Treatment of Hay Fever PowerPoint Presentation 2022
VerifiedAdded on 2022/10/15
|26
|2081
|16
Presentation
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Development of
JNJ20190729, a new H4
receptor antagonist for
treatment of Hay fever
JNJ20190729, a new H4
receptor antagonist for
treatment of Hay fever
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Allergic Rhinitis- Causes and Treatment
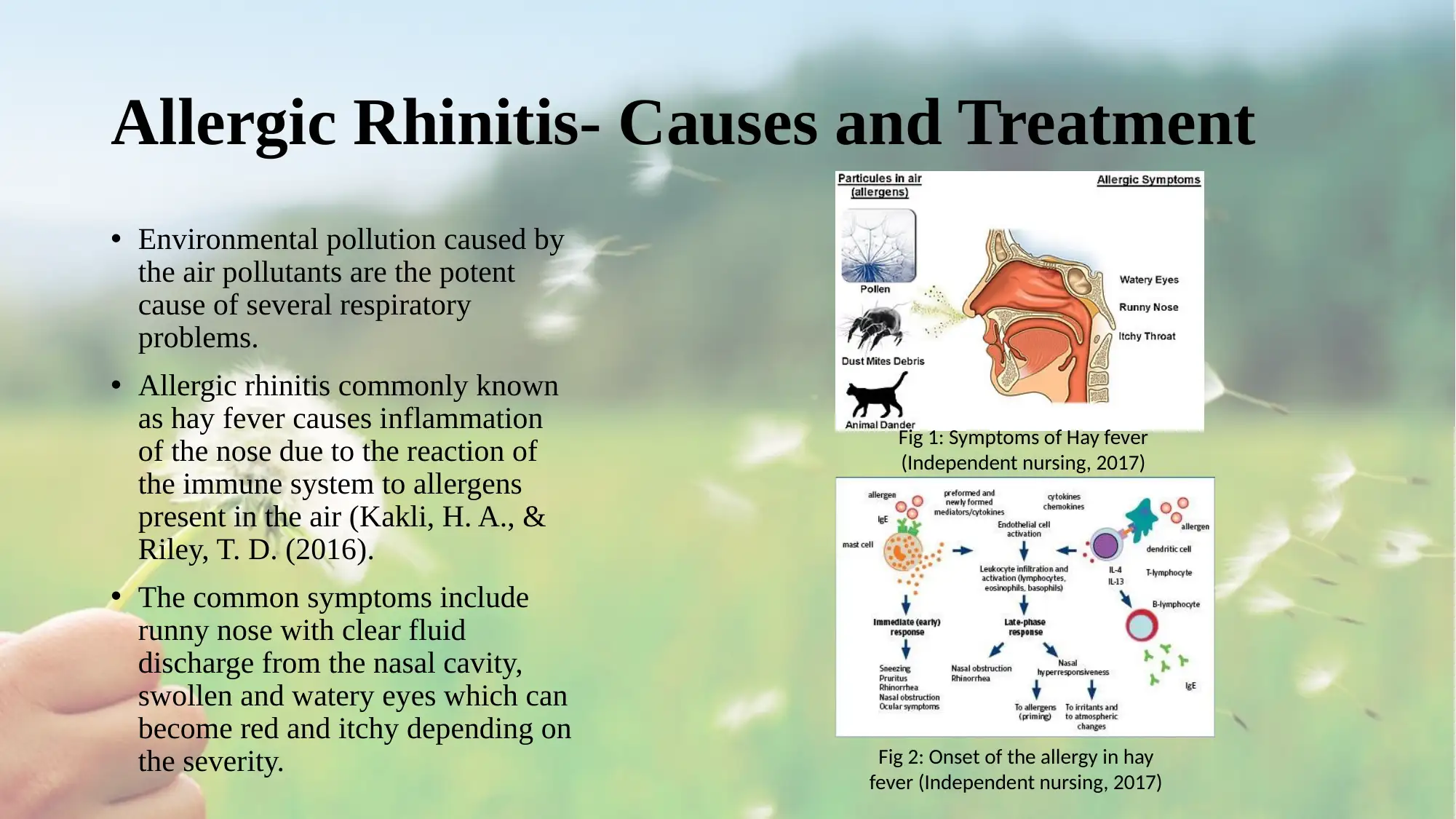
• Environmental pollution caused by
the air pollutants are the potent
cause of several respiratory
problems.
• Allergic rhinitis commonly known
as hay fever causes inflammation
of the nose due to the reaction of
the immune system to allergens
present in the air (Kakli, H. A., &
Riley, T. D. (2016).
• The common symptoms include
runny nose with clear fluid
discharge from the nasal cavity,
swollen and watery eyes which can
become red and itchy depending on
the severity.
Fig 1: Symptoms of Hay fever
(Independent nursing, 2017)
Fig 2: Onset of the allergy in hay
fever (Independent nursing, 2017)
• Environmental pollution caused by
the air pollutants are the potent
cause of several respiratory
problems.
• Allergic rhinitis commonly known
as hay fever causes inflammation
of the nose due to the reaction of
the immune system to allergens
present in the air (Kakli, H. A., &
Riley, T. D. (2016).
• The common symptoms include
runny nose with clear fluid
discharge from the nasal cavity,
swollen and watery eyes which can
become red and itchy depending on
the severity.
