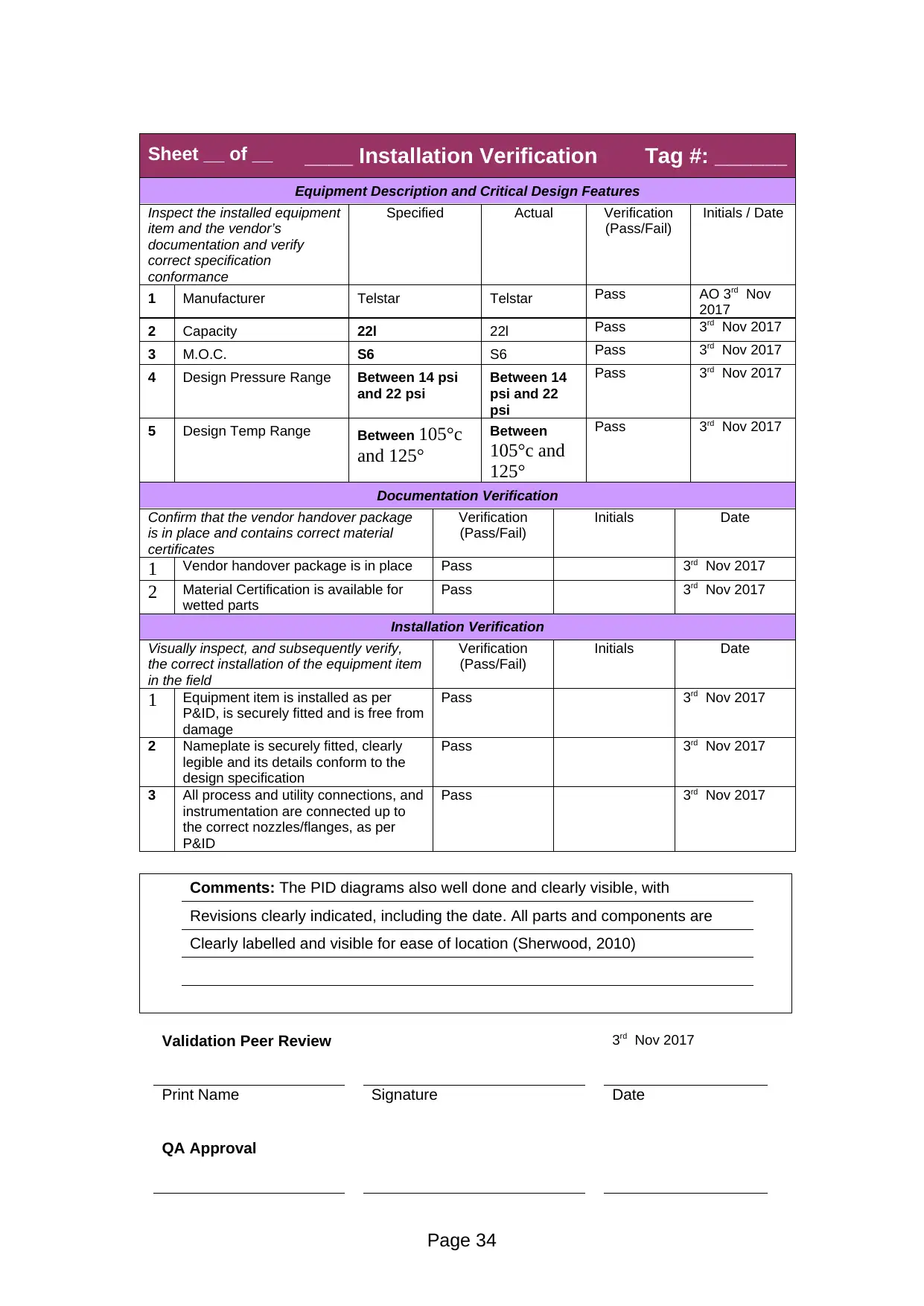
Equipment Description and Critical Design Features
Inspect the installed equipment
item and the vendor’s
documentation and verify
correct specification
conformance
Specified Actual Verification
(Pass/Fail)
Initials / Date
1 Manufacturer Telstar Telstar Pass AO 3rd Nov
2017
2 Capacity 22l 22l Pass 3rd Nov 2017
3 M.O.C. S6 S6 Pass 3rd Nov 2017
4 Design Pressure Range Between 14 psi
and 22 psi
Between 14
psi and 22
psi
Pass 3rd Nov 2017
5 Design Temp Range Between 105°c
and 125°
Between
105°c and
125°
Pass 3rd Nov 2017
Documentation Verification
Confirm that the vendor handover package
is in place and contains correct material
certificates
Verification
(Pass/Fail)
Initials Date
1 Vendor handover package is in place Pass 3rd Nov 2017
2 Material Certification is available for
wetted parts
Pass 3rd Nov 2017
Installation Verification
Visually inspect, and subsequently verify,
the correct installation of the equipment item
in the field
Verification
(Pass/Fail)
Initials Date
1 Equipment item is installed as per
P&ID, is securely fitted and is free from
damage
Pass 3rd Nov 2017
2 Nameplate is securely fitted, clearly
legible and its details conform to the
design specification
Pass 3rd Nov 2017
3 All process and utility connections, and
instrumentation are connected up to
the correct nozzles/flanges, as per
P&ID
Pass 3rd Nov 2017
Comments: The PID diagrams also well done and clearly visible, with
Revisions clearly indicated, including the date. All parts and components are
Clearly labelled and visible for ease of location (Sherwood, 2010)
Validation Peer Review 3rd Nov 2017
Print Name Signature Date
QA Approval
Page 34
Paraphrase This Document

Page 35
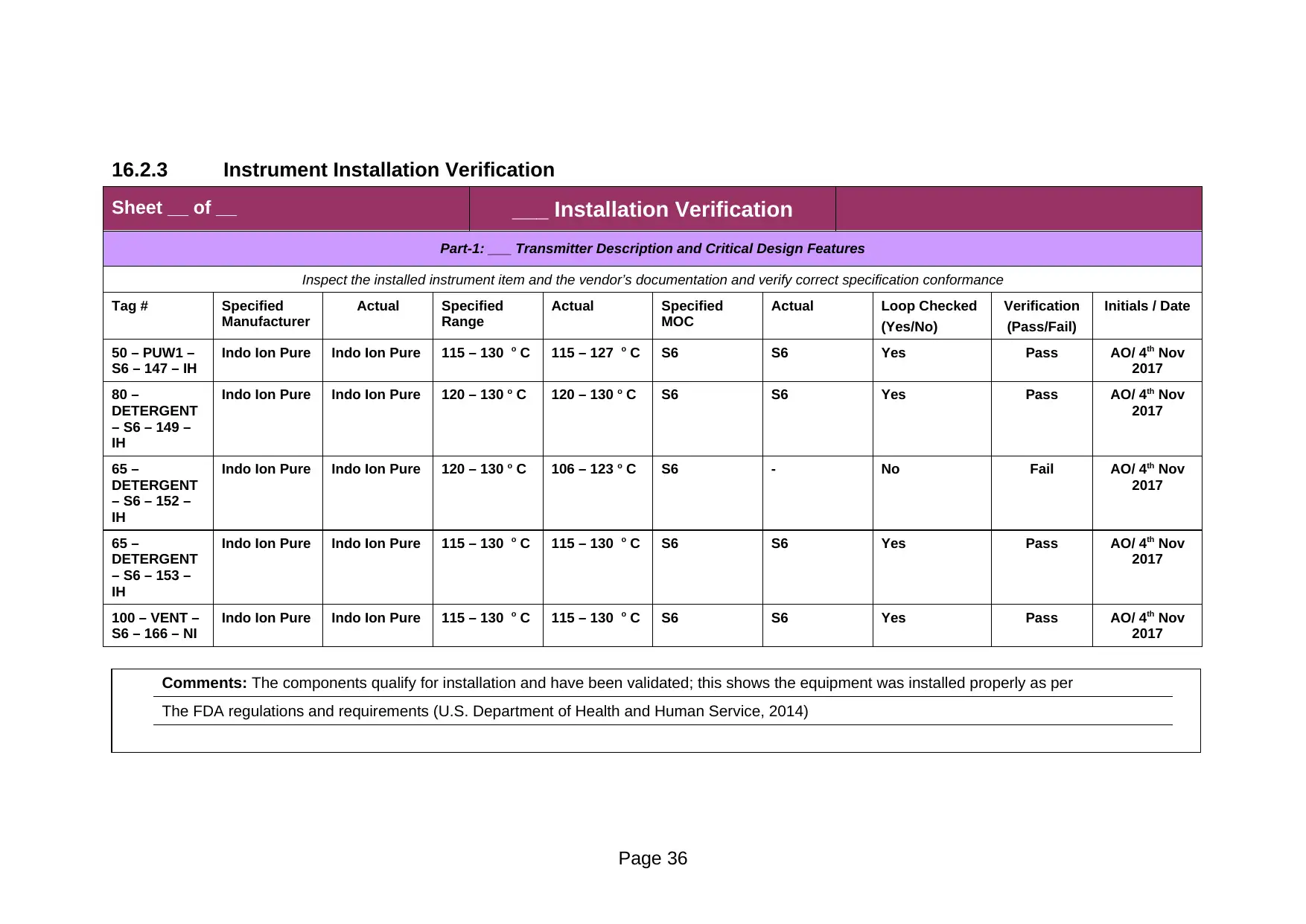
Sheet __ of __ ___ Installation Verification
Part-1: ___ Transmitter Description and Critical Design Features
Inspect the installed instrument item and the vendor’s documentation and verify correct specification conformance
Tag # Specified
Manufacturer
Actual Specified
Range
Actual Specified
MOC
Actual Loop Checked
(Yes/No)
Verification
(Pass/Fail)
Initials / Date
50 – PUW1 –
S6 – 147 – IH
Indo Ion Pure Indo Ion Pure 115 – 130 o C 115 – 127 o C S6 S6 Yes Pass AO/ 4th Nov
2017
80 –
DETERGENT
– S6 – 149 –
IH
Indo Ion Pure Indo Ion Pure 120 – 130 o C 120 – 130 o C S6 S6 Yes Pass AO/ 4th Nov
2017
65 –
DETERGENT
– S6 – 152 –
IH
Indo Ion Pure Indo Ion Pure 120 – 130 o C 106 – 123 o C S6 - No Fail AO/ 4th Nov
2017
65 –
DETERGENT
– S6 – 153 –
IH
Indo Ion Pure Indo Ion Pure 115 – 130 o C 115 – 130 o C S6 S6 Yes Pass AO/ 4th Nov
2017
100 – VENT –
S6 – 166 – NI
Indo Ion Pure Indo Ion Pure 115 – 130 o C 115 – 130 o C S6 S6 Yes Pass AO/ 4th Nov
2017
Comments: The components qualify for installation and have been validated; this shows the equipment was installed properly as per
The FDA regulations and requirements (U.S. Department of Health and Human Service, 2014)
Page 36
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Print Name Signature Date
QA Approval
Print Name Signature Date
Page 37
Paraphrase This Document
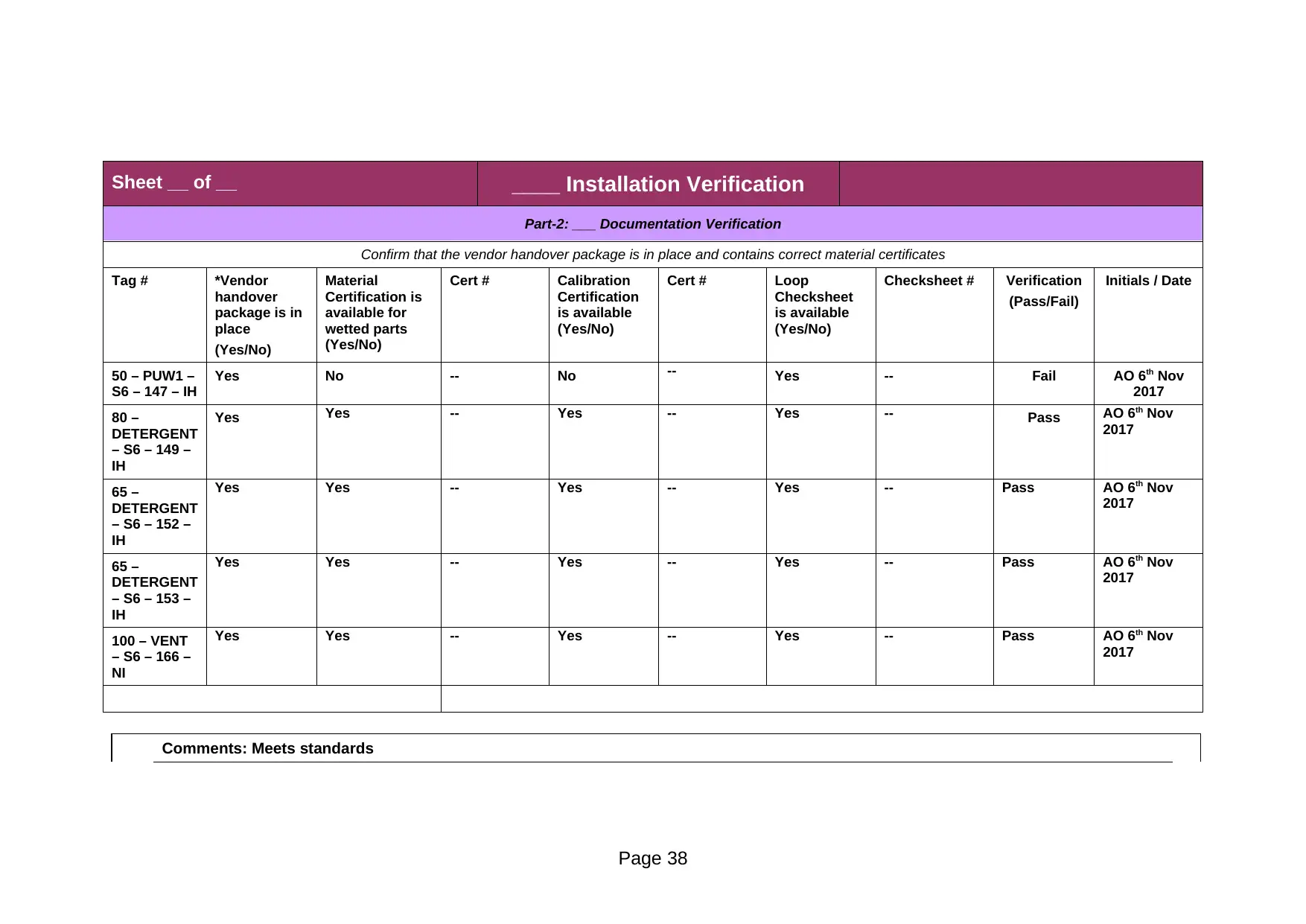
Part-2: ___ Documentation Verification
Confirm that the vendor handover package is in place and contains correct material certificates
Tag # *Vendor
handover
package is in
place
(Yes/No)
Material
Certification is
available for
wetted parts
(Yes/No)
Cert # Calibration
Certification
is available
(Yes/No)
Cert # Loop
Checksheet
is available
(Yes/No)
Checksheet # Verification
(Pass/Fail)
Initials / Date
50 – PUW1 –
S6 – 147 – IH
Yes No -- No -- Yes -- Fail AO 6th Nov
2017
80 –
DETERGENT
– S6 – 149 –
IH
Yes Yes -- Yes -- Yes -- Pass AO 6th Nov
2017
65 –
DETERGENT
– S6 – 152 –
IH
Yes Yes -- Yes -- Yes -- Pass AO 6th Nov