Fig 1: Symptoms of Hay fever
(Independent nursing, 2017)
Fig 2: Onset of the allergy in hay
fever (Independent nursing, 2017)
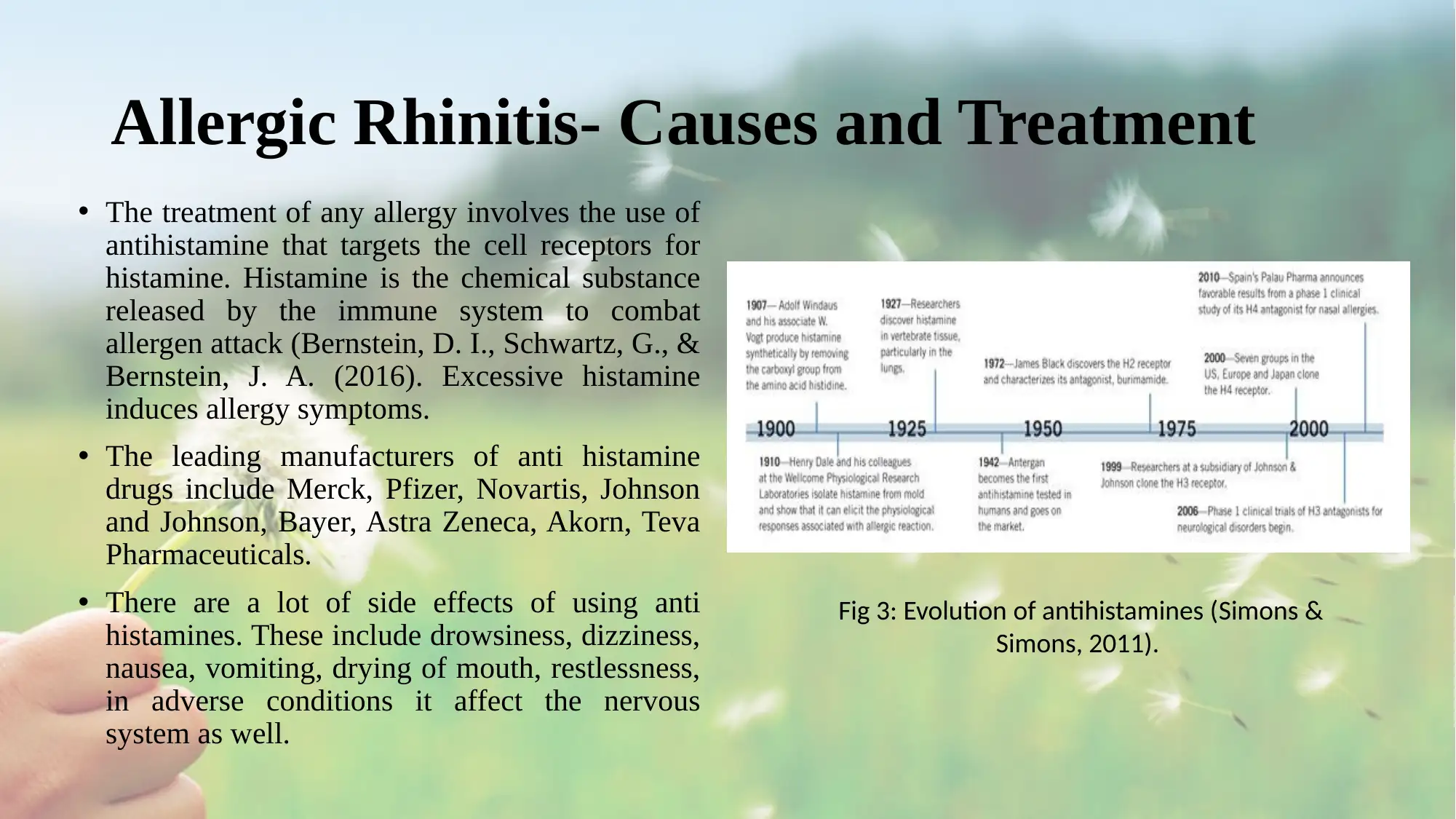
• The treatment of any allergy involves the use of
antihistamine that targets the cell receptors for
histamine. Histamine is the chemical substance
released by the immune system to combat
allergen attack (Bernstein, D. I., Schwartz, G., &
Bernstein, J. A. (2016). Excessive histamine
induces allergy symptoms.
• The leading manufacturers of anti histamine
drugs include Merck, Pfizer, Novartis, Johnson
and Johnson, Bayer, Astra Zeneca, Akorn, Teva
Pharmaceuticals.
• There are a lot of side effects of using anti
histamines. These include drowsiness, dizziness,
nausea, vomiting, drying of mouth, restlessness,
in adverse conditions it affect the nervous
system as well.
Allergic Rhinitis- Causes and Treatment
Fig 3: Evolution of antihistamines (Simons &
Simons, 2011).
antihistamine that targets the cell receptors for
histamine. Histamine is the chemical substance
released by the immune system to combat
allergen attack (Bernstein, D. I., Schwartz, G., &
Bernstein, J. A. (2016). Excessive histamine
induces allergy symptoms.
• The leading manufacturers of anti histamine
drugs include Merck, Pfizer, Novartis, Johnson
and Johnson, Bayer, Astra Zeneca, Akorn, Teva
Pharmaceuticals.
• There are a lot of side effects of using anti
histamines. These include drowsiness, dizziness,
nausea, vomiting, drying of mouth, restlessness,
in adverse conditions it affect the nervous
system as well.
Allergic Rhinitis- Causes and Treatment
Fig 3: Evolution of antihistamines (Simons &
Simons, 2011).

H4 Receptor- A good target
• H4 is the fourth histamine receptor that is exclusively expressed on hematopoietic
cells that promotes the development of the symptoms of allergy as well as asthma
(Liu et al., 2014).
• The H1 receptor antagonists are the mainstay but they fail to show efficacy in
reduction of the allergy symptoms.
• H4 is the fourth histamine receptor that is exclusively expressed on hematopoietic
cells that promotes the development of the symptoms of allergy as well as asthma
(Liu et al., 2014).
• The H1 receptor antagonists are the mainstay but they fail to show efficacy in
reduction of the allergy symptoms.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Screening of the H4 antagonist
• H4 is a target for allergy and asthma. Hence asthma and allergy can be treated by
targeting the H4 receptor.
• The drugs that were considered for screening had three known antagonists
VUF-6002, JNJ777120 and thioperamide.
• The structurally similar compounds of the antagonist had been retrieved from
PubChem and database was formed. Some compounds that had high docking
scores were retrieved.
• All of the compounds proved to interact with the Asp64 target of the H4 receptor
(Ehling et al., 2016).
• H4 is a target for allergy and asthma. Hence asthma and allergy can be treated by
targeting the H4 receptor.
• The drugs that were considered for screening had three known antagonists
VUF-6002, JNJ777120 and thioperamide.
• The structurally similar compounds of the antagonist had been retrieved from
PubChem and database was formed. Some compounds that had high docking
scores were retrieved.
• All of the compounds proved to interact with the Asp64 target of the H4 receptor
(Ehling et al., 2016).

Drawbacks of some data obtained
• According to the literature review there has been a lot of H4 receptor antagonists
reported but a very less number amongst them has been studied in humans due to
the hindrance faced in targeting a suitable ligand inclining to all the properties
important for analysis in the clinic.
• According to the literature review there has been a lot of H4 receptor antagonists
reported but a very less number amongst them has been studied in humans due to
the hindrance faced in targeting a suitable ligand inclining to all the properties
important for analysis in the clinic.
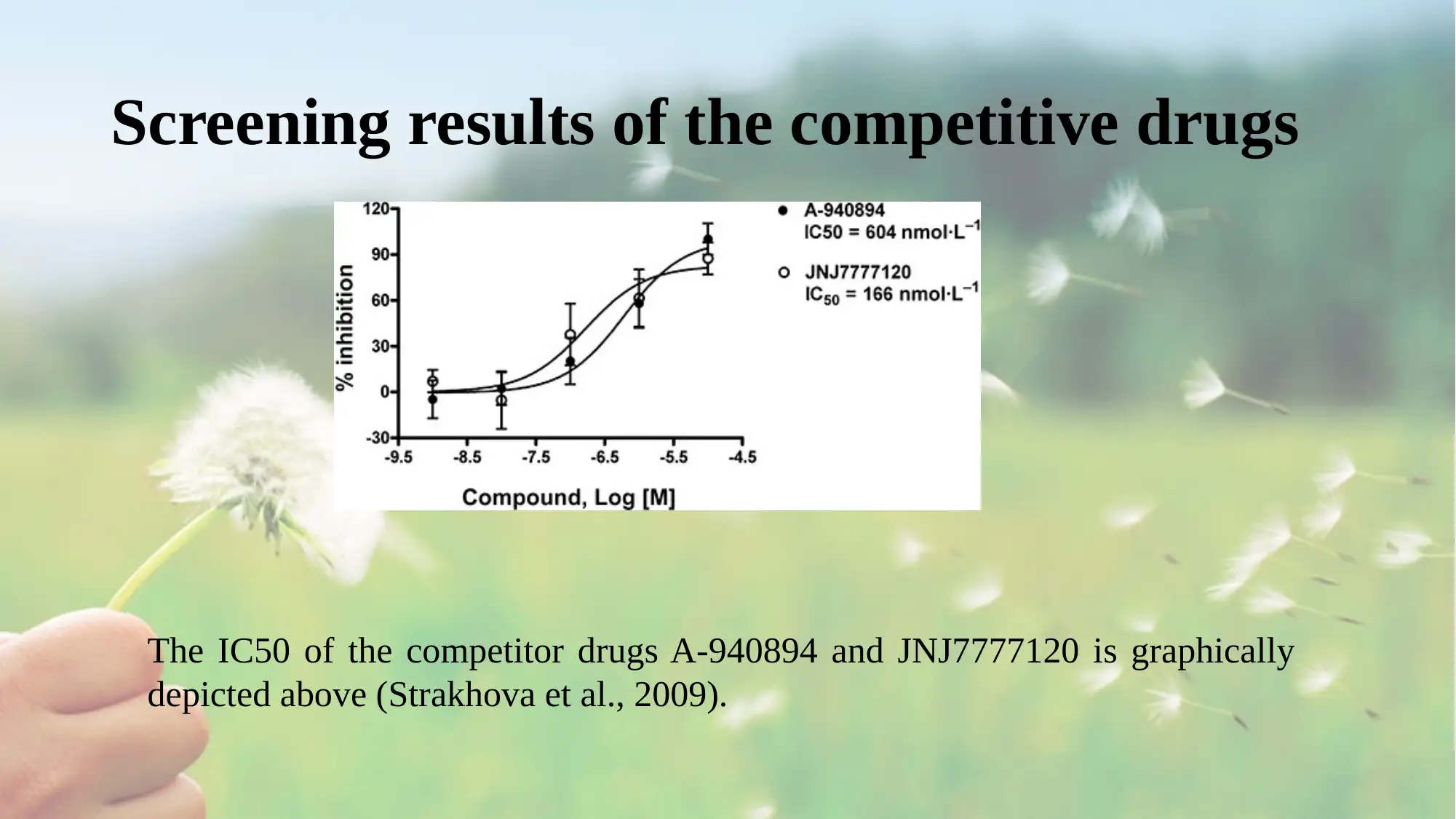
Screening results of the competitive drugs
The IC50 of the competitor drugs A-940894 and JNJ7777120 is graphically
depicted above (Strakhova et al., 2009).