2017
65 –
DETERGENT
– S6 – 153 –
IH
Yes Yes -- Yes -- Yes -- Pass AO 6th Nov
2017
100 – VENT
– S6 – 166 –
NI
Yes Yes -- Yes -- Yes -- Pass AO 6th Nov
2017
Comments: Meets standards
Page 38

Print Name Signature Date
QA Approval
Print Name Signature Date
Page 39
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
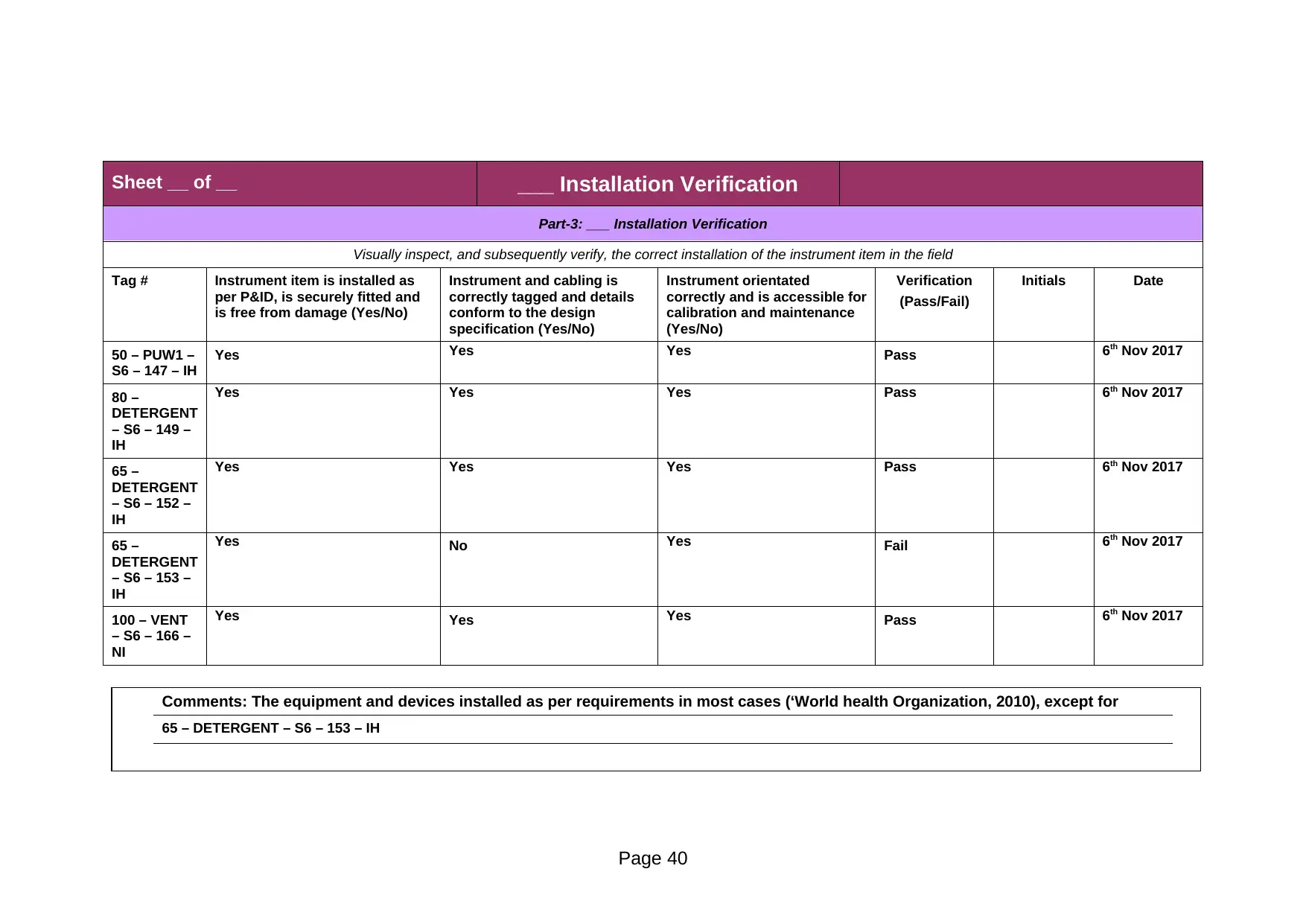
Part-3: ___ Installation Verification
Visually inspect, and subsequently verify, the correct installation of the instrument item in the field
Tag # Instrument item is installed as
per P&ID, is securely fitted and
is free from damage (Yes/No)
Instrument and cabling is
correctly tagged and details
conform to the design
specification (Yes/No)
Instrument orientated
correctly and is accessible for
calibration and maintenance
(Yes/No)
Verification
(Pass/Fail)
Initials Date
50 – PUW1 –
S6 – 147 – IH
Yes Yes Yes Pass 6th Nov 2017
80 –
DETERGENT
– S6 – 149 –
IH
Yes Yes Yes Pass 6th Nov 2017
65 –
DETERGENT
– S6 – 152 –
IH
Yes Yes Yes Pass 6th Nov 2017
65 –
DETERGENT
– S6 – 153 –
IH
Yes No Yes Fail 6th Nov 2017
100 – VENT
– S6 – 166 –
NI
Yes Yes Yes Pass 6th Nov 2017
Comments: The equipment and devices installed as per requirements in most cases (‘World health Organization, 2010), except for
65 – DETERGENT – S6 – 153 – IH
Page 40
Paraphrase This Document

Print Name Signature Date
QA Approval
Print Name Signature Date
Page 41
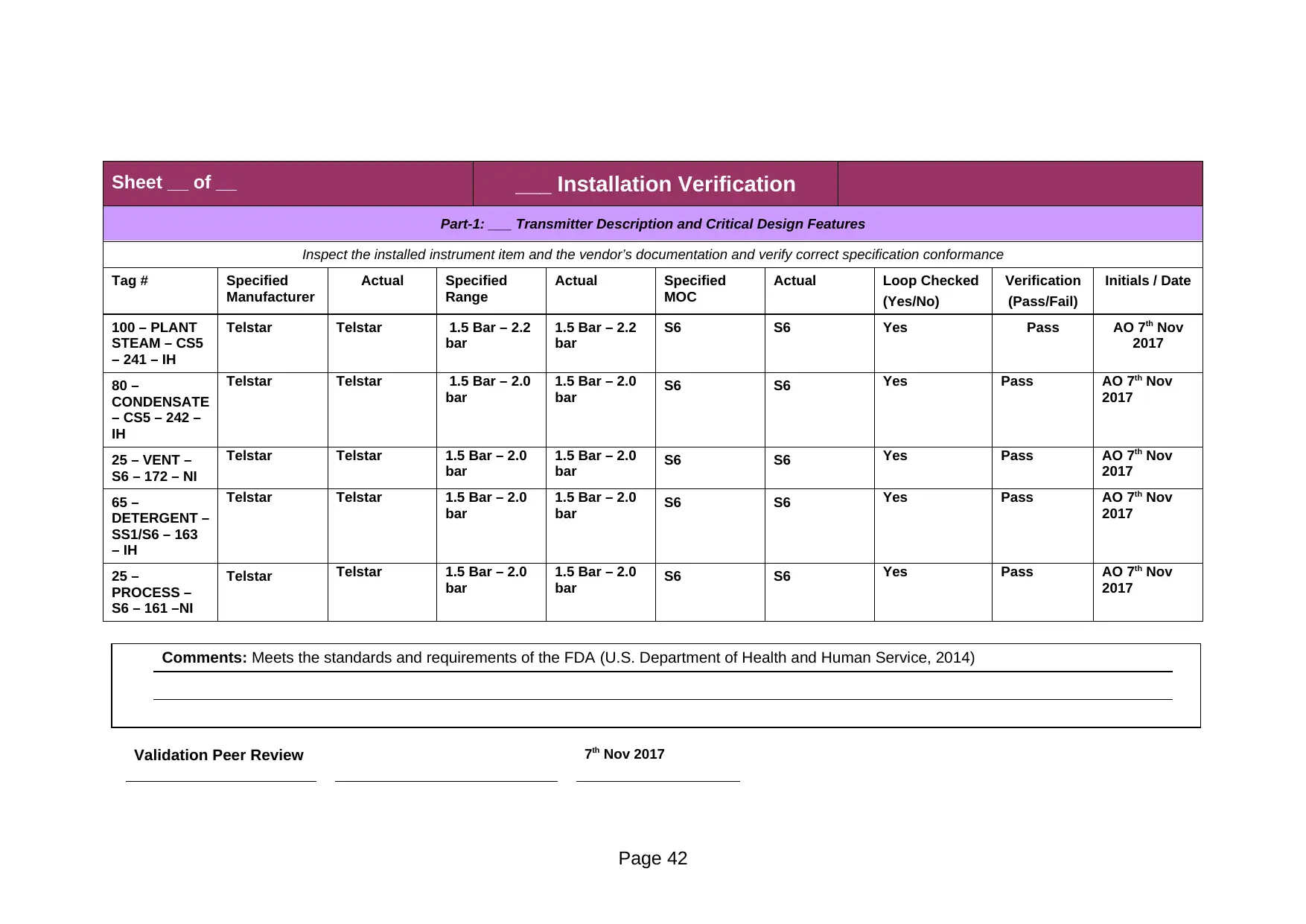
Part-1: ___ Transmitter Description and Critical Design Features
Inspect the installed instrument item and the vendor’s documentation and verify correct specification conformance
Tag # Specified
Manufacturer
Actual Specified
Range
Actual Specified
MOC
Actual Loop Checked
(Yes/No)
Verification
(Pass/Fail)
Initials / Date
100 – PLANT
STEAM – CS5
– 241 – IH
Telstar Telstar 1.5 Bar – 2.2
bar
1.5 Bar – 2.2
bar
S6 S6 Yes Pass AO 7th Nov
2017
80 –
CONDENSATE
– CS5 – 242 –
IH
Telstar Telstar 1.5 Bar – 2.0
bar
1.5 Bar – 2.0
bar S6 S6 Yes Pass AO 7th Nov
2017
25 – VENT –
S6 – 172 – NI
Telstar Telstar 1.5 Bar – 2.0
bar
1.5 Bar – 2.0
bar S6 S6 Yes Pass AO 7th Nov
2017
65 –
DETERGENT –
SS1/S6 – 163
– IH
Telstar Telstar 1.5 Bar – 2.0
bar
1.5 Bar – 2.0
bar S6 S6 Yes Pass AO 7th Nov
2017
25 –
PROCESS –
S6 – 161 –NI
Telstar Telstar 1.5 Bar – 2.0
bar
1.5 Bar – 2.0
bar S6 S6 Yes Pass AO 7th Nov
2017
Comments: Meets the standards and requirements of the FDA (U.S. Department of Health and Human Service, 2014)
Validation Peer Review 7th Nov 2017
Page 42
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

QA Approval
Print Name Signature Date
Page 43
Paraphrase This Document
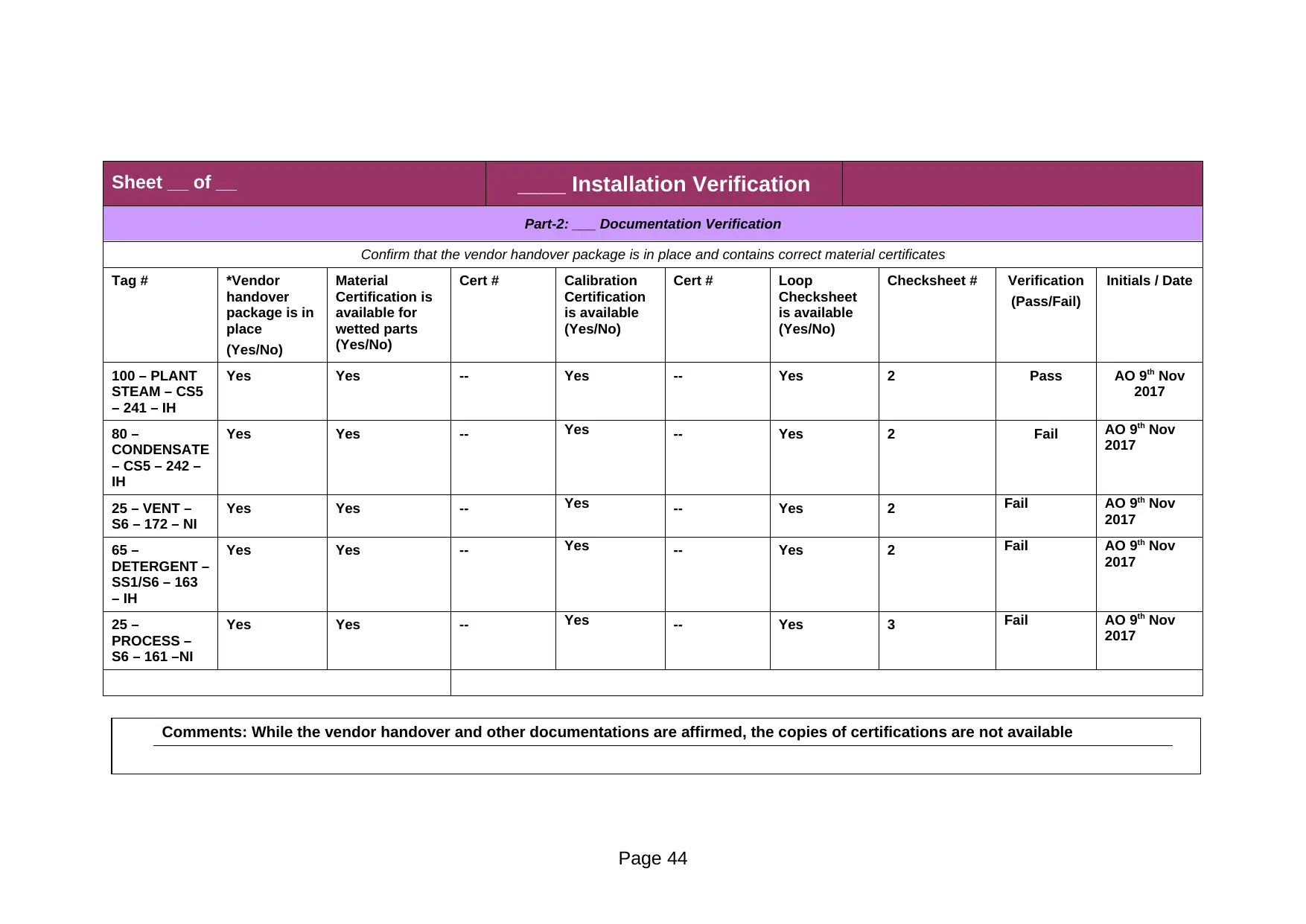
Part-2: ___ Documentation Verification
Confirm that the vendor handover package is in place and contains correct material certificates
Tag # *Vendor
handover
package is in
place
(Yes/No)
Material
Certification is
available for
wetted parts
(Yes/No)
Cert # Calibration
Certification
is available
(Yes/No)
Cert # Loop
Checksheet
is available
(Yes/No)
Checksheet # Verification
(Pass/Fail)
Initials / Date
100 – PLANT
STEAM – CS5
– 241 – IH
Yes Yes -- Yes -- Yes 2 Pass AO 9th Nov
2017
80 –
CONDENSATE
– CS5 – 242 –
IH
Yes Yes -- Yes -- Yes 2 Fail AO 9th Nov
2017
25 – VENT –
S6 – 172 – NI
Yes Yes -- Yes -- Yes 2 Fail AO 9th Nov
2017
65 –
DETERGENT –
SS1/S6 – 163
– IH
Yes Yes -- Yes -- Yes 2 Fail AO 9th Nov
2017
25 –
PROCESS –
S6 – 161 –NI
Yes Yes -- Yes -- Yes 3 Fail AO 9th Nov
2017
Comments: While the vendor handover and other documentations are affirmed, the copies of certifications are not available
Page 44

Print Name Signature Date
QA Approval
Print Name Signature Date
Page 45
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
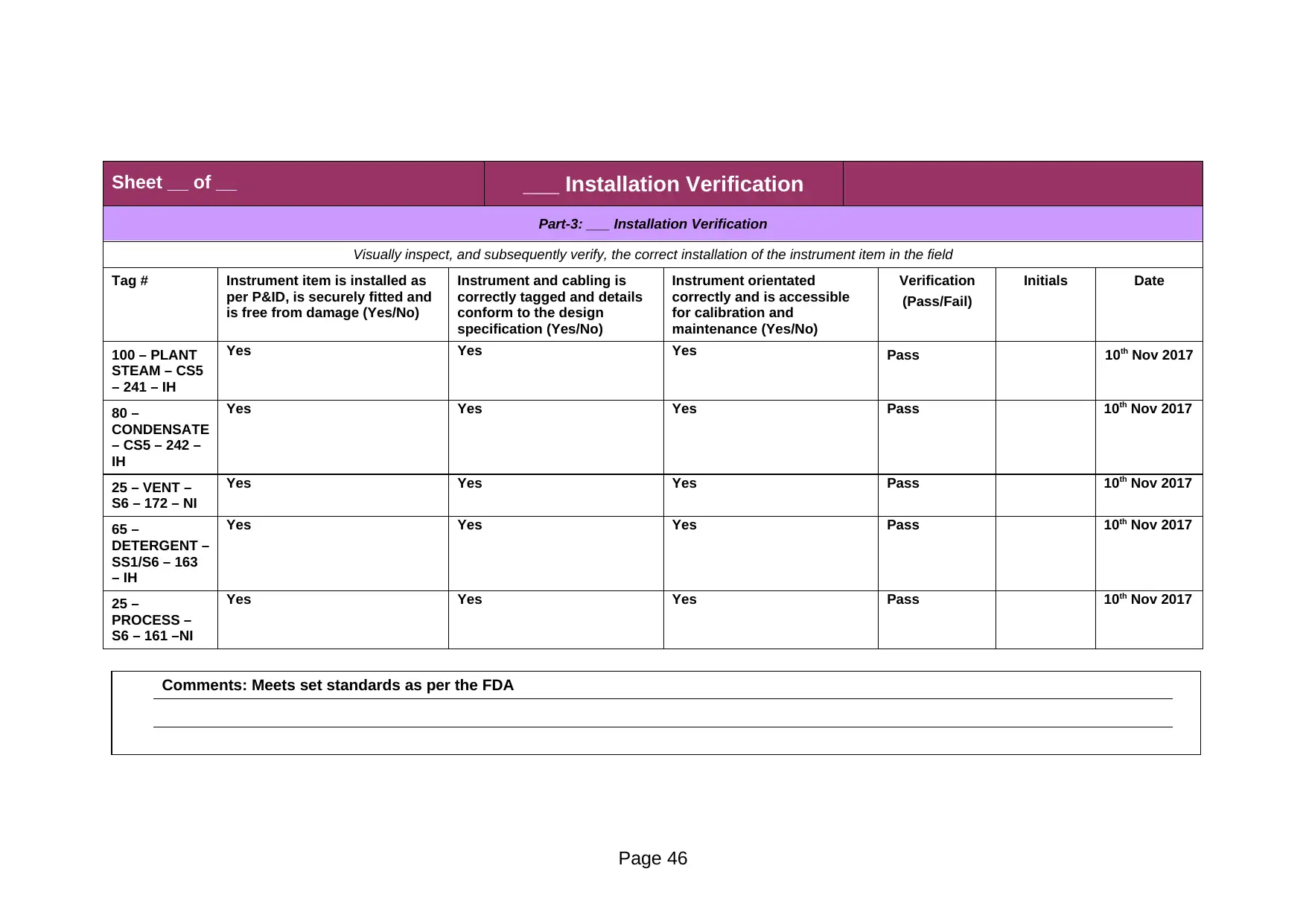