The IC50 of the competitor drugs A-940894 and JNJ7777120 is graphically
depicted above (Strakhova et al., 2009).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Drugs that are a potent competitor
• JNJ777120 is used extensively in in vitro cell models and animal models that
concluded that H4 antagonists can have anti inflammatory properties. It can be
considered as a potent competitor.
• JNJ777120 is used extensively in in vitro cell models and animal models that
concluded that H4 antagonists can have anti inflammatory properties. It can be
considered as a potent competitor.

The animal model selected
• Male rats (Sprague-Dawley) were used as the animal model.
• The animals were acclaimed in the lab for a week before the
onset of the experiment
• They were housed in three with free access to food and
water.
• All efforts were made to fulfil the 3Rs principle of the
ethical values (Dettori et al., 2018)
Sprague-Dawley Rats
• Male rats (Sprague-Dawley) were used as the animal model.
• The animals were acclaimed in the lab for a week before the
onset of the experiment
• They were housed in three with free access to food and
water.
• All efforts were made to fulfil the 3Rs principle of the
ethical values (Dettori et al., 2018)
Sprague-Dawley Rats

• Venous blood was taken from the healthy host inclining to the ethical policies.
• The eosinophil were extracted from the blood and isolated.
• The activity of the drugs were tested after administering various amounts to the
extracted blood.
The animal model selected
• The eosinophil were extracted from the blood and isolated.
• The activity of the drugs were tested after administering various amounts to the
extracted blood.
The animal model selected
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Results of the drug testing model
• The H4 antagonist was found to successfully bind to the human as well as rat H4
receptors.
• It showed about 50 fold selective binding for human H4 receptor.
• However, depending on the dosage this antagonist promoted blocking of
histamine evoked calcium responses.
• It also inhibited changes in the shape of the bone marrow induced mast calls.
• The H4 antagonist was found to successfully bind to the human as well as rat H4
receptors.
• It showed about 50 fold selective binding for human H4 receptor.
• However, depending on the dosage this antagonist promoted blocking of
histamine evoked calcium responses.
• It also inhibited changes in the shape of the bone marrow induced mast calls.
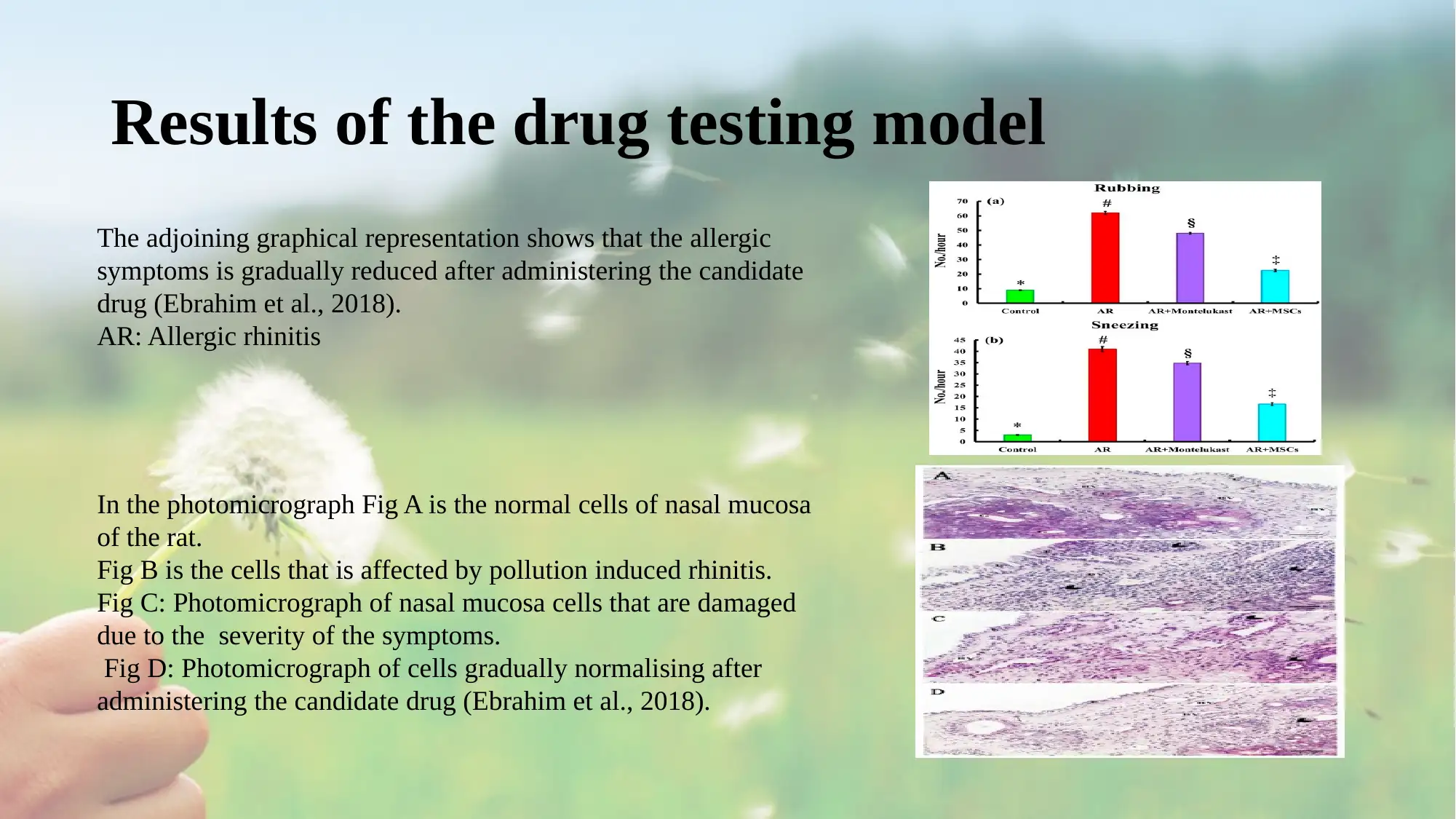
Results of the drug testing model
The adjoining graphical representation shows that the allergic
symptoms is gradually reduced after administering the candidate
drug (Ebrahim et al., 2018).