Part-3: ___ Installation Verification
Visually inspect, and subsequently verify, the correct installation of the instrument item in the field
Tag # Instrument item is installed as
per P&ID, is securely fitted and
is free from damage (Yes/No)
Instrument and cabling is
correctly tagged and details
conform to the design
specification (Yes/No)
Instrument orientated
correctly and is accessible
for calibration and
maintenance (Yes/No)
Verification
(Pass/Fail)
Initials Date
100 – PLANT
STEAM – CS5
– 241 – IH
Yes Yes Yes Pass 10th Nov 2017
80 –
CONDENSATE
– CS5 – 242 –
IH
Yes Yes Yes Pass 10th Nov 2017
25 – VENT –
S6 – 172 – NI
Yes Yes Yes Pass 10th Nov 2017
65 –
DETERGENT –
SS1/S6 – 163
– IH
Yes Yes Yes Pass 10th Nov 2017
25 –
PROCESS –
S6 – 161 –NI
Yes Yes Yes Pass 10th Nov 2017
Comments: Meets set standards as per the FDA
Page 46
Paraphrase This Document

Print Name Signature Date
QA Approval
Print Name Signature Date
Page 47
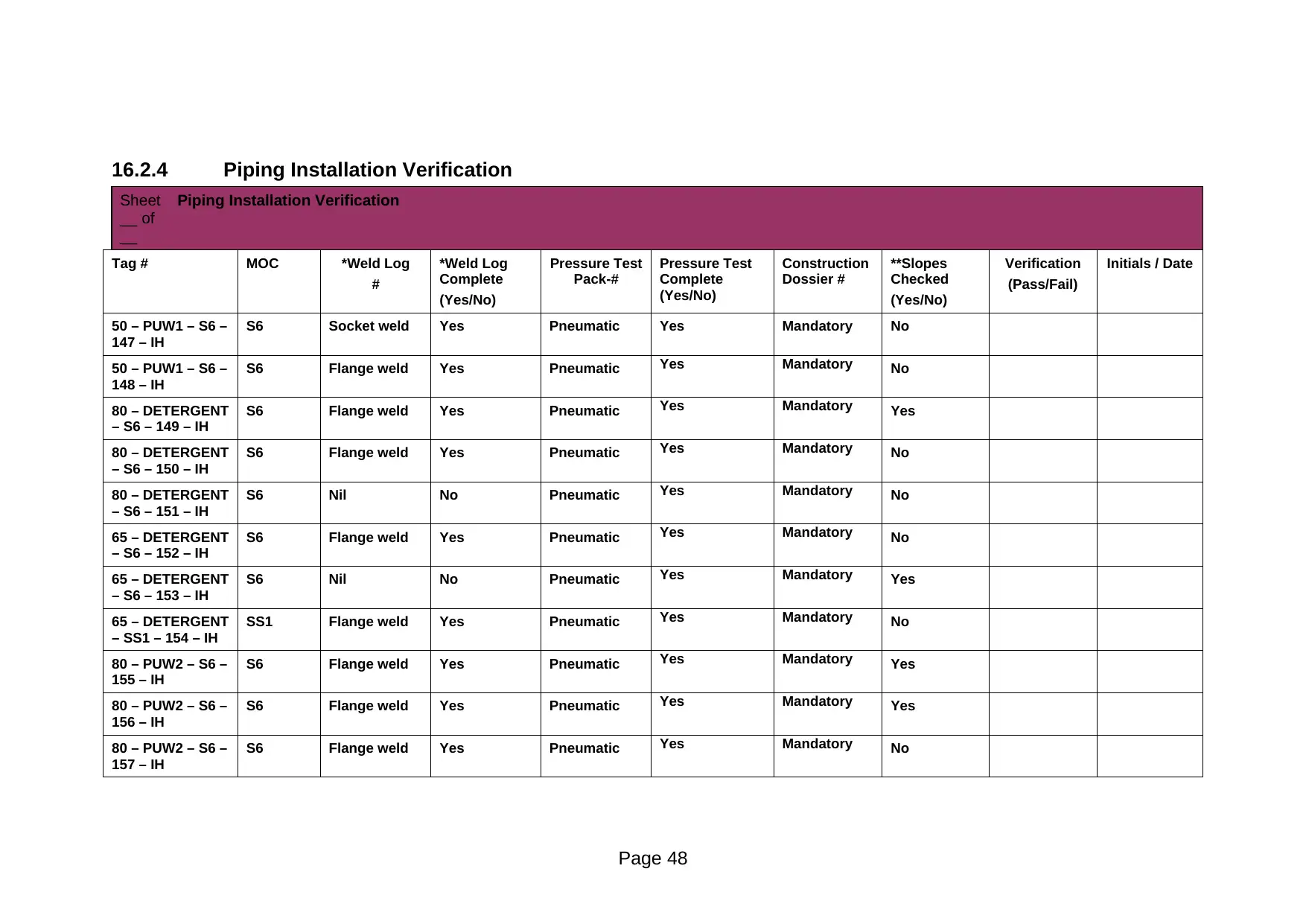
Sheet
__ of
__
Piping Installation Verification
Tag # MOC *Weld Log
#
*Weld Log
Complete
(Yes/No)
Pressure Test
Pack-#
Pressure Test
Complete
(Yes/No)
Construction
Dossier #
**Slopes
Checked
(Yes/No)
Verification
(Pass/Fail)
Initials / Date
50 – PUW1 – S6 –
147 – IH
S6 Socket weld Yes Pneumatic Yes Mandatory No
50 – PUW1 – S6 –
148 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory No
80 – DETERGENT
– S6 – 149 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory Yes
80 – DETERGENT
– S6 – 150 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory No
80 – DETERGENT
– S6 – 151 – IH
S6 Nil No Pneumatic Yes Mandatory No
65 – DETERGENT
– S6 – 152 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory No
65 – DETERGENT
– S6 – 153 – IH
S6 Nil No Pneumatic Yes Mandatory Yes
65 – DETERGENT
– SS1 – 154 – IH
SS1 Flange weld Yes Pneumatic Yes Mandatory No
80 – PUW2 – S6 –
155 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory Yes
80 – PUW2 – S6 –
156 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory Yes
80 – PUW2 – S6 –
157 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory No
Page 48
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
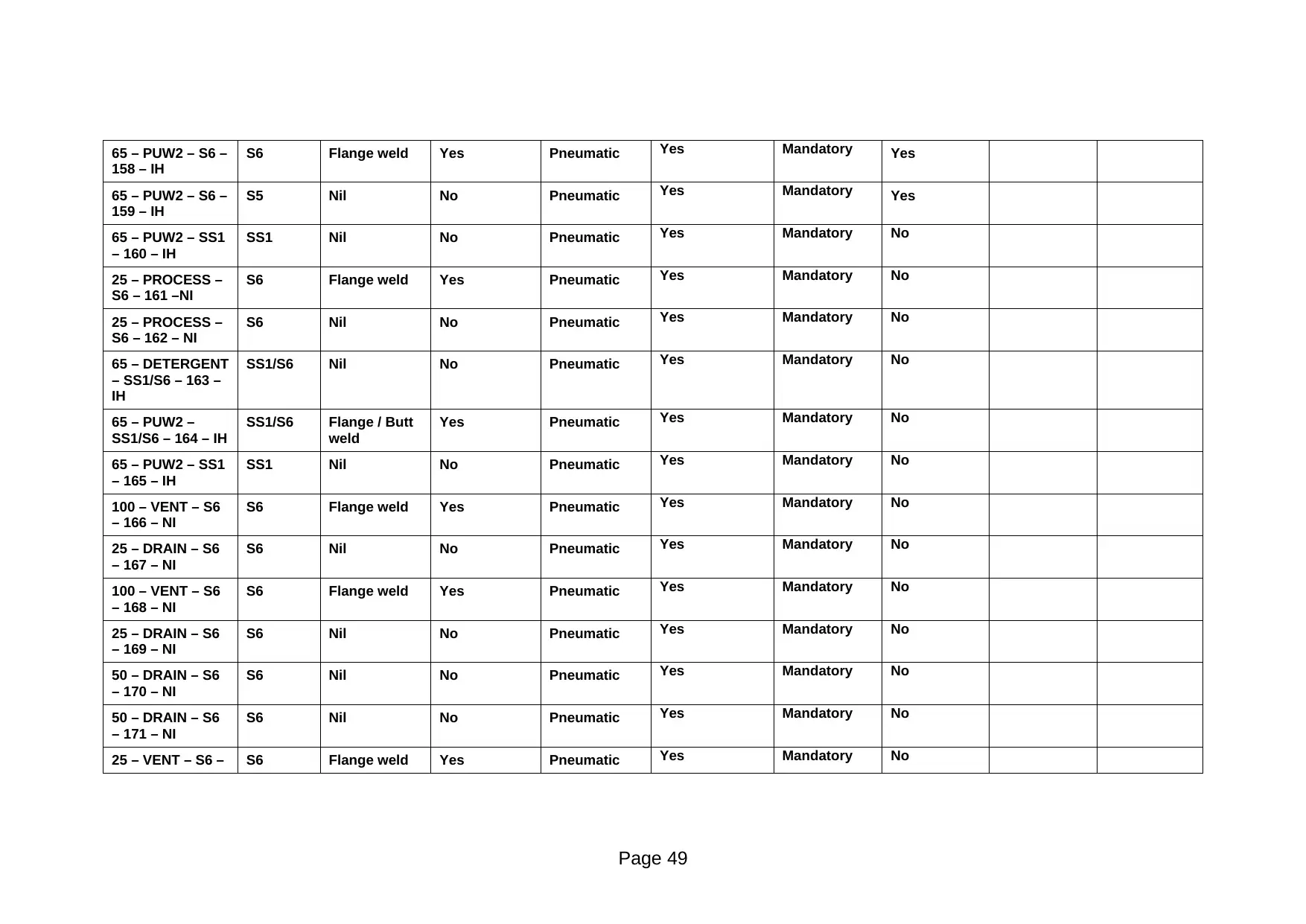
158 – IH
S6 Flange weld Yes Pneumatic Yes Mandatory Yes
65 – PUW2 – S6 –
159 – IH
S5 Nil No Pneumatic Yes Mandatory Yes
65 – PUW2 – SS1
– 160 – IH
SS1 Nil No Pneumatic Yes Mandatory No
25 – PROCESS –
S6 – 161 –NI
S6 Flange weld Yes Pneumatic Yes Mandatory No
25 – PROCESS –
S6 – 162 – NI
S6 Nil No Pneumatic Yes Mandatory No
65 – DETERGENT
– SS1/S6 – 163 –
IH
SS1/S6 Nil No Pneumatic Yes Mandatory No
65 – PUW2 –
SS1/S6 – 164 – IH
SS1/S6 Flange / Butt
weld
Yes Pneumatic Yes Mandatory No
65 – PUW2 – SS1
– 165 – IH
SS1 Nil No Pneumatic Yes Mandatory No
100 – VENT – S6
– 166 – NI
S6 Flange weld Yes Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 167 – NI
S6 Nil No Pneumatic Yes Mandatory No
100 – VENT – S6
– 168 – NI
S6 Flange weld Yes Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 169 – NI
S6 Nil No Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 170 – NI
S6 Nil No Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 171 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – VENT – S6 – S6 Flange weld Yes Pneumatic Yes Mandatory No
Page 49
Paraphrase This Document
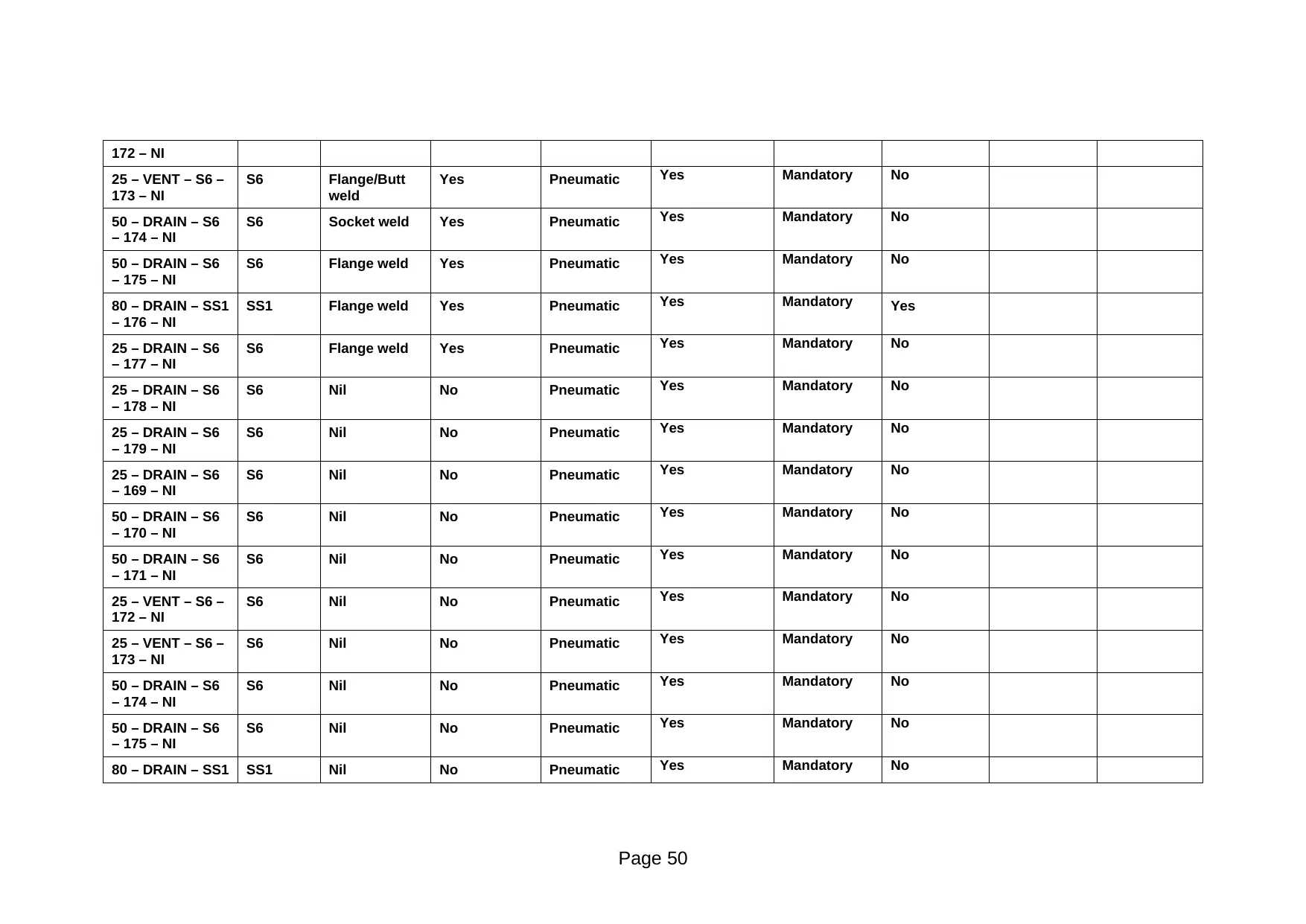
25 – VENT – S6 –
173 – NI
S6 Flange/Butt
weld
Yes Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 174 – NI
S6 Socket weld Yes Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 175 – NI
S6 Flange weld Yes Pneumatic Yes Mandatory No
80 – DRAIN – SS1
– 176 – NI
SS1 Flange weld Yes Pneumatic Yes Mandatory Yes
25 – DRAIN – S6
– 177 – NI
S6 Flange weld Yes Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 178 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 179 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 169 – NI
S6 Nil No Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 170 – NI
S6 Nil No Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 171 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – VENT – S6 –
172 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – VENT – S6 –
173 – NI
S6 Nil No Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 174 – NI
S6 Nil No Pneumatic Yes Mandatory No
50 – DRAIN – S6
– 175 – NI
S6 Nil No Pneumatic Yes Mandatory No
80 – DRAIN – SS1 SS1 Nil No Pneumatic Yes Mandatory No
Page 50
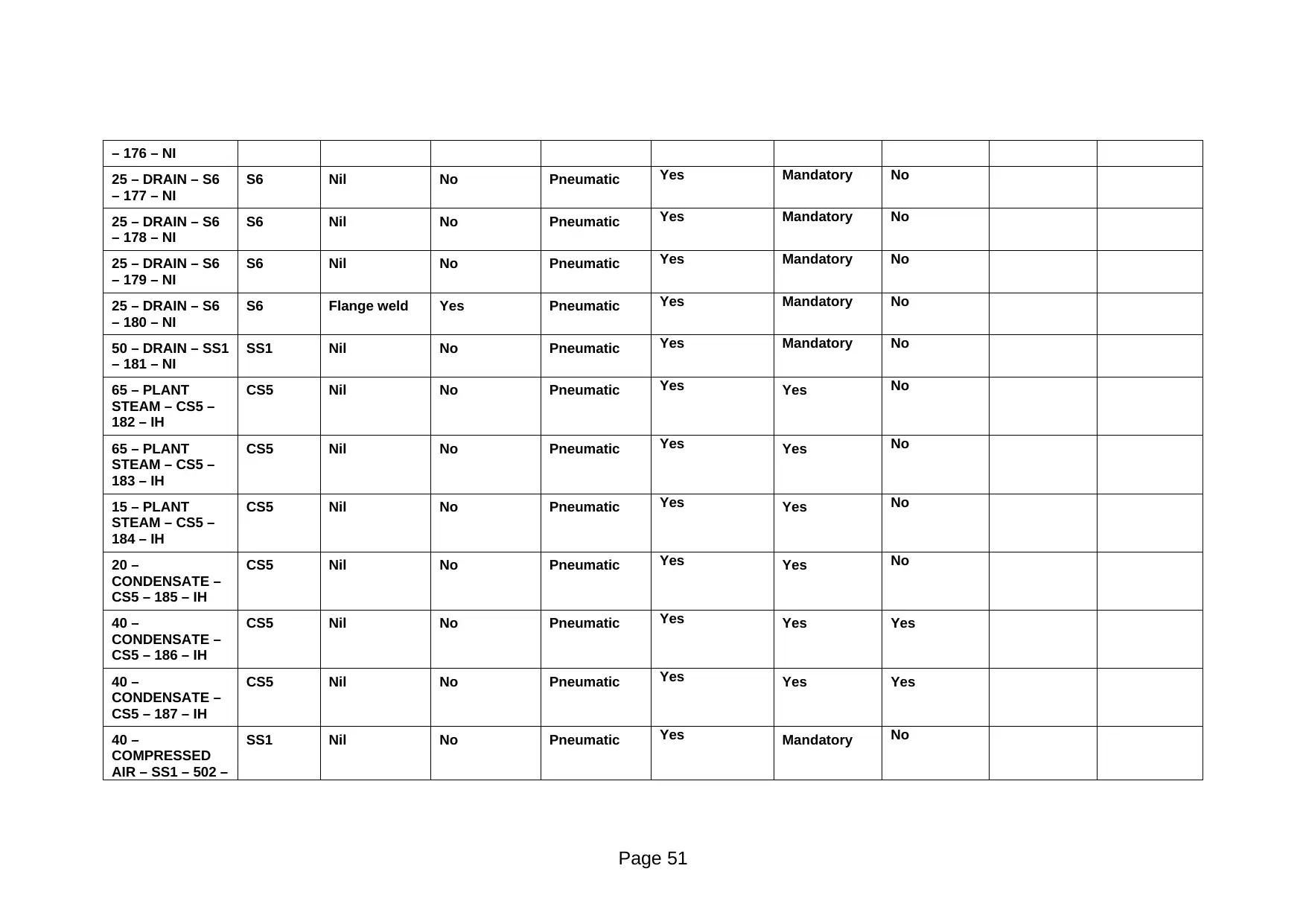
25 – DRAIN – S6
– 177 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 178 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 179 – NI
S6 Nil No Pneumatic Yes Mandatory No
25 – DRAIN – S6
– 180 – NI
S6 Flange weld Yes Pneumatic Yes Mandatory No
50 – DRAIN – SS1
– 181 – NI
SS1 Nil No Pneumatic Yes Mandatory No
65 – PLANT
STEAM – CS5 –
182 – IH
CS5 Nil No Pneumatic Yes Yes No
65 – PLANT
STEAM – CS5 –
183 – IH
CS5 Nil No Pneumatic Yes Yes No
15 – PLANT
STEAM – CS5 –
184 – IH
CS5 Nil No Pneumatic Yes Yes No
20 –
CONDENSATE –
CS5 – 185 – IH
CS5 Nil No Pneumatic Yes Yes No
40 –
CONDENSATE –
CS5 – 186 – IH
CS5 Nil No Pneumatic Yes Yes Yes
40 –
CONDENSATE –
CS5 – 187 – IH
CS5 Nil No Pneumatic Yes Yes Yes
40 –
COMPRESSED
AIR – SS1 – 502 –
SS1 Nil No Pneumatic Yes Mandatory No
Page 51
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
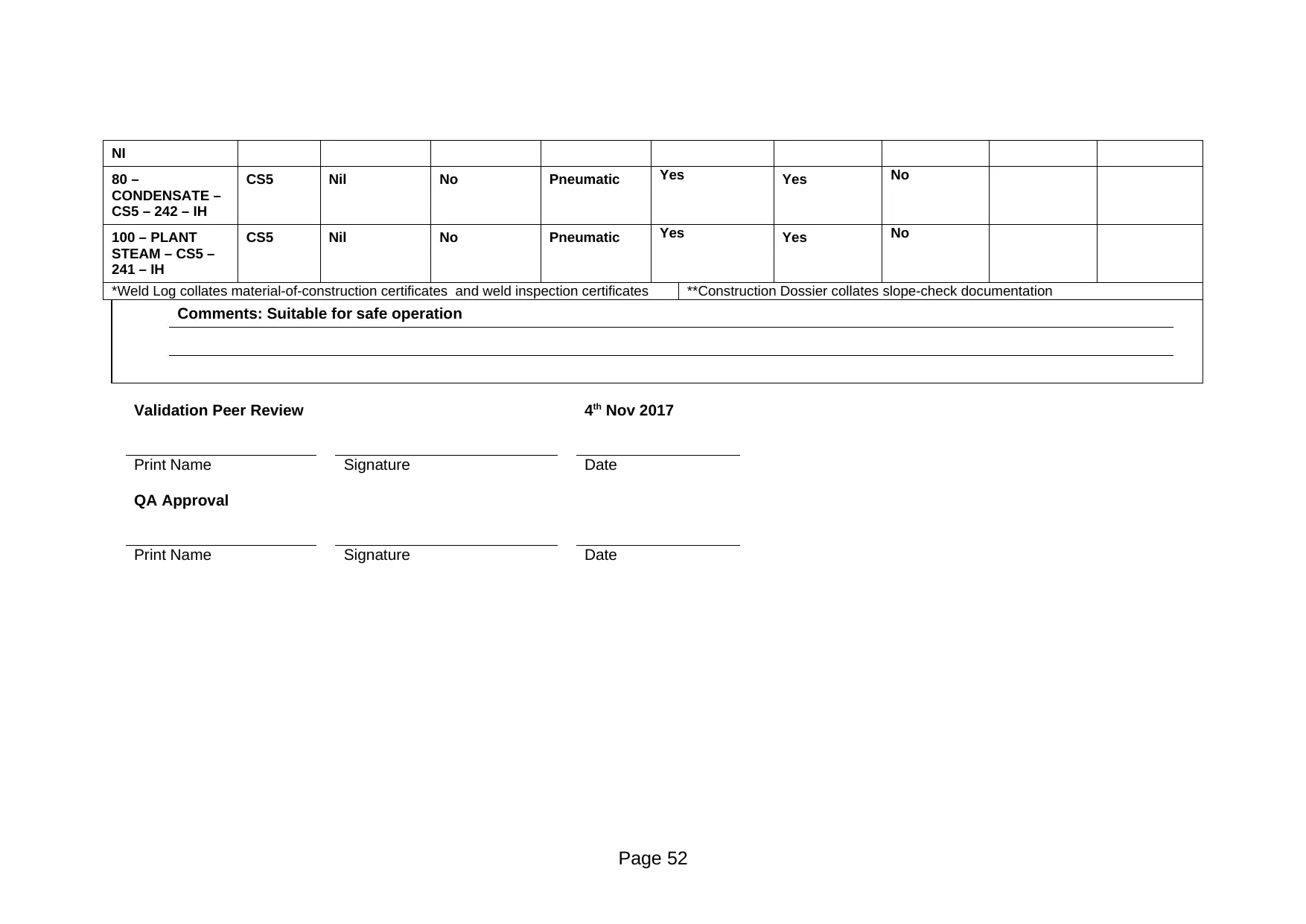
80 –
CONDENSATE –
CS5 – 242 – IH
CS5 Nil No Pneumatic Yes Yes No
100 – PLANT
STEAM – CS5 –
241 – IH
CS5 Nil No Pneumatic Yes Yes No
*Weld Log collates material-of-construction certificates and weld inspection certificates **Construction Dossier collates slope-check documentation
Comments: Suitable for safe operation
Validation Peer Review 4th Nov 2017
Print Name Signature Date
QA Approval
Print Name Signature Date
Page 52
Paraphrase This Document
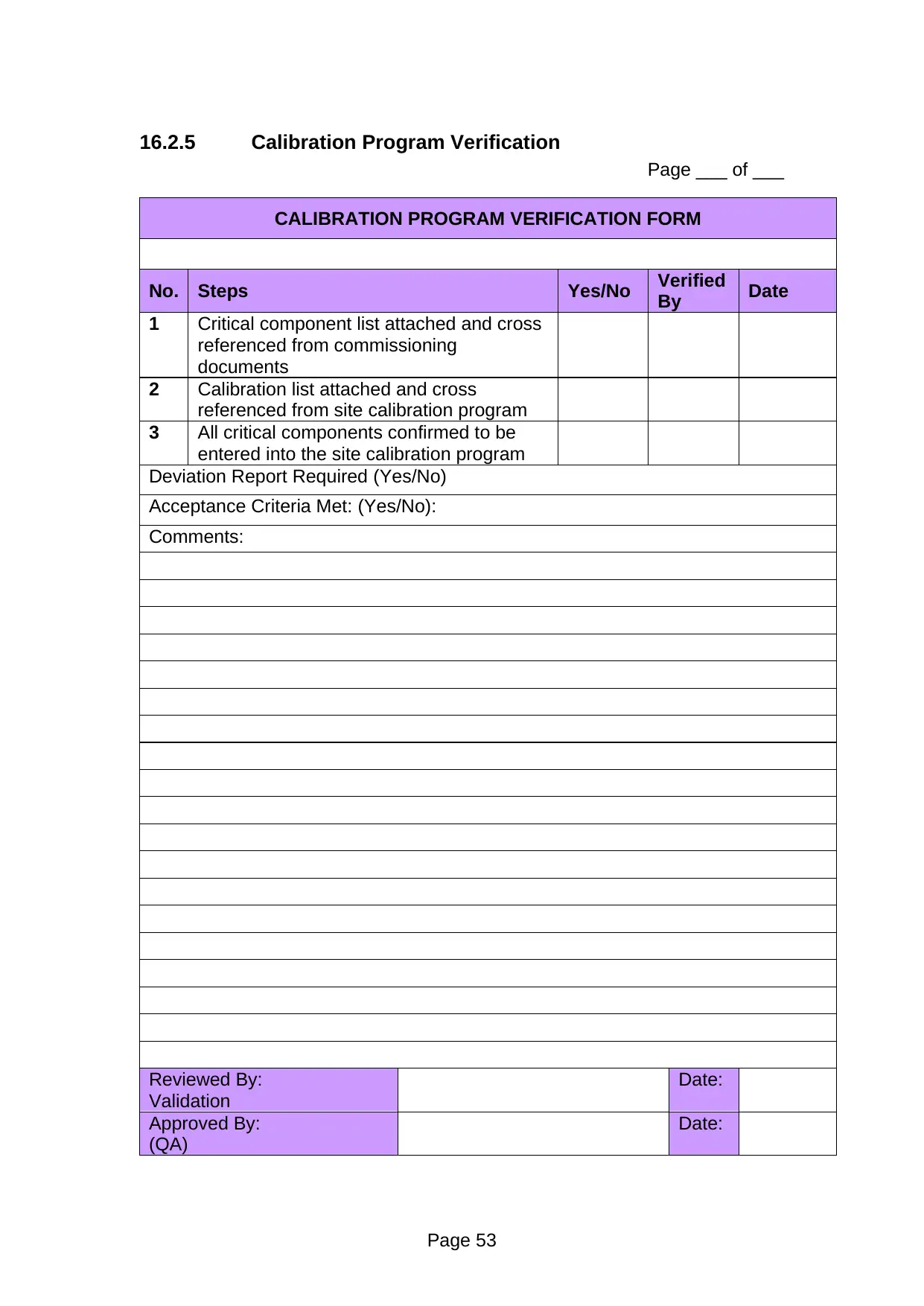
Page ___ of ___
CALIBRATION PROGRAM VERIFICATION FORM
No. Steps Yes/No Verified
By Date
1 Critical component list attached and cross
referenced from commissioning
documents
2 Calibration list attached and cross
referenced from site calibration program
3 All critical components confirmed to be
entered into the site calibration program
Deviation Report Required (Yes/No)
Acceptance Criteria Met: (Yes/No):
Comments:
Reviewed By:
Validation
Date:
Approved By:
(QA)
Date:
Page 53
Page ___ of ___
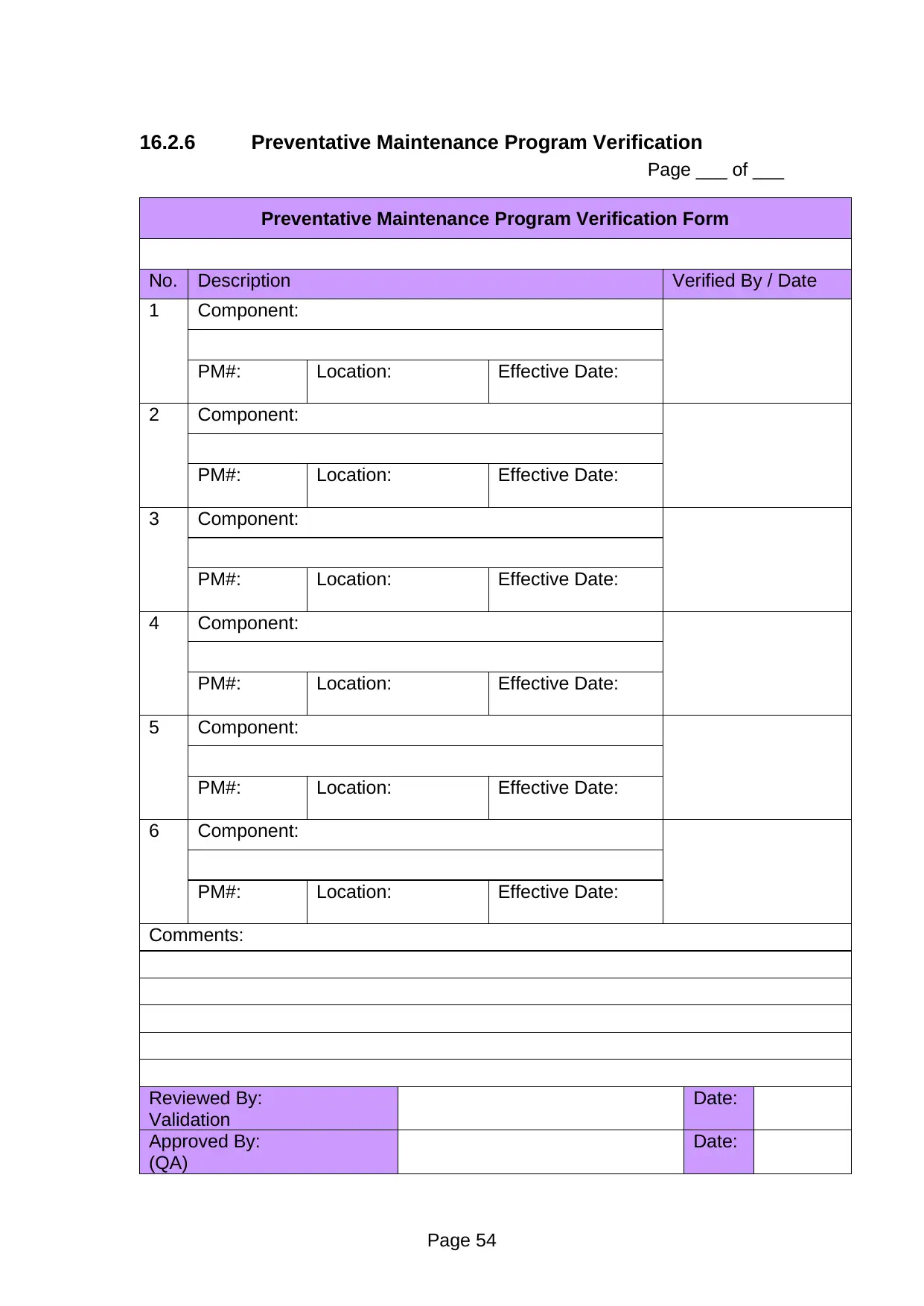
Preventative Maintenance Program Verification Form
No. Description Verified By / Date
1 Component:
PM#: Location: Effective Date:
2 Component:
PM#: Location: Effective Date:
3 Component:
PM#: Location: Effective Date:
4 Component:
PM#: Location: Effective Date:
5 Component:
PM#: Location: Effective Date:
6 Component:
PM#: Location: Effective Date:
Comments:
Reviewed By:
Validation
Date:
Approved By:
(QA)
Date:
Page 54
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
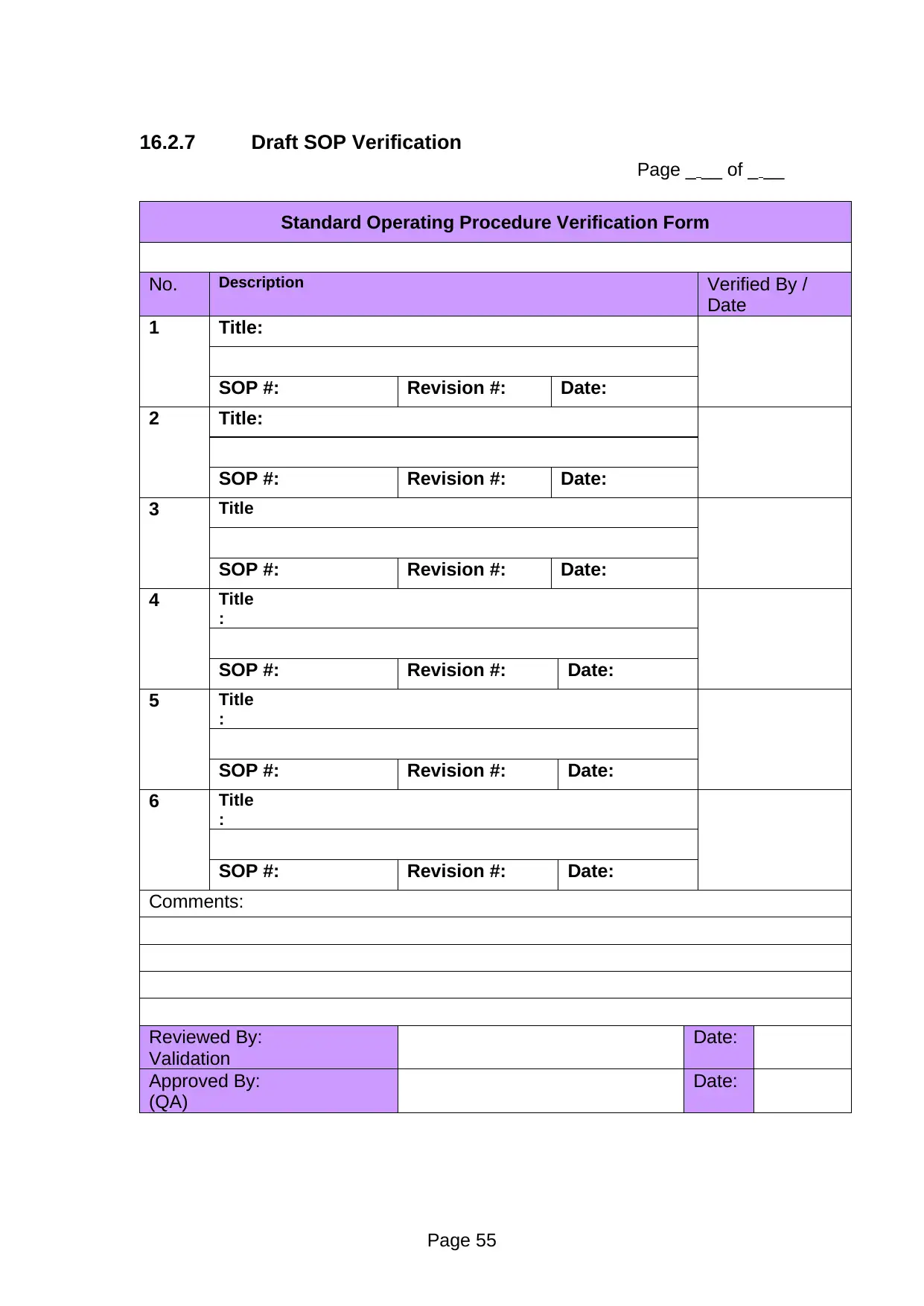
Page _ __ of _ __
Standard Operating Procedure Verification Form
No. Description Verified By /
Date
1 Title:
SOP #: Revision #: Date:
2 Title:
SOP #: Revision #: Date:
3 Title
SOP #: Revision #: Date:
4 Title
:
SOP #: Revision #: Date:
5 Title
:
SOP #: Revision #: Date:
6 Title
:
SOP #: Revision #: Date:
Comments:
Reviewed By:
Validation
Date:
Approved By:
(QA)
Date:
Page 55
Paraphrase This Document
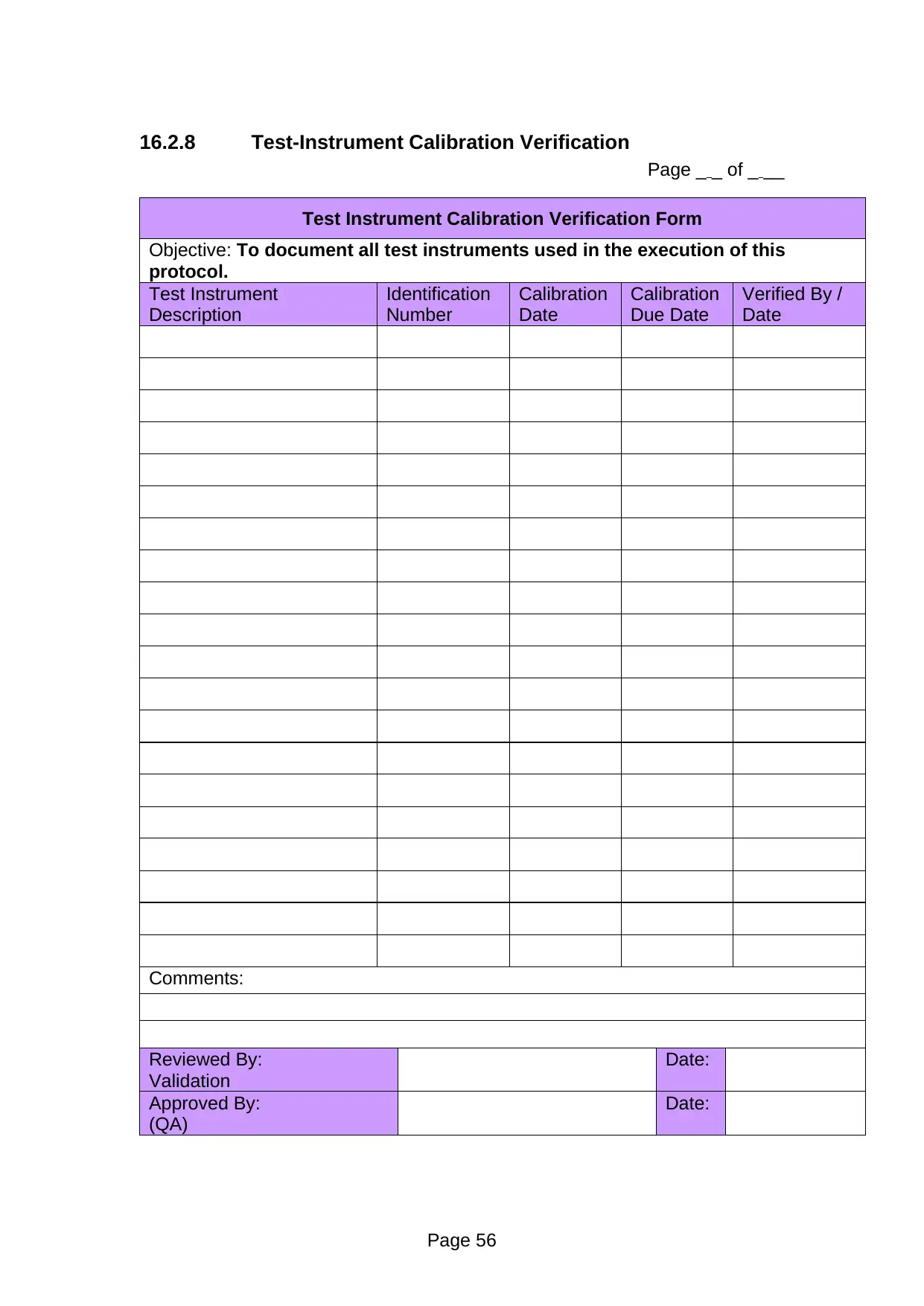
Page _ _ of _ __
Test Instrument Calibration Verification Form
Objective: To document all test instruments used in the execution of this
protocol.