AR: Allergic rhinitis
In the photomicrograph Fig A is the normal cells of nasal mucosa
of the rat.
Fig B is the cells that is affected by pollution induced rhinitis.
Fig C: Photomicrograph of nasal mucosa cells that are damaged
due to the severity of the symptoms.
Fig D: Photomicrograph of cells gradually normalising after
administering the candidate drug (Ebrahim et al., 2018).
The adjoining graphical representation shows that the allergic
symptoms is gradually reduced after administering the candidate
drug (Ebrahim et al., 2018).
AR: Allergic rhinitis
In the photomicrograph Fig A is the normal cells of nasal mucosa
of the rat.
Fig B is the cells that is affected by pollution induced rhinitis.
Fig C: Photomicrograph of nasal mucosa cells that are damaged
due to the severity of the symptoms.
Fig D: Photomicrograph of cells gradually normalising after
administering the candidate drug (Ebrahim et al., 2018).

The observed side effects
• The side effects observed involved drowsiness and minimal activity of one rat that
was administered with comparatively higher dosage.
• There was no adverse effects observed in the tested animal model but only one
discontinuation case due to adverse effects.
• The side effects observed involved drowsiness and minimal activity of one rat that
was administered with comparatively higher dosage.
• There was no adverse effects observed in the tested animal model but only one
discontinuation case due to adverse effects.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
• It was evident from the results that the drug was proven to be a good choice to
inhibit the adverse effects of the pollution-induced rhinitis.
• The adverse effects were minimal and out of the 100 selected members only one
showed adverse effect.
• The drug was effect in reducing the physiological changes that is the nasal
mucous membrane was in the process of being restored to normal after being
adversely damaged due to continuous sneezing.
• The rubbing of eyes and sneezing was also reduced after administering the drug.
Efficacy vs. side effects of the Drug
inhibit the adverse effects of the pollution-induced rhinitis.
• The adverse effects were minimal and out of the 100 selected members only one
showed adverse effect.