Test Instrument
Description
Identification
Number
Calibration
Date
Calibration
Due Date
Verified By /
Date
Comments:
Reviewed By:
Validation
Date:
Approved By:
(QA)
Date:
Page 56

16.3.1 Hot Detergent System – Detergent Dosing Control
16.3.2 Hot Detergent System – Temperature Control
16.3.3 Hot Detergent System – Flow Control
16.3.4 Hot Detergent System – Level Control
16.3.5 Hot PUW System – Temperature Control
16.3.6 Hot PUW System – Flow Control
16.3.7 Hot PUW System – Level Control
Page 57
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
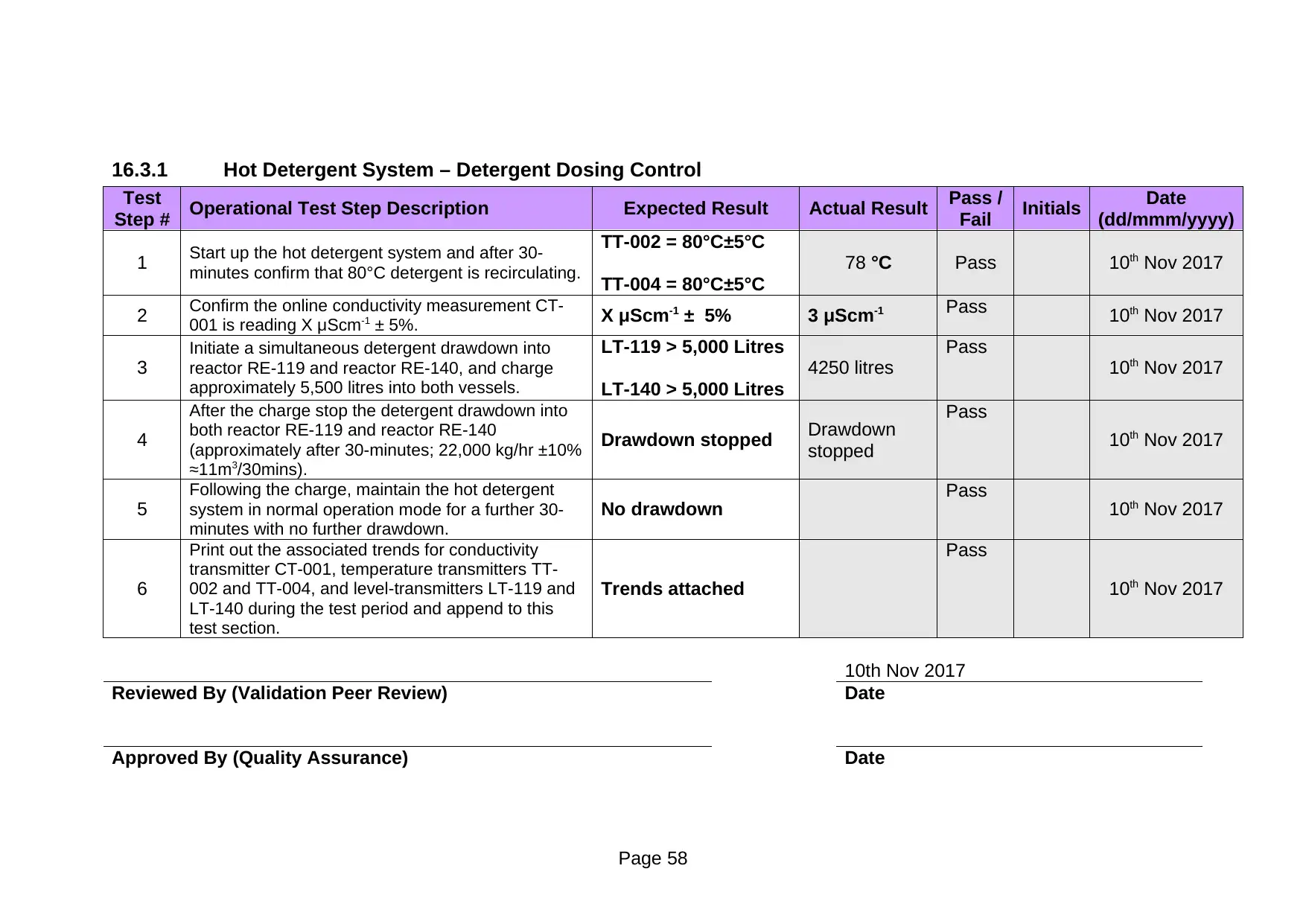
Test
Step # Operational Test Step Description Expected Result Actual Result Pass /
Fail Initials Date
(dd/mmm/yyyy)
1 Start up the hot detergent system and after 30-
minutes confirm that 80°C detergent is recirculating.
TT-002 = 80°C±5°C
TT-004 = 80°C±5°C
78 °C Pass 10th Nov 2017
2 Confirm the online conductivity measurement CT-
001 is reading X μScm-1 ± 5%. X μScm-1 ± 5% 3 μScm-1 Pass 10th Nov 2017
3
Initiate a simultaneous detergent drawdown into
reactor RE-119 and reactor RE-140, and charge
approximately 5,500 litres into both vessels.
LT-119 > 5,000 Litres
LT-140 > 5,000 Litres
4250 litres
Pass
10th Nov 2017
4
After the charge stop the detergent drawdown into
both reactor RE-119 and reactor RE-140
(approximately after 30-minutes; 22,000 kg/hr ±10%
≈11m3/30mins).
Drawdown stopped Drawdown
stopped
Pass
10th Nov 2017
5
Following the charge, maintain the hot detergent
system in normal operation mode for a further 30-
minutes with no further drawdown.
No drawdown Pass 10th Nov 2017
6
Print out the associated trends for conductivity
transmitter CT-001, temperature transmitters TT-
002 and TT-004, and level-transmitters LT-119 and
LT-140 during the test period and append to this
test section.
Trends attached
Pass
10th Nov 2017
10th Nov 2017
Reviewed By (Validation Peer Review) Date
Approved By (Quality Assurance) Date
Page 58
Paraphrase This Document
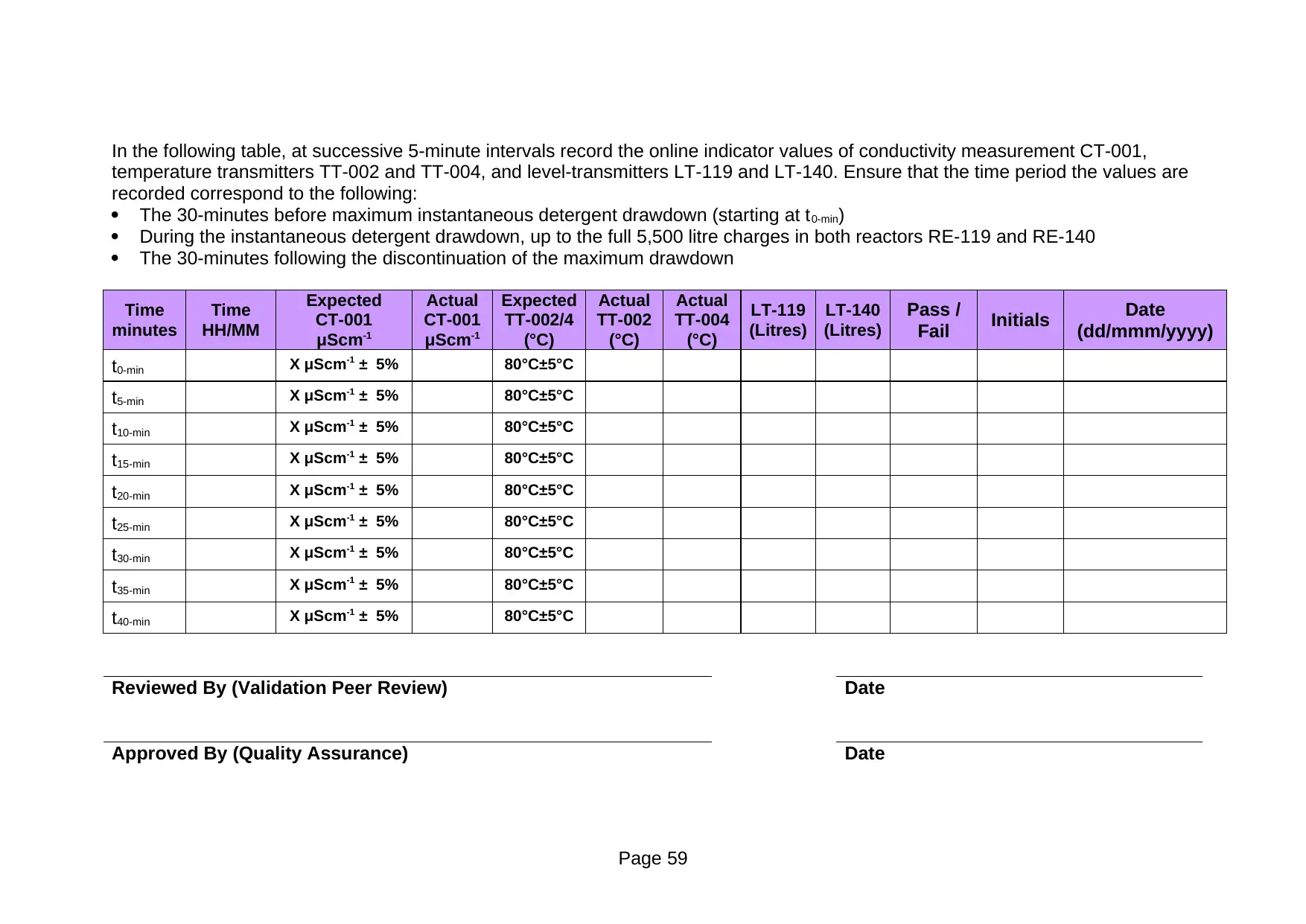
temperature transmitters TT-002 and TT-004, and level-transmitters LT-119 and LT-140. Ensure that the time period the values are
recorded correspond to the following:
The 30-minutes before maximum instantaneous detergent drawdown (starting at t0-min)
During the instantaneous detergent drawdown, up to the full 5,500 litre charges in both reactors RE-119 and RE-140
The 30-minutes following the discontinuation of the maximum drawdown
Time
minutes
Time
HH/MM
Expected
CT-001
μScm-1
Actual
CT-001
μScm-1
Expected
TT-002/4
(°C)
Actual
TT-002
(°C)
Actual
TT-004
(°C)
LT-119
(Litres)
LT-140
(Litres)
Pass /
Fail Initials Date
(dd/mmm/yyyy)
t0-min X μScm-1 ± 5% 80°C±5°C
t5-min X μScm-1 ± 5% 80°C±5°C
t10-min X μScm-1 ± 5% 80°C±5°C
t15-min X μScm-1 ± 5% 80°C±5°C
t20-min X μScm-1 ± 5% 80°C±5°C
t25-min X μScm-1 ± 5% 80°C±5°C
t30-min X μScm-1 ± 5% 80°C±5°C
t35-min X μScm-1 ± 5% 80°C±5°C
t40-min X μScm-1 ± 5% 80°C±5°C
Reviewed By (Validation Peer Review) Date
Approved By (Quality Assurance) Date
Page 59
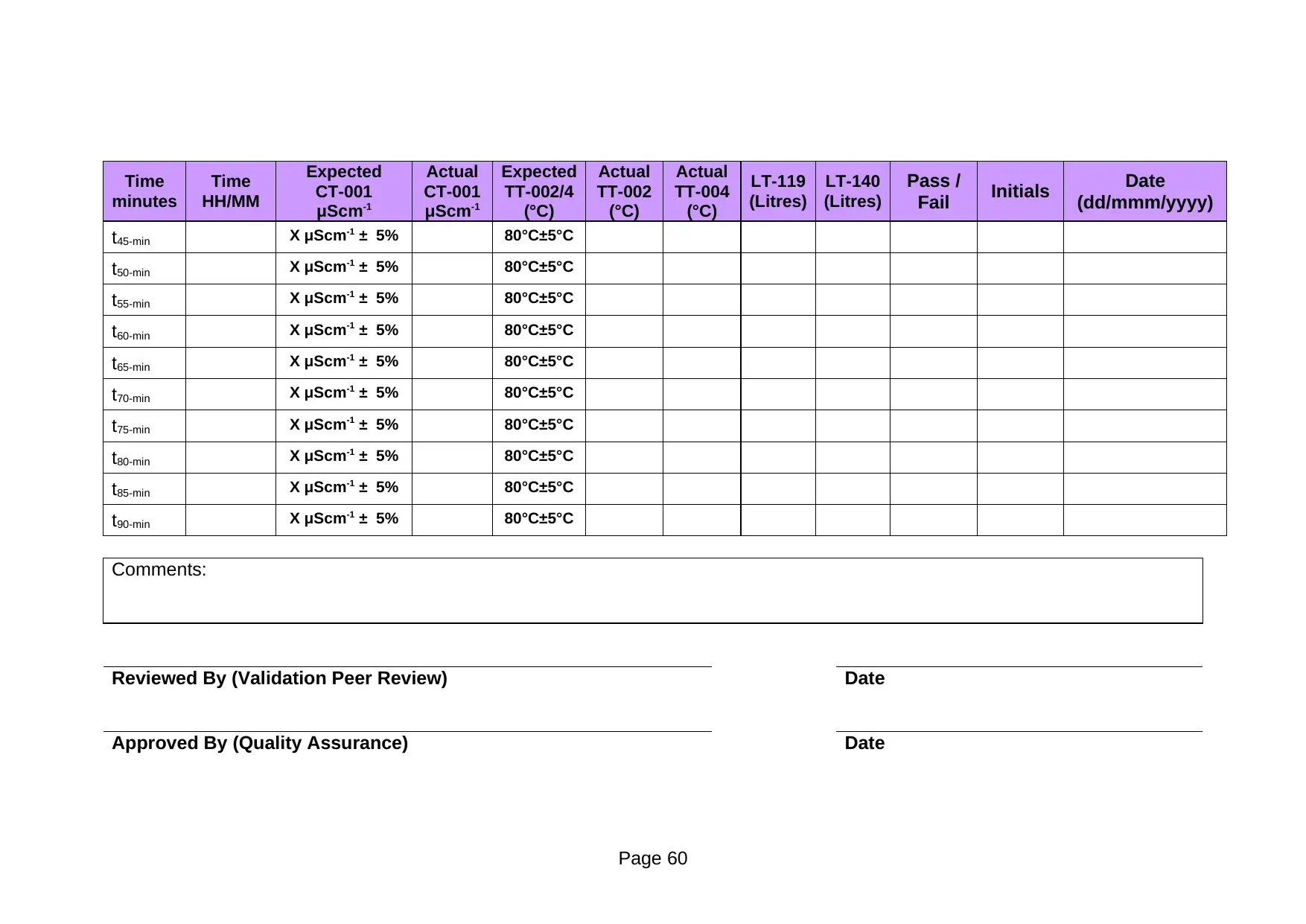
minutes
Time
HH/MM
Expected
CT-001
μScm-1
Actual
CT-001
μScm-1
Expected
TT-002/4
(°C)
Actual
TT-002
(°C)
Actual
TT-004
(°C)
LT-119
(Litres)
LT-140
(Litres)
Pass /
Fail Initials Date
(dd/mmm/yyyy)
t45-min X μScm-1 ± 5% 80°C±5°C
t50-min X μScm-1 ± 5% 80°C±5°C
t55-min X μScm-1 ± 5% 80°C±5°C
t60-min X μScm-1 ± 5% 80°C±5°C
t65-min X μScm-1 ± 5% 80°C±5°C
t70-min X μScm-1 ± 5% 80°C±5°C
t75-min X μScm-1 ± 5% 80°C±5°C
t80-min X μScm-1 ± 5% 80°C±5°C
t85-min X μScm-1 ± 5% 80°C±5°C
t90-min X μScm-1 ± 5% 80°C±5°C
Comments:
Reviewed By (Validation Peer Review) Date
Approved By (Quality Assurance) Date
Page 60
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Page 61
Paraphrase This Document

Page 62

Page 63
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Page 64
Paraphrase This Document

Page 65

Page 66
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Page _ __ of _ __
Standard Operating Procedure Verification Form
No. Description Verified By /
Date
1 Title:
SOP #: Revision #: Date:
2 Title:
SOP #: Revision #: Date:
3 Title
SOP #: Revision #: Date:
4 Title
:
SOP #: Revision #: Date:
5 Title
:
SOP #: Revision #: Date:
6 Title
:
SOP #: Revision #: Date:
Comments:
Reviewed By:
Validation
Date:
Approved By:
(QA)
Date:
Page 67
Paraphrase This Document
Deviation # Deviation Summary Statement
Deviation
Resolved
(YES / No)
Date Verified
Date
Approved
Related
Change #
(if applicable)
Comments
Reviewed By:
(Validation)
Date:
*Reviewed By:
(QA)
Date:
*Must be approved by QA if any deviations are deemed a ‘Direct Impact System’
Page 68
Deviation Details Deviation#
Building / Area Equipment /
System
Protocol Section # Protocol Test
Descriptor
Deviation Category
(Please Circle) 1 2 3 *4 *5
DEVIATION DESCRIPTION
2. Impact Assessment of Deviation
System GMP Category
(Please Circle)
Component GMP Category
(Please Circle)
*Direct Indirect No Impact Critical Non-Critical
Affected Items Yes/No Affected Items Yes/No
User Requirement Functional or Detailed Design
Specifications
Testing Already Completed System Documentation
Testing Protocols Drawings
Validation Plan Standard Operating Procedures
Training Other
Details of Affected Items:
REFERENCES (AFFECTED ITEMS)
Document # Title / Description Revision
3. Resolution Description
4. Resolution Verification
Verified By: (Originator) Date:
Approved By: (Validation) Date:
*Approved By: (QA) Date:
*Must be approved by QA only if a ‘Direct Impact System’
Page 69
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Change
Control #
P&ID /
System #
Summary Description of
change
Direct /
Indirect /
No-Impact
Date Raised
(dd/mmm/yyyy)
Pre-Approval
Date
(dd/mmm/yyyy)
Post-Approval
Date
(dd/mmm/yyyy)
COMMENTS:
Reviewed By:
(Validation
Engineering)
Date:
Page 70
Paraphrase This Document
Change Control Form
Project Title: CHANGE #:
Project No: P&ID /
System:
Attachments
(Yes/No): Date:
SECTION 1: OBSERVATIONS AND PROPOSED CHANGE (to be completed by 1Project Engineer)
ORIGINAL DESIGN: PROPOSED CHANGE:
2HAZOP TO BE UPDATED (Yes / No)
SECTION 2: IMPACT ASSESSMENT (to be completed by 1Engineering Validation)
AFFECTED APPROVED ITEMS Yes/No AFFECTED APPROVED ITEMS Yes/No
3Validation Plan 3Project URS
3System Level URS 3System Impact Assessment
3Component Impact Assessment 3, 4Qualification Protocol(s)
4Commissioning Protocol(s) 4Other
3, 5DIRECT 5INDIRECT 5NO IMPACT
3SYSTEM IMPACT Yes / No Yes / No Yes / No
Page 71
1Engineering Validation)
3, 5OPER. CRITICAL 3, 5PRODUCT CONTACT 5NON CRITICAL
3COMPONENT IMPACT Yes / No Yes / No Yes / No
5CONSTRUCTION 5COMMISSIONING 3, 5QUALIFICATION
PROJECT PHASE Yes / No Yes / No Yes / No
SECTION 3: TESTING REQUIRED (to be completed by 1Engineering Validation)
4TESTING REQUIRED 4DOCUMENT UPDATES REQUIRED
Number of Test Pages Attached
SECTION 4: COST CALCULATIONS AND SCHEDULE (to be completed by 1Project Engineer)
6COST OF CHANGE 6IMPACT ON SCHEDULE (time)
SECTION 5: PRE-APPROVALS
PROJECT DISCIPLINE PRINT NAME SIGNATURE DATE
PROJECT ENGINEER
PROJECT MANAGER
EHS
VALIDATION
QA
SECTION 6: REJECT CHANGE
PRINT NAME SIGNATURE DATE
CHANGE REJECTED BY
REASON FOR REJECTING CHANGE
Page 72
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
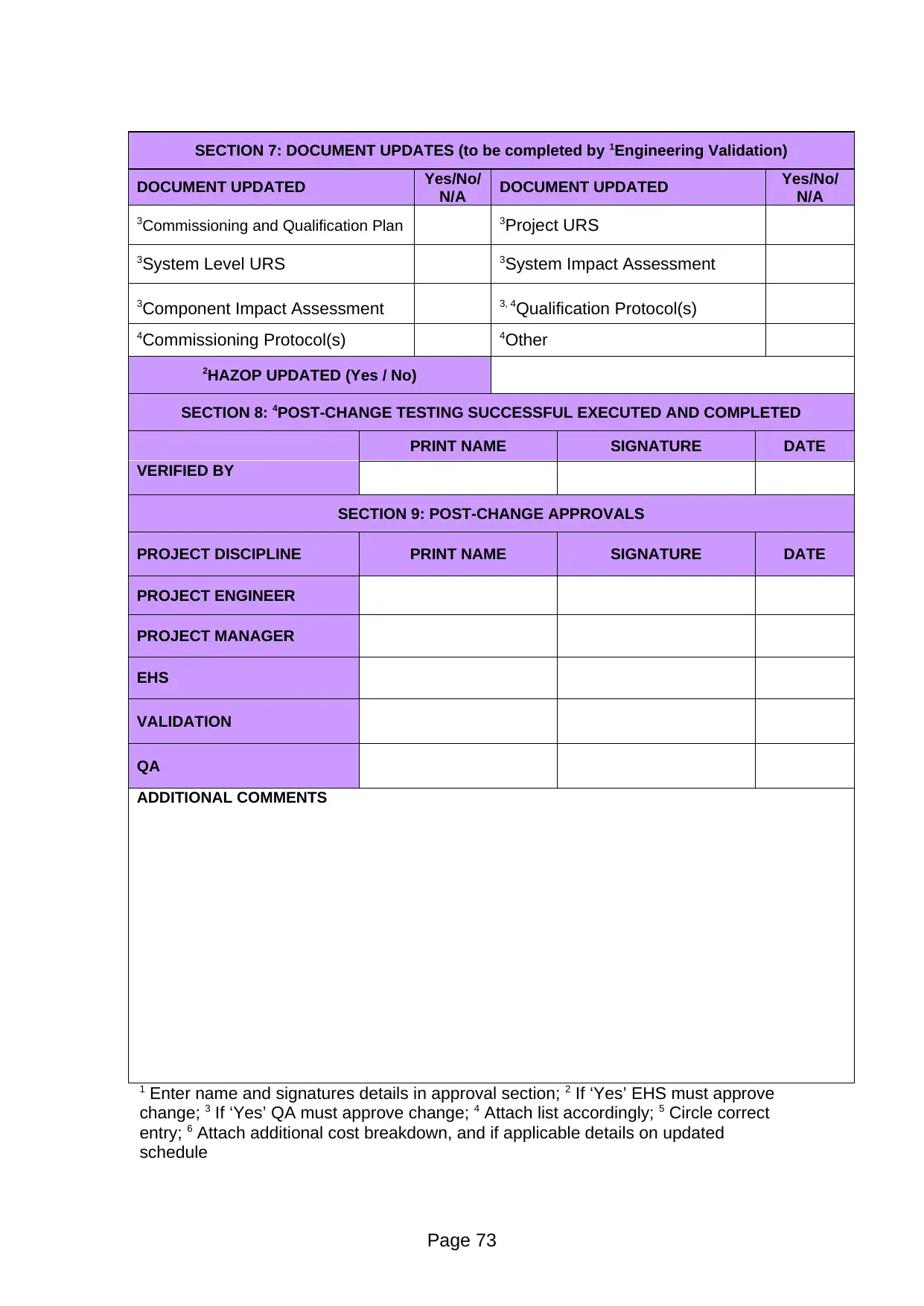
DOCUMENT UPDATED Yes/No/
N/A DOCUMENT UPDATED Yes/No/
N/A
3Commissioning and Qualification Plan 3Project URS
3System Level URS 3System Impact Assessment
3Component Impact Assessment 3, 4Qualification Protocol(s)
4Commissioning Protocol(s) 4Other
2HAZOP UPDATED (Yes / No)
SECTION 8: 4POST-CHANGE TESTING SUCCESSFUL EXECUTED AND COMPLETED
PRINT NAME SIGNATURE DATE
VERIFIED BY
SECTION 9: POST-CHANGE APPROVALS
PROJECT DISCIPLINE PRINT NAME SIGNATURE DATE
PROJECT ENGINEER
PROJECT MANAGER
EHS
VALIDATION
QA
ADDITIONAL COMMENTS
1 Enter name and signatures details in approval section; 2 If ‘Yes’ EHS must approve
change; 3 If ‘Yes’ QA must approve change; 4 Attach list accordingly; 5 Circle correct
entry; 6 Attach additional cost breakdown, and if applicable details on updated
schedule
Page 73
Paraphrase This Document
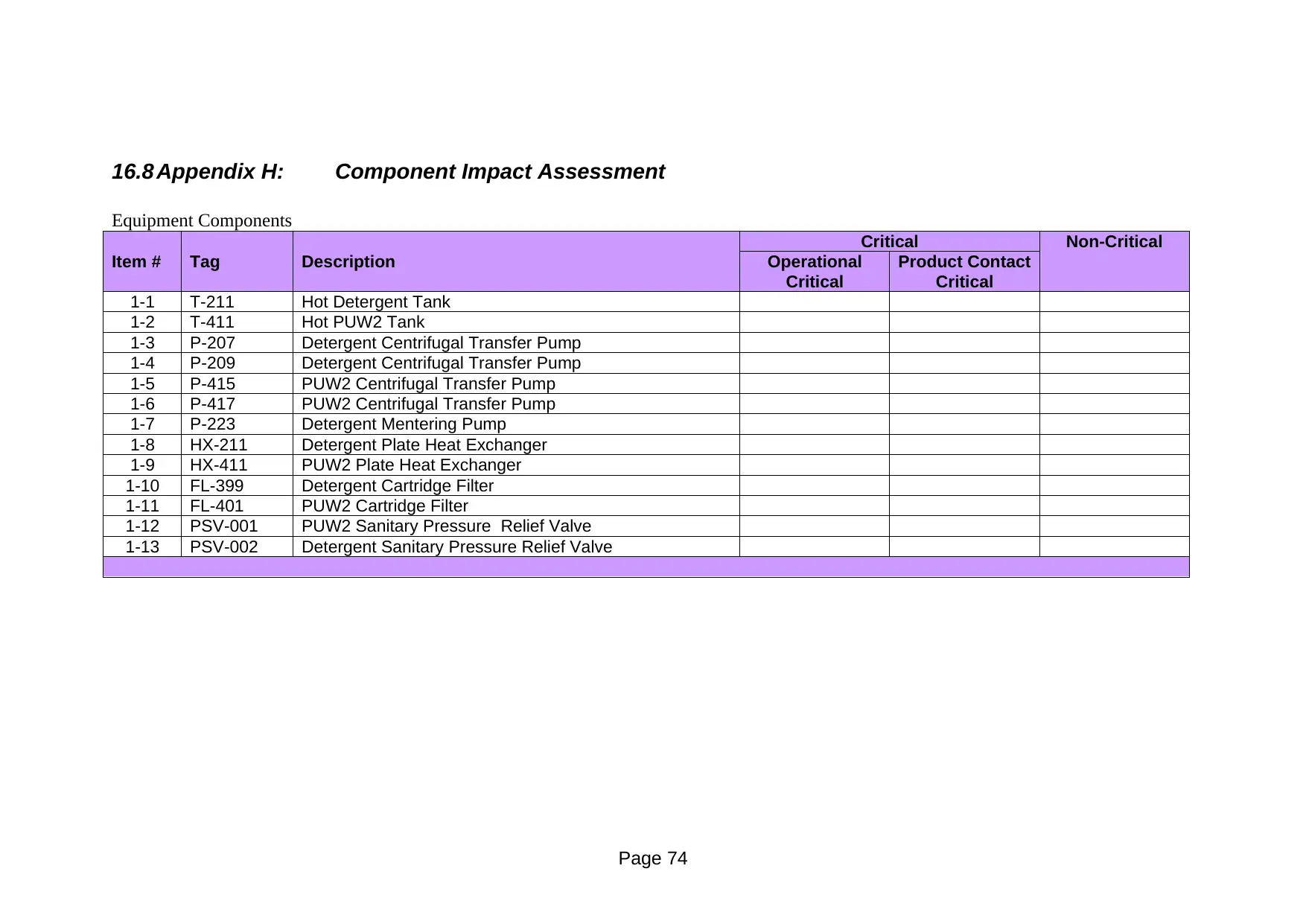
Equipment Components
Critical Non-Critical
Item # Tag Description Operational
Critical
Product Contact
Critical
1-1 T-211 Hot Detergent Tank
1-2 T-411 Hot PUW2 Tank
1-3 P-207 Detergent Centrifugal Transfer Pump
1-4 P-209 Detergent Centrifugal Transfer Pump
1-5 P-415 PUW2 Centrifugal Transfer Pump
1-6 P-417 PUW2 Centrifugal Transfer Pump
1-7 P-223 Detergent Mentering Pump
1-8 HX-211 Detergent Plate Heat Exchanger
1-9 HX-411 PUW2 Plate Heat Exchanger
1-10 FL-399 Detergent Cartridge Filter
1-11 FL-401 PUW2 Cartridge Filter
1-12 PSV-001 PUW2 Sanitary Pressure Relief Valve
1-13 PSV-002 Detergent Sanitary Pressure Relief Valve
Page 74
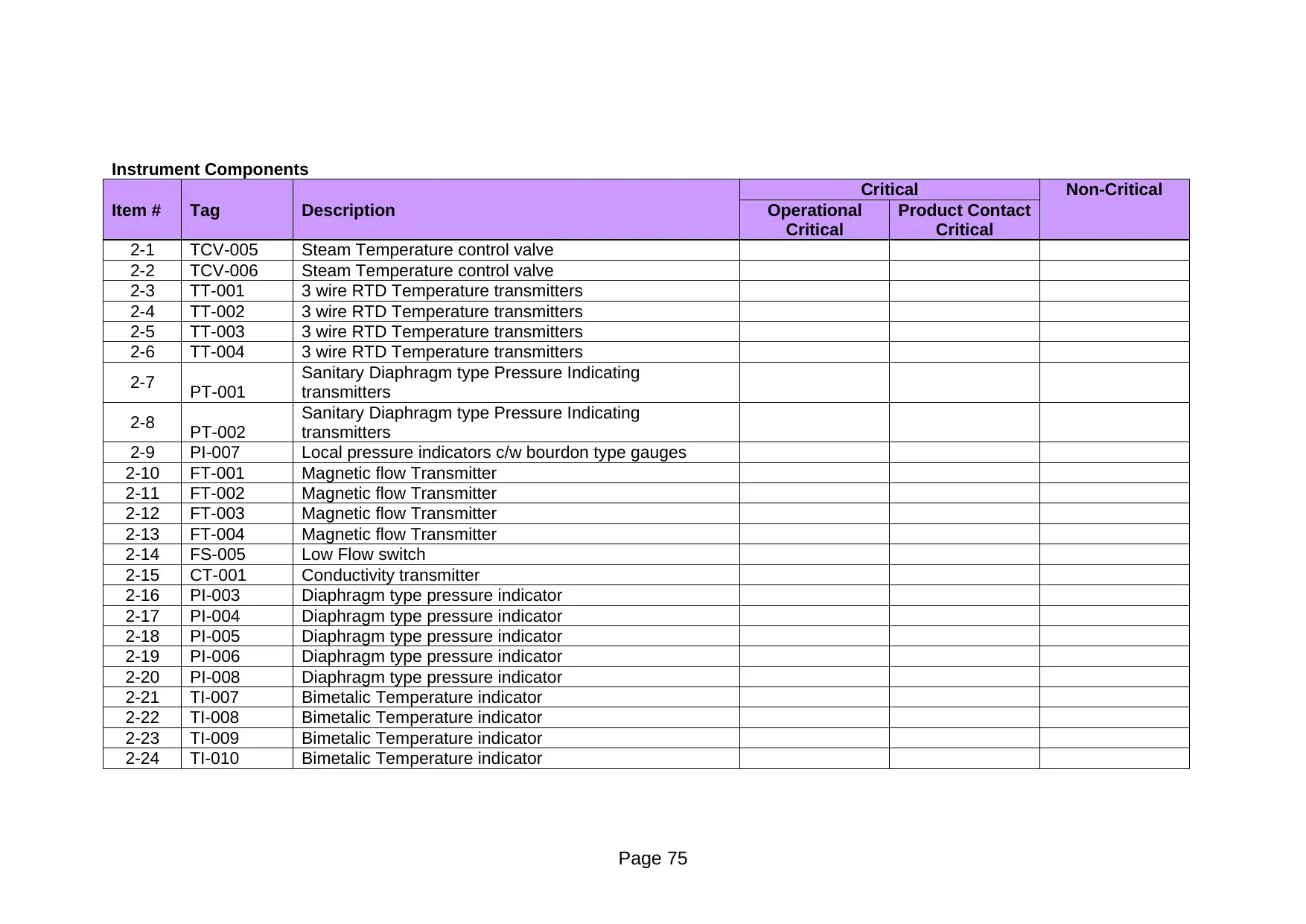
Critical Non-Critical
Item # Tag Description Operational
Critical
Product Contact
Critical
2-1 TCV-005 Steam Temperature control valve
2-2 TCV-006 Steam Temperature control valve
2-3 TT-001 3 wire RTD Temperature transmitters
2-4 TT-002 3 wire RTD Temperature transmitters
2-5 TT-003 3 wire RTD Temperature transmitters
2-6 TT-004 3 wire RTD Temperature transmitters
2-7 PT-001
Sanitary Diaphragm type Pressure Indicating
transmitters
2-8 PT-002
Sanitary Diaphragm type Pressure Indicating
transmitters
2-9 PI-007 Local pressure indicators c/w bourdon type gauges
2-10 FT-001 Magnetic flow Transmitter
2-11 FT-002 Magnetic flow Transmitter
2-12 FT-003 Magnetic flow Transmitter
2-13 FT-004 Magnetic flow Transmitter
2-14 FS-005 Low Flow switch
2-15 CT-001 Conductivity transmitter
2-16 PI-003 Diaphragm type pressure indicator
2-17 PI-004 Diaphragm type pressure indicator
2-18 PI-005 Diaphragm type pressure indicator
2-19 PI-006 Diaphragm type pressure indicator
2-20 PI-008 Diaphragm type pressure indicator
2-21 TI-007 Bimetalic Temperature indicator
2-22 TI-008 Bimetalic Temperature indicator
2-23 TI-009 Bimetalic Temperature indicator
2-24 TI-010 Bimetalic Temperature indicator
Page 75
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
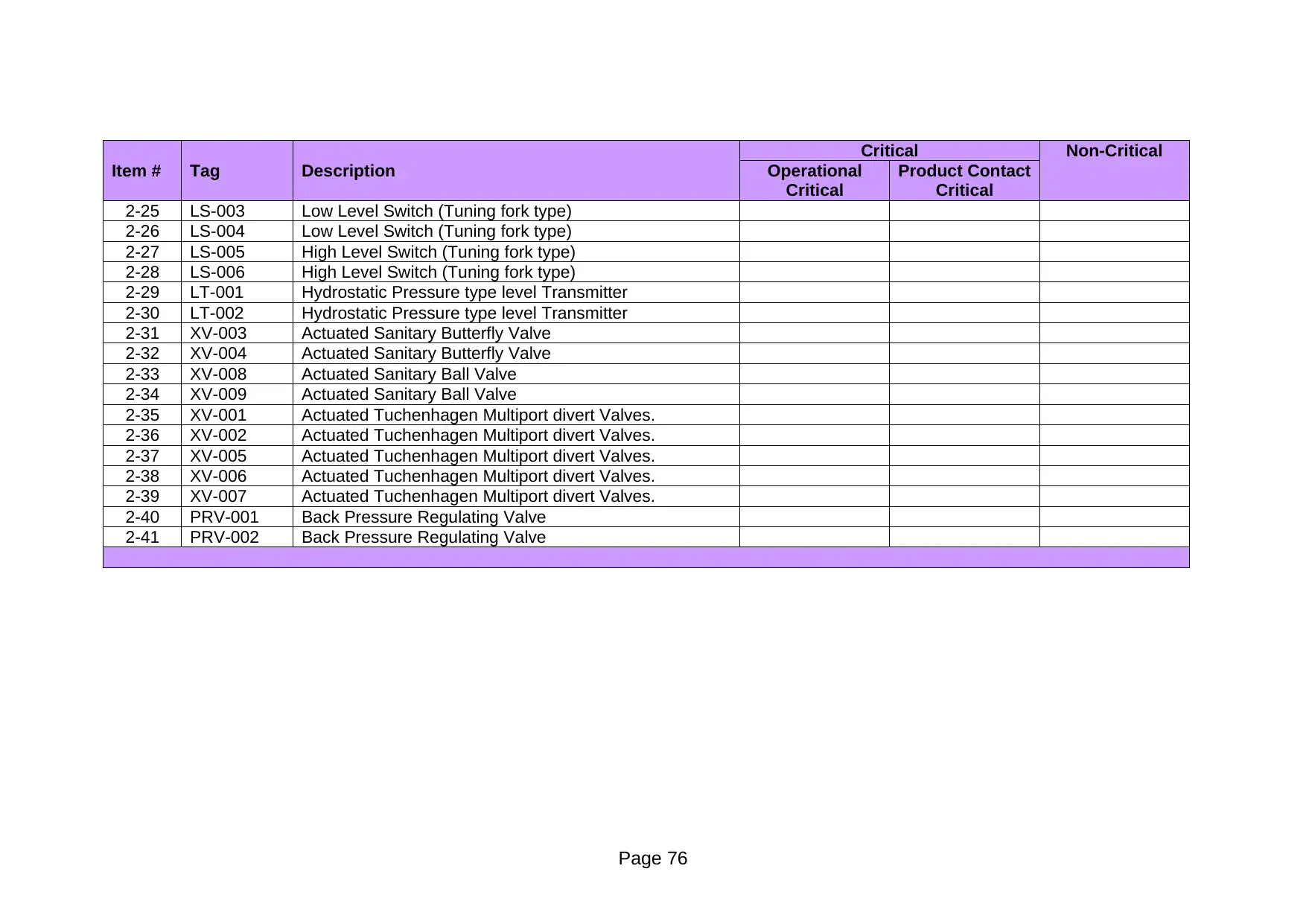
Item # Tag Description Operational
Critical
Product Contact
Critical
2-25 LS-003 Low Level Switch (Tuning fork type)
2-26 LS-004 Low Level Switch (Tuning fork type)
2-27 LS-005 High Level Switch (Tuning fork type)
2-28 LS-006 High Level Switch (Tuning fork type)
2-29 LT-001 Hydrostatic Pressure type level Transmitter
2-30 LT-002 Hydrostatic Pressure type level Transmitter
2-31 XV-003 Actuated Sanitary Butterfly Valve
2-32 XV-004 Actuated Sanitary Butterfly Valve
2-33 XV-008 Actuated Sanitary Ball Valve
2-34 XV-009 Actuated Sanitary Ball Valve
2-35 XV-001 Actuated Tuchenhagen Multiport divert Valves.
2-36 XV-002 Actuated Tuchenhagen Multiport divert Valves.
2-37 XV-005 Actuated Tuchenhagen Multiport divert Valves.
2-38 XV-006 Actuated Tuchenhagen Multiport divert Valves.
2-39 XV-007 Actuated Tuchenhagen Multiport divert Valves.