• The drug was effect in reducing the physiological changes that is the nasal
mucous membrane was in the process of being restored to normal after being
adversely damaged due to continuous sneezing.
• The rubbing of eyes and sneezing was also reduced after administering the drug.
Efficacy vs. side effects of the Drug
Efficacy vs. side effects of the Drug
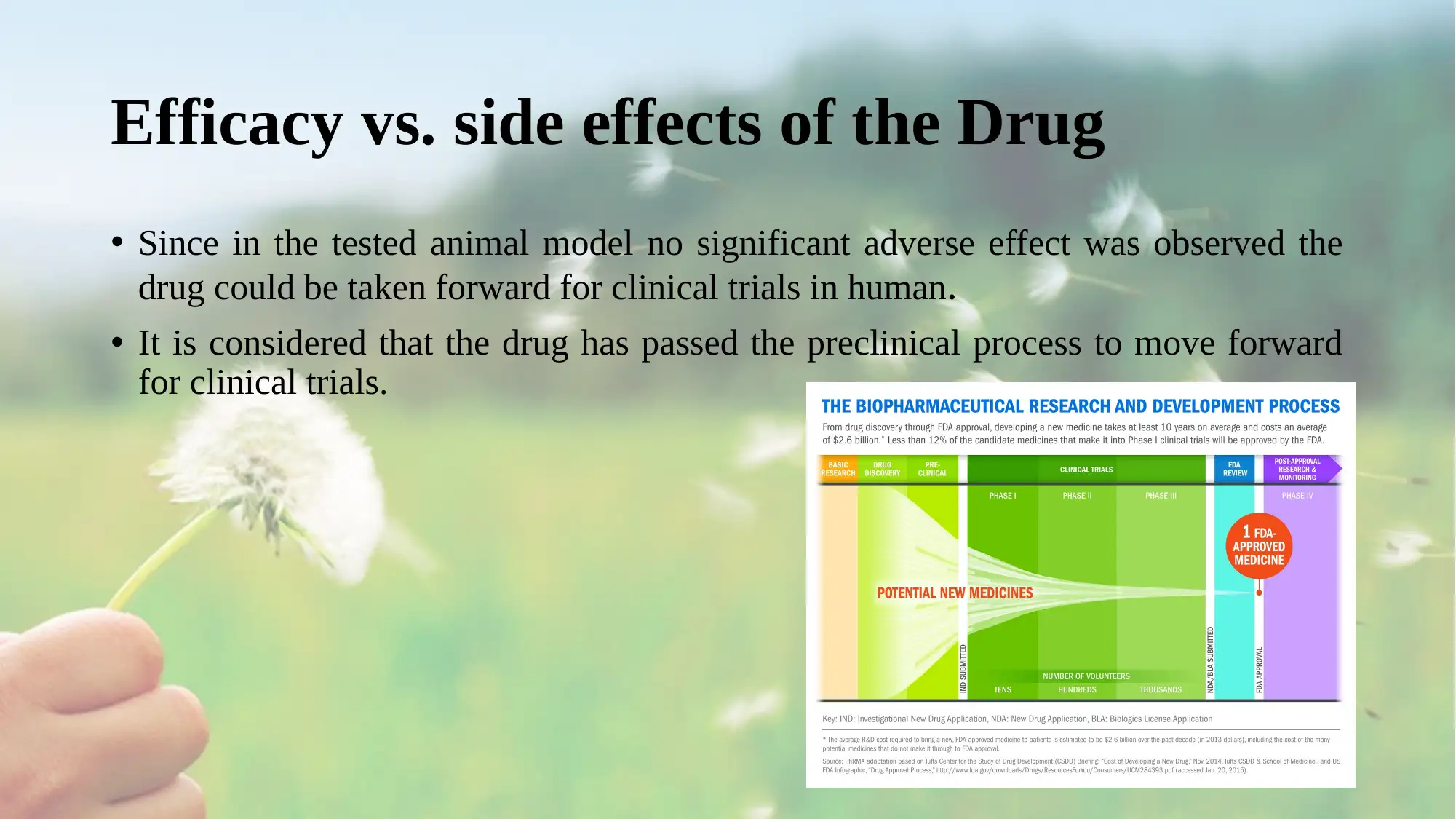
• Since in the tested animal model no significant adverse effect was observed the
drug could be taken forward for clinical trials in human.
• It is considered that the drug has passed the preclinical process to move forward
for clinical trials.
• Since in the tested animal model no significant adverse effect was observed the
drug could be taken forward for clinical trials in human.
• It is considered that the drug has passed the preclinical process to move forward
for clinical trials.

The Gene Expression
• The cells models for experiment included Human Embryonic Kidney cells,
HEK293K cells.
• The other cells that can be considered for the screening is COS 7 cells.
• The required materials for the experiment was obtained from Invitrogen.
• The HEK293 cells were purchased from ATCC.
• The action of the candidate drug was compared with that of JNJ777120
• The cells models for experiment included Human Embryonic Kidney cells,
HEK293K cells.
• The other cells that can be considered for the screening is COS 7 cells.
• The required materials for the experiment was obtained from Invitrogen.
• The HEK293 cells were purchased from ATCC.
• The action of the candidate drug was compared with that of JNJ777120
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Gene expression analysis
• The human H4 receptor cDNA was cloned using RT-PCR
technique and then sub-cloned in pCI-neo expression vector.
• The cell lines that were stable was established exploiting
fluorimetric imaging plate reader assay and other standard
molecular techniques.
• The cells that were expressing H4 receptors were harvested
and homogenized.
• These cells were incubated with histamine in the presence and
absence of the antagonist ligands that have affinity for H4
ligands.
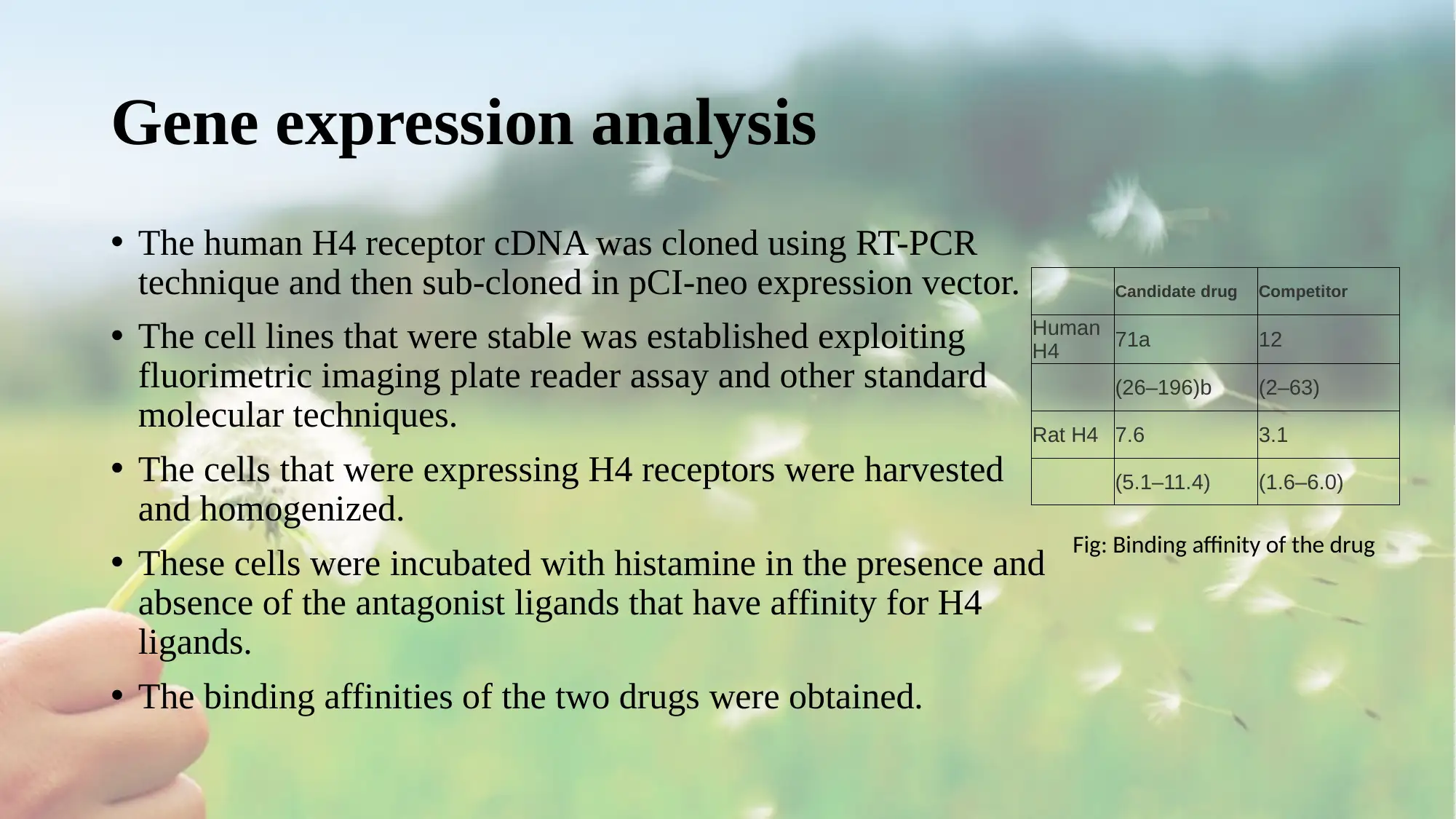
• The binding affinities of the two drugs were obtained.