2-40 PRV-001 Back Pressure Regulating Valve
2-41 PRV-002 Back Pressure Regulating Valve
Page 76
Paraphrase This Document
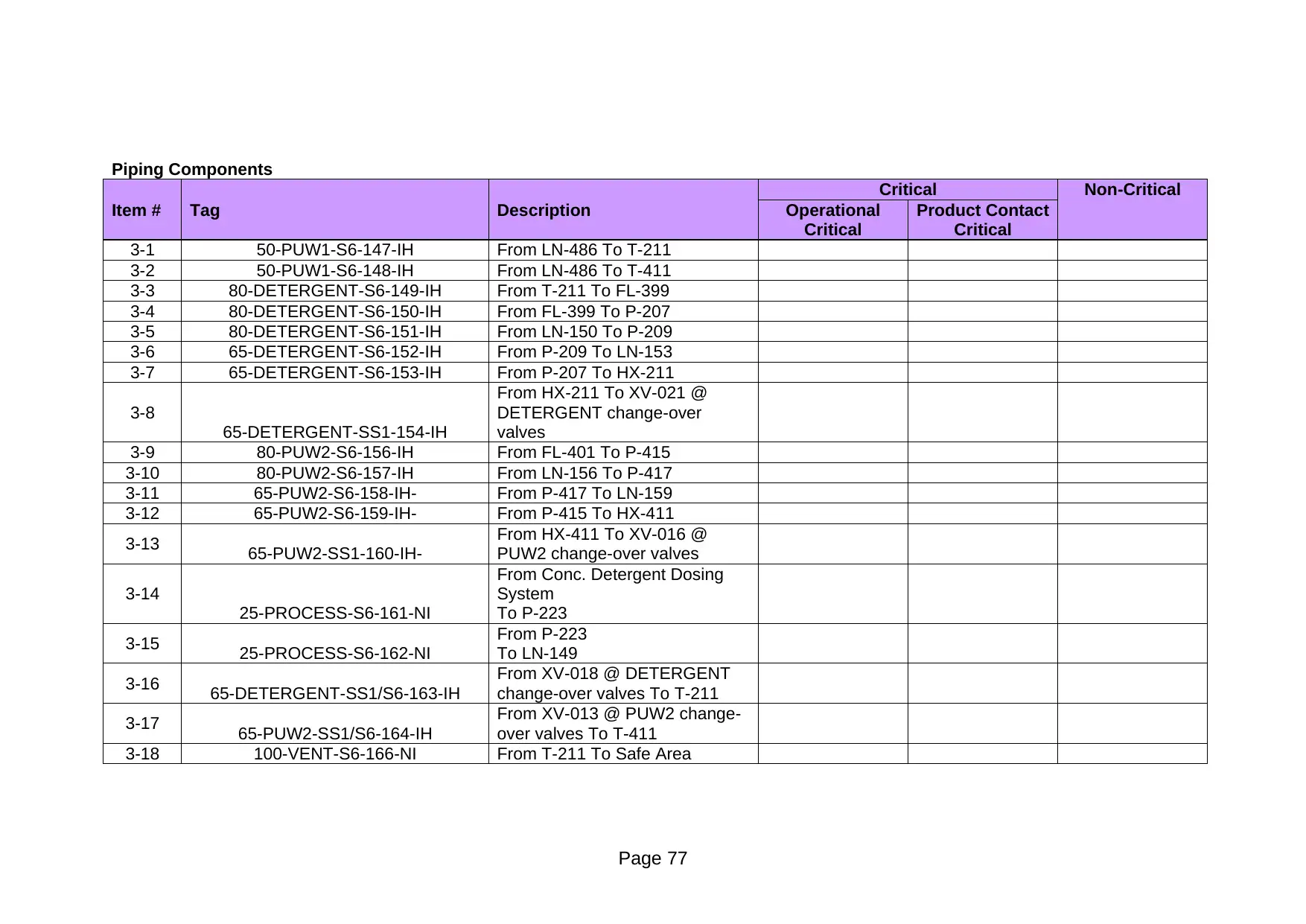
Critical Non-Critical
Item # Tag Description Operational
Critical
Product Contact
Critical
3-1 50-PUW1-S6-147-IH From LN-486 To T-211
3-2 50-PUW1-S6-148-IH From LN-486 To T-411
3-3 80-DETERGENT-S6-149-IH From T-211 To FL-399
3-4 80-DETERGENT-S6-150-IH From FL-399 To P-207
3-5 80-DETERGENT-S6-151-IH From LN-150 To P-209
3-6 65-DETERGENT-S6-152-IH From P-209 To LN-153
3-7 65-DETERGENT-S6-153-IH From P-207 To HX-211
3-8
65-DETERGENT-SS1-154-IH
From HX-211 To XV-021 @
DETERGENT change-over
valves
3-9 80-PUW2-S6-156-IH From FL-401 To P-415
3-10 80-PUW2-S6-157-IH From LN-156 To P-417
3-11 65-PUW2-S6-158-IH- From P-417 To LN-159
3-12 65-PUW2-S6-159-IH- From P-415 To HX-411
3-13 65-PUW2-SS1-160-IH-
From HX-411 To XV-016 @
PUW2 change-over valves
3-14
25-PROCESS-S6-161-NI
From Conc. Detergent Dosing
System
To P-223
3-15 25-PROCESS-S6-162-NI
From P-223
To LN-149
3-16 65-DETERGENT-SS1/S6-163-IH
From XV-018 @ DETERGENT
change-over valves To T-211
3-17 65-PUW2-SS1/S6-164-IH
From XV-013 @ PUW2 change-
over valves To T-411
3-18 100-VENT-S6-166-NI From T-211 To Safe Area
Page 77
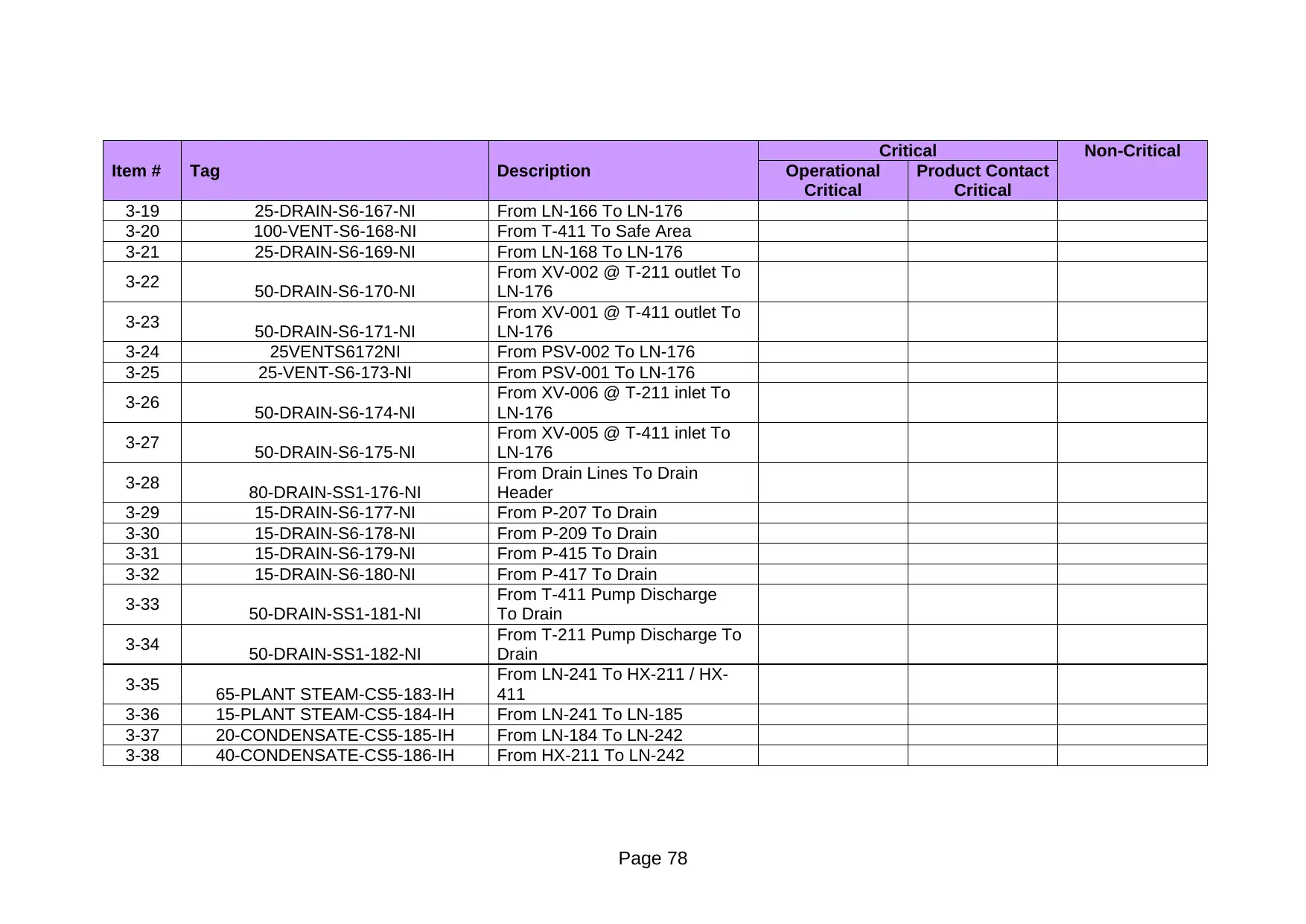
Item # Tag Description Operational
Critical
Product Contact
Critical
3-19 25-DRAIN-S6-167-NI From LN-166 To LN-176
3-20 100-VENT-S6-168-NI From T-411 To Safe Area
3-21 25-DRAIN-S6-169-NI From LN-168 To LN-176
3-22 50-DRAIN-S6-170-NI
From XV-002 @ T-211 outlet To
LN-176
3-23 50-DRAIN-S6-171-NI
From XV-001 @ T-411 outlet To
LN-176
3-24 25VENTS6172NI From PSV-002 To LN-176
3-25 25-VENT-S6-173-NI From PSV-001 To LN-176
3-26 50-DRAIN-S6-174-NI
From XV-006 @ T-211 inlet To
LN-176
3-27 50-DRAIN-S6-175-NI
From XV-005 @ T-411 inlet To
LN-176
3-28 80-DRAIN-SS1-176-NI
From Drain Lines To Drain
Header
3-29 15-DRAIN-S6-177-NI From P-207 To Drain
3-30 15-DRAIN-S6-178-NI From P-209 To Drain
3-31 15-DRAIN-S6-179-NI From P-415 To Drain
3-32 15-DRAIN-S6-180-NI From P-417 To Drain
3-33 50-DRAIN-SS1-181-NI
From T-411 Pump Discharge
To Drain
3-34 50-DRAIN-SS1-182-NI
From T-211 Pump Discharge To
Drain
3-35 65-PLANT STEAM-CS5-183-IH
From LN-241 To HX-211 / HX-
411
3-36 15-PLANT STEAM-CS5-184-IH From LN-241 To LN-185
3-37 20-CONDENSATE-CS5-185-IH From LN-184 To LN-242
3-38 40-CONDENSATE-CS5-186-IH From HX-211 To LN-242
Page 78
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
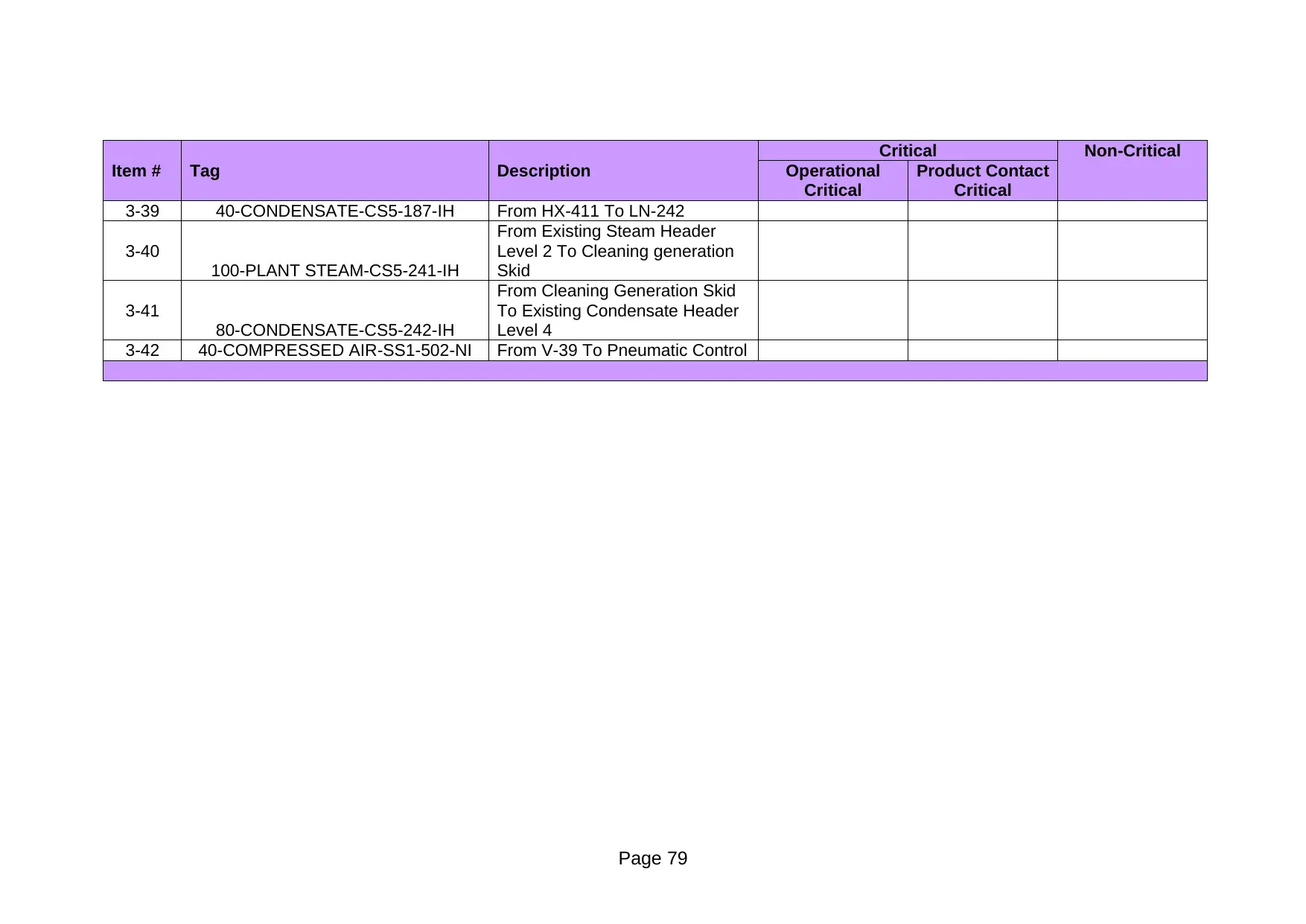
Item # Tag Description Operational
Critical
Product Contact
Critical
3-39 40-CONDENSATE-CS5-187-IH From HX-411 To LN-242
3-40
100-PLANT STEAM-CS5-241-IH
From Existing Steam Header
Level 2 To Cleaning generation
Skid
3-41
80-CONDENSATE-CS5-242-IH
From Cleaning Generation Skid
To Existing Condensate Header
Level 4
3-42 40-COMPRESSED AIR-SS1-502-NI From V-39 To Pneumatic Control
Page 79
Paraphrase This Document
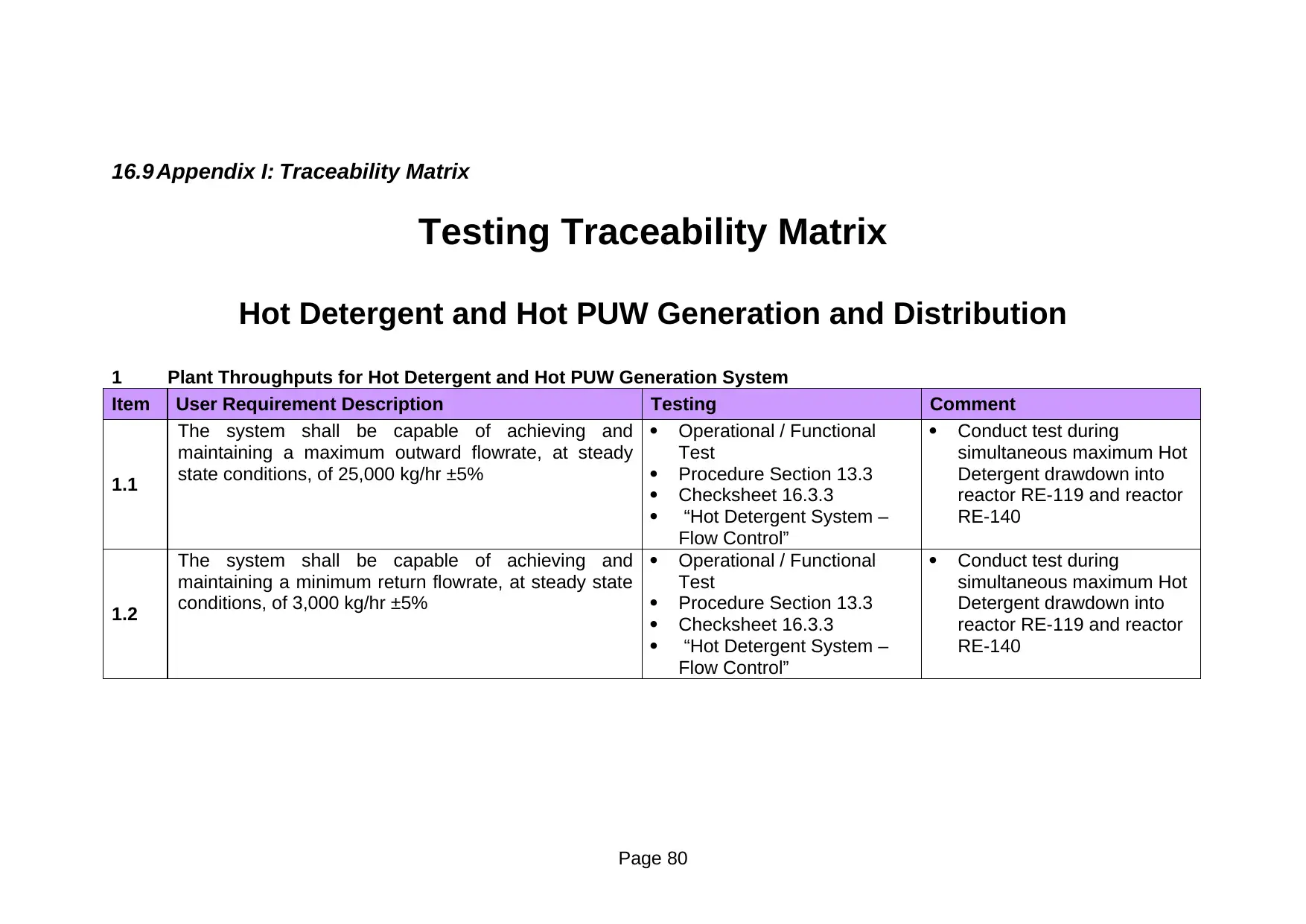
Testing Traceability Matrix
Hot Detergent and Hot PUW Generation and Distribution
1 Plant Throughputs for Hot Detergent and Hot PUW Generation System
Item User Requirement Description Testing Comment
1.1
The system shall be capable of achieving and
maintaining a maximum outward flowrate, at steady
state conditions, of 25,000 kg/hr ±5%
Operational / Functional
Test
Procedure Section 13.3
Checksheet 16.3.3
“Hot Detergent System –
Flow Control”
Conduct test during
simultaneous maximum Hot
Detergent drawdown into
reactor RE-119 and reactor
RE-140
1.2
The system shall be capable of achieving and
maintaining a minimum return flowrate, at steady state
conditions, of 3,000 kg/hr ±5%
Operational / Functional
Test
Procedure Section 13.3
Checksheet 16.3.3
“Hot Detergent System –
Flow Control”
Conduct test during
simultaneous maximum Hot
Detergent drawdown into
reactor RE-119 and reactor
RE-140
Page 80
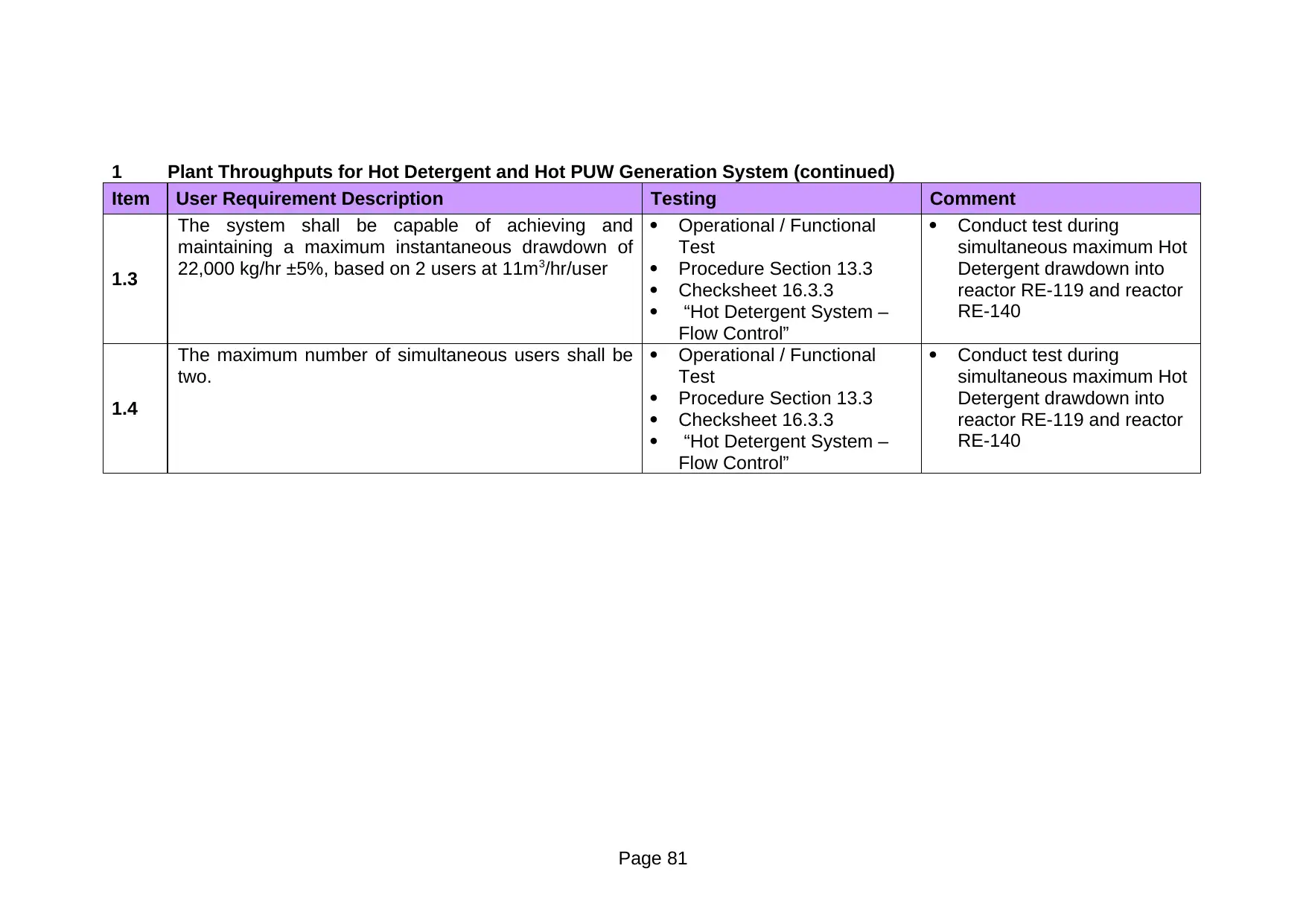
Item User Requirement Description Testing Comment
1.3
The system shall be capable of achieving and
maintaining a maximum instantaneous drawdown of
22,000 kg/hr ±5%, based on 2 users at 11m3/hr/user
Operational / Functional
Test
Procedure Section 13.3
Checksheet 16.3.3
“Hot Detergent System –
Flow Control”
Conduct test during
simultaneous maximum Hot
Detergent drawdown into
reactor RE-119 and reactor
RE-140
1.4
The maximum number of simultaneous users shall be
two.