Candidate drug Competitor
Human
H4 71a 12
(26–196)b (2–63)
Rat H4 7.6 3.1
(5.1–11.4) (1.6–6.0)
Fig: Binding affinity of the drug
• The human H4 receptor cDNA was cloned using RT-PCR
technique and then sub-cloned in pCI-neo expression vector.
• The cell lines that were stable was established exploiting
fluorimetric imaging plate reader assay and other standard
molecular techniques.
• The cells that were expressing H4 receptors were harvested
and homogenized.
• These cells were incubated with histamine in the presence and
absence of the antagonist ligands that have affinity for H4
ligands.
• The binding affinities of the two drugs were obtained.
Candidate drug Competitor
Human
H4 71a 12
(26–196)b (2–63)
Rat H4 7.6 3.1
(5.1–11.4) (1.6–6.0)
Fig: Binding affinity of the drug

• An increased level of IFN-gamma was observed on administration of
JNJ7777120 but no such effects were observed after administering the
candidate drug.
• Both the candidate drug as well as JNJ7777120 showed no effect on
the T-cells.
Gene Expression Results
JNJ7777120 but no such effects were observed after administering the
candidate drug.
• Both the candidate drug as well as JNJ7777120 showed no effect on
the T-cells.
Gene Expression Results

Gene Expression Results
• These studies confirm that the candidate drug affects the H4 receptors
and prevents the histamine from binding to the receptor.
• Thus this confirms that inability for histamine to bind to its receptors
prevents the onset of the symptoms of pollution induced allergic
rhinitis.
• These studies confirm that the candidate drug affects the H4 receptors
and prevents the histamine from binding to the receptor.
• Thus this confirms that inability for histamine to bind to its receptors
prevents the onset of the symptoms of pollution induced allergic
rhinitis.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

• JNJ7777120 drug in higher dosage seemed to affect the nervous
system of the target sinitis was also seen. The adverse side effects
were not seen with the candidate drug and hence it is proposed for
clinical trials.
Gene Expression Results
system of the target sinitis was also seen. The adverse side effects
were not seen with the candidate drug and hence it is proposed for
clinical trials.
Gene Expression Results

The Clinical Trials- Experimental design
• A clinical trial was performed to establish a knowledge whether montelukast is
safe and tolerated in a controlled manner. The pharmacokinetics and also the
effectivity of perennial arthritis rhinitis treatment was measured.
• The trial was tested among 87 Japanese paediatric patients aged between 1 to 15
years.
• A clinical trial was performed to establish a knowledge whether montelukast is
safe and tolerated in a controlled manner. The pharmacokinetics and also the
effectivity of perennial arthritis rhinitis treatment was measured.
• The trial was tested among 87 Japanese paediatric patients aged between 1 to 15
years.

• There were no serious adverse effects observed and almost all reportedly had
improved health conditions with maximum effectivity observed in patients
administered with the drug for 12 weeks.
• The drug was well tolerated in kids of the age limit 1 to 9 years but reduced
exposure was observed in 10-15 years age group which can be the result of the
difference in their body weight (Okubo et al., 2016).
• There was one discontinuation observed due to the adverse effects which mainly
involved pharyngitis and sinitis.
The Clinical Trials- Phenotypic measures
improved health conditions with maximum effectivity observed in patients
administered with the drug for 12 weeks.
• The drug was well tolerated in kids of the age limit 1 to 9 years but reduced
exposure was observed in 10-15 years age group which can be the result of the
difference in their body weight (Okubo et al., 2016).
• There was one discontinuation observed due to the adverse effects which mainly
involved pharyngitis and sinitis.
The Clinical Trials- Phenotypic measures
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

• The adverse effects observed were mild in intensity and the only discontinuation
case had moderate level of adverse effects.
• Global evaluation of the effectivity of the drug proved that the health conditions
of the patient improvement in the rhinitis symptoms in different age groups.
• It was proved to be tolerant in the tested age group of 1 to 15 years with minimal
side effects.