Operational / Functional
Test
Procedure Section 13.3
Checksheet 16.3.3
“Hot Detergent System –
Flow Control”
Conduct test during
simultaneous maximum Hot
Detergent drawdown into
reactor RE-119 and reactor
RE-140
Page 81
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
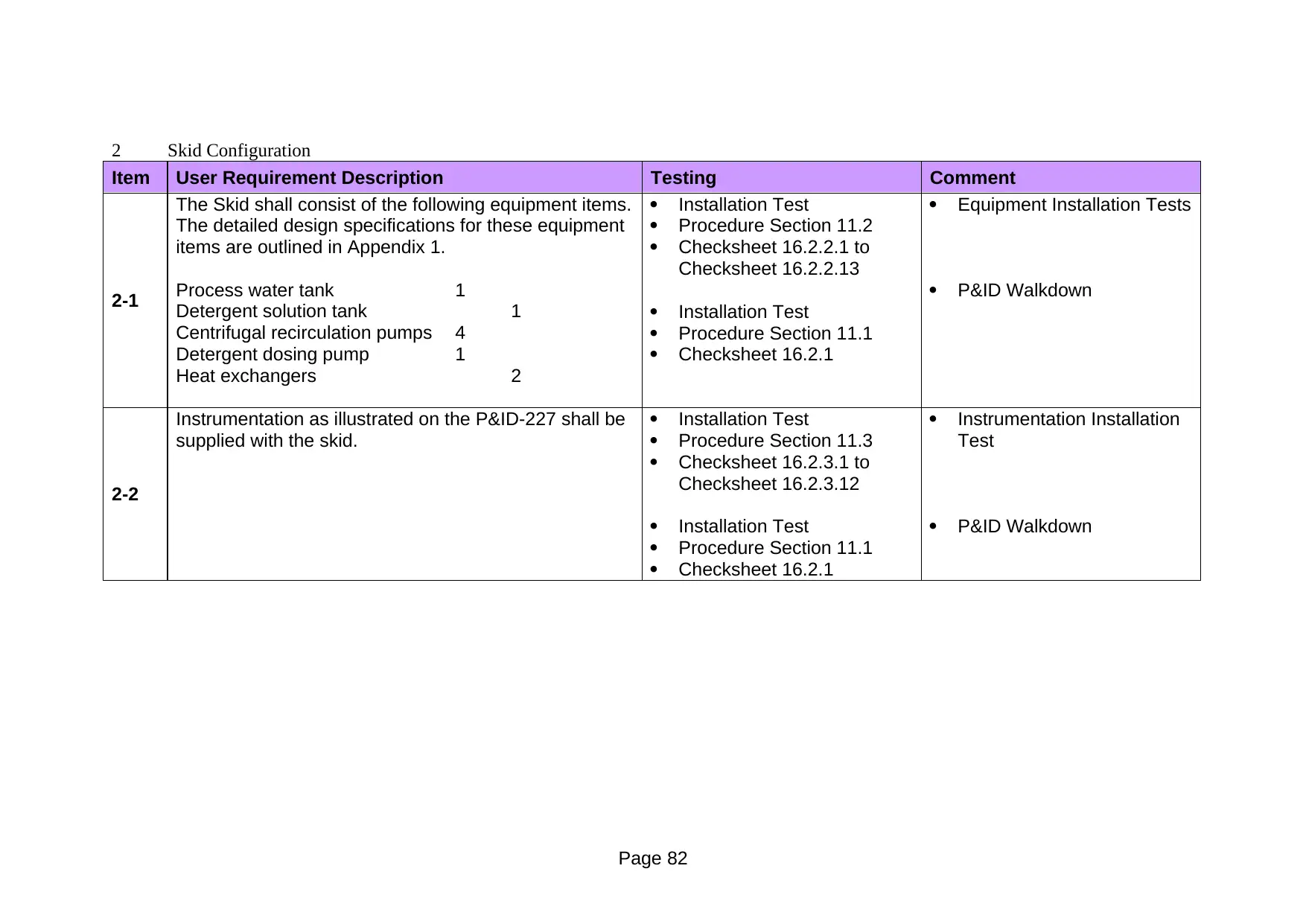
Item User Requirement Description Testing Comment
2-1
The Skid shall consist of the following equipment items.
The detailed design specifications for these equipment
items are outlined in Appendix 1.
Process water tank 1
Detergent solution tank 1
Centrifugal recirculation pumps 4
Detergent dosing pump 1
Heat exchangers 2
Installation Test
Procedure Section 11.2
Checksheet 16.2.2.1 to
Checksheet 16.2.2.13
Installation Test
Procedure Section 11.1
Checksheet 16.2.1
Equipment Installation Tests
P&ID Walkdown
2-2
Instrumentation as illustrated on the P&ID-227 shall be
supplied with the skid.
Installation Test
Procedure Section 11.3
Checksheet 16.2.3.1 to
Checksheet 16.2.3.12
Installation Test
Procedure Section 11.1
Checksheet 16.2.1
Instrumentation Installation
Test
P&ID Walkdown
Page 82
Paraphrase This Document
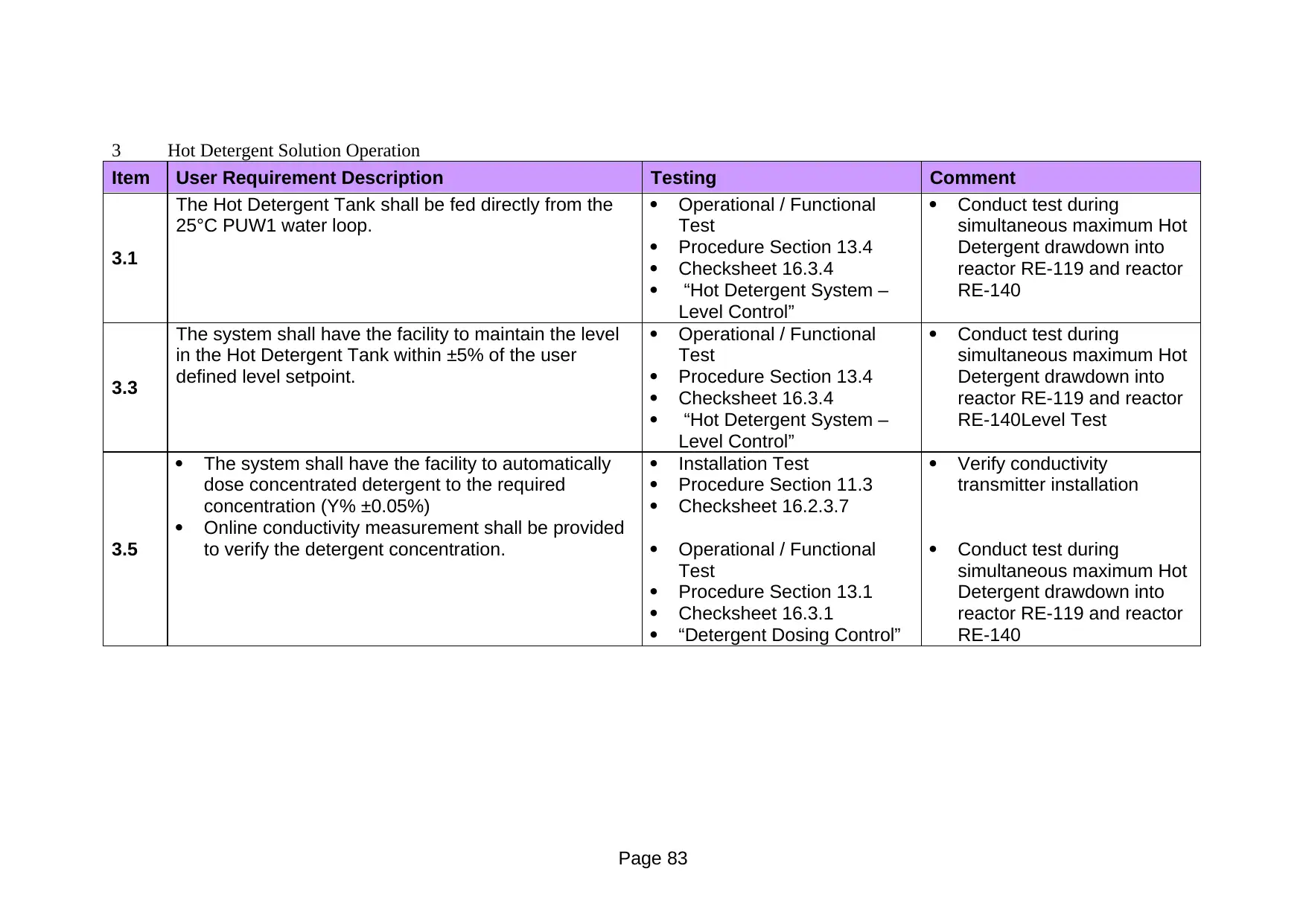
Item User Requirement Description Testing Comment
3.1
The Hot Detergent Tank shall be fed directly from the
25°C PUW1 water loop.
Operational / Functional
Test
Procedure Section 13.4
Checksheet 16.3.4
“Hot Detergent System –
Level Control”
Conduct test during
simultaneous maximum Hot
Detergent drawdown into
reactor RE-119 and reactor
RE-140
3.3
The system shall have the facility to maintain the level
in the Hot Detergent Tank within ±5% of the user
defined level setpoint.
Operational / Functional
Test
Procedure Section 13.4
Checksheet 16.3.4
“Hot Detergent System –
Level Control”
Conduct test during
simultaneous maximum Hot
Detergent drawdown into
reactor RE-119 and reactor
RE-140Level Test
3.5
The system shall have the facility to automatically
dose concentrated detergent to the required
concentration (Y% ±0.05%)
Online conductivity measurement shall be provided
to verify the detergent concentration.
Installation Test
Procedure Section 11.3
Checksheet 16.2.3.7
Operational / Functional
Test
Procedure Section 13.1
Checksheet 16.3.1
“Detergent Dosing Control”
Verify conductivity
transmitter installation
Conduct test during
simultaneous maximum Hot
Detergent drawdown into
reactor RE-119 and reactor
RE-140
Page 83
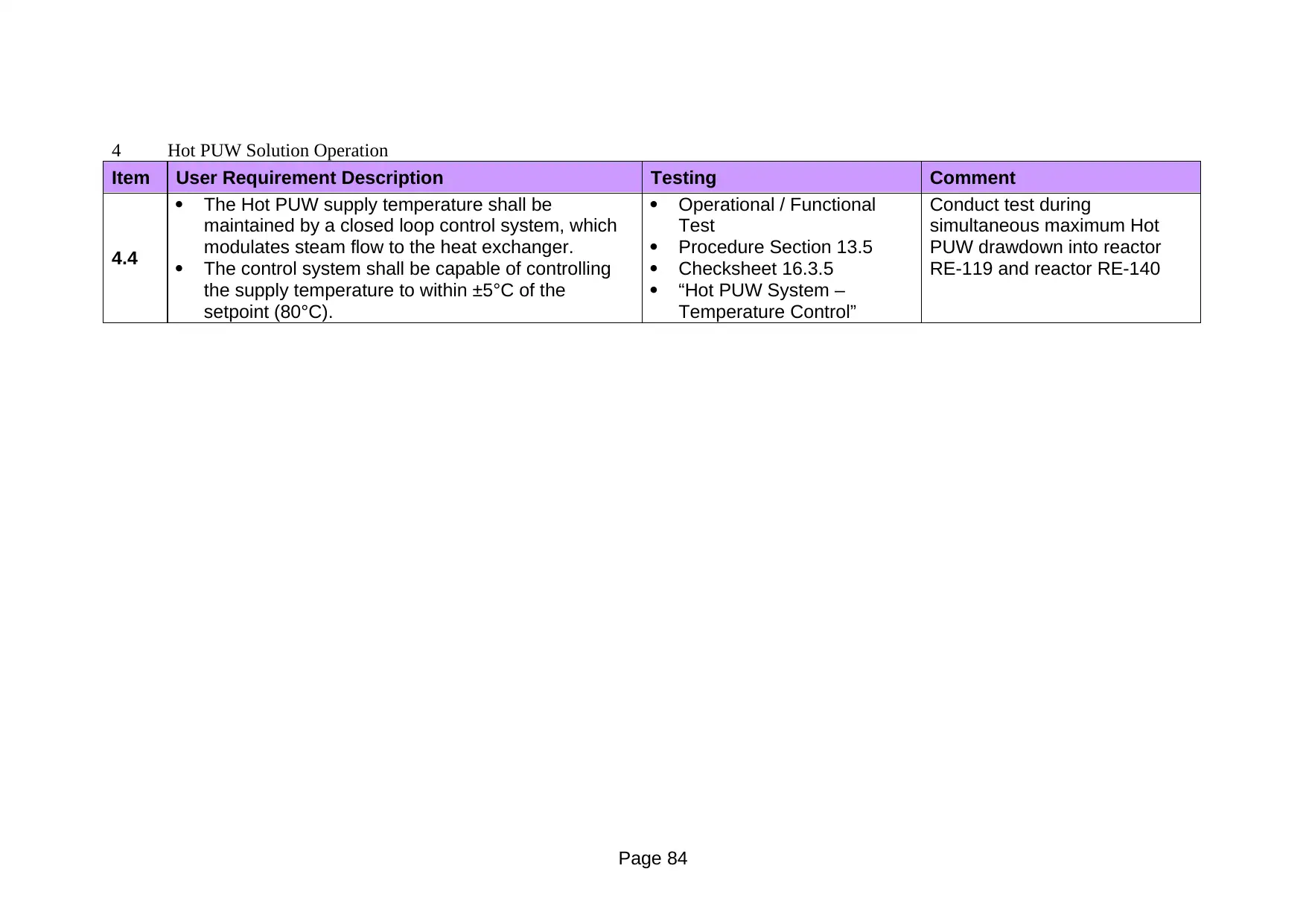
Item User Requirement Description Testing Comment
4.4
The Hot PUW supply temperature shall be
maintained by a closed loop control system, which
modulates steam flow to the heat exchanger.
The control system shall be capable of controlling
the supply temperature to within ±5°C of the
setpoint (80°C).
Operational / Functional
Test
Procedure Section 13.5
Checksheet 16.3.5
“Hot PUW System –
Temperature Control”
Conduct test during
simultaneous maximum Hot
PUW drawdown into reactor
RE-119 and reactor RE-140
Page 84
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Chartier, Y. (2014). Safe management of wastes from health-care activities. Geneva: World
Health Organization.
Meier, F. A., & Meier, C. A. (2011). Instrumentation and control systems documentation.
Research Triangle Park, N.C: International Society of Automation.
Sherwood, D. R. (2010). Piping guide: For the design and drafting of industrial piping systems.
New York: Construction Trades Pr.
U.S. Department of Health and Human Service. (2014). Inspections, Compliance, Enforcement,
and Criminal Investigations (p. 9). Silver Spring: US Food and Drug Administration.
Winters, G. L., & Nutt, M. J. (2011). Stainless steels for medical and surgical applications. West
Conshohocken, Pa: ASTM International.
World Health Organization. (2011). Quality assurance of pharmaceuticals: A compendium of
guidelines and related materials. Geneva: World Health Organization.
Page 85
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
© 2024 | Zucol Services PVT LTD | All rights reserved.