The Clinical Trials- Benefits of the drug
case had moderate level of adverse effects.
• Global evaluation of the effectivity of the drug proved that the health conditions
of the patient improvement in the rhinitis symptoms in different age groups.
• It was proved to be tolerant in the tested age group of 1 to 15 years with minimal
side effects.
The Clinical Trials- Benefits of the drug

• Behavioural adverse effect was noted in experience post marketing
with respect to the selected drug.
• However, it showed symptomatic effectiveness in the treatment of hay
fever. It has proven efficacy in night time symptoms and in reducing
inflammation.
The Clinical Trials- Placebo
with respect to the selected drug.
• However, it showed symptomatic effectiveness in the treatment of hay
fever. It has proven efficacy in night time symptoms and in reducing
inflammation.
The Clinical Trials- Placebo

Benefits of the drug, Montelukast
• It is a novel medication considered as a viable alternative that targets the cysteinyl
leukotriene receptor 1.
• It is non sedating in nature and has a safety profile history for all starting from
6months of age.
• When exploited in monotherapy it has similar benefits that is observed in other
antihistamines and it serves as an intranasal corticosteroid when administered
along with other antihistamine.
• It is a novel medication considered as a viable alternative that targets the cysteinyl
leukotriene receptor 1.
• It is non sedating in nature and has a safety profile history for all starting from
6months of age.
• When exploited in monotherapy it has similar benefits that is observed in other
antihistamines and it serves as an intranasal corticosteroid when administered
along with other antihistamine.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
• Okubo, K., Inoue, Y., Numaguchi, H., Tanaka, K., Saito, I., Oshima, N., ... & Philip, G. (2016). Montelukast in the treatment of
perennial allergic rhinitis in paediatric Japanese patients; an open-label clinical trial. Journal of drug assessment, 5(1), 6-14.
• Kakli, H. A., & Riley, T. D. (2016). Allergic rhinitis. Primary Care: Clinics in Office Practice, 43(3), 465-475.
• Bernstein, D. I., Schwartz, G., & Bernstein, J. A. (2016). Allergic rhinitis: mechanisms and treatment. Immunology and Allergy
Clinics, 36(2), 261-278.
• Liu, W. L. (2014). Histamine H4 receptor antagonists for the treatment of inflammatory disorders. Drug discovery today, 19(8),
1222-1225.
• Ehling, S., Roßbach, K., Dunston, S. M., Stark, H., & Bäumer, W. (2016). Allergic inflammation is augmented via histamine H4
receptor activation: the role of natural killer cells in vitro and in vivo. Journal of dermatological science, 83(2), 106-115.
• Dettori, I., Gaviano, L., Melani, A., Lucarini, L., Durante, M., Masini, E., & Pedata, F. (2018). A selective histamine H4 receptor
antagonist, JNJ7777120, is protective in a rat model of transient cerebral ischemia. Frontiers in pharmacology, 9, 1231.
• Strakhova, M. I., Cuff, C. A., Manelli, A. M., Carr, T. L., Witte, D. G., Baranowski, J. L., ... & Adair, R. M. (2009). In vitro and in vivo
characterization of A 940894: a potent histamine H4 receptor antagonist with anti inflammatory properties.‐ ‐ British journal of
pharmacology, 157(1), 44-54.
• Ebrahim, N., Mandour, Y. M. H., Farid, A. S., Nafie, E., Mohamed, A. Z., Safwat, M., ... & Refae, A. (2018). Adipose Tissue-Derived
Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model. International journal of molecular
sciences, 20(4), 873.
• Okubo, K., Inoue, Y., Numaguchi, H., Tanaka, K., Saito, I., Oshima, N., ... & Philip, G. (2016). Montelukast in the treatment of
perennial allergic rhinitis in paediatric Japanese patients; an open-label clinical trial. Journal of drug assessment, 5(1), 6-14.
• Kakli, H. A., & Riley, T. D. (2016). Allergic rhinitis. Primary Care: Clinics in Office Practice, 43(3), 465-475.
• Bernstein, D. I., Schwartz, G., & Bernstein, J. A. (2016). Allergic rhinitis: mechanisms and treatment. Immunology and Allergy
Clinics, 36(2), 261-278.
• Liu, W. L. (2014). Histamine H4 receptor antagonists for the treatment of inflammatory disorders. Drug discovery today, 19(8),
1222-1225.
• Ehling, S., Roßbach, K., Dunston, S. M., Stark, H., & Bäumer, W. (2016). Allergic inflammation is augmented via histamine H4
receptor activation: the role of natural killer cells in vitro and in vivo. Journal of dermatological science, 83(2), 106-115.
• Dettori, I., Gaviano, L., Melani, A., Lucarini, L., Durante, M., Masini, E., & Pedata, F. (2018). A selective histamine H4 receptor
antagonist, JNJ7777120, is protective in a rat model of transient cerebral ischemia. Frontiers in pharmacology, 9, 1231.
• Strakhova, M. I., Cuff, C. A., Manelli, A. M., Carr, T. L., Witte, D. G., Baranowski, J. L., ... & Adair, R. M. (2009). In vitro and in vivo
characterization of A 940894: a potent histamine H4 receptor antagonist with anti inflammatory properties.‐ ‐ British journal of
pharmacology, 157(1), 44-54.
• Ebrahim, N., Mandour, Y. M. H., Farid, A. S., Nafie, E., Mohamed, A. Z., Safwat, M., ... & Refae, A. (2018). Adipose Tissue-Derived
Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat Model. International journal of molecular
sciences, 20(4), 873.
1 out of 26
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
