Tremelimumab: Clinical Trials, Efficacy, Safety, and Future Research
VerifiedAdded on 2023/04/22
|10
|940
|62
Literature Review
AI Summary
This literature review provides an overview of Tremelimumab, a humanized anti-CTLA-4 antibody used in cancer immunotherapy. It discusses the drug's function, mechanism of action, and clinical trials involving various cancers such as melanoma, mesothelioma, non-small cell lung cancer, and hepatocellular carcinoma. The review highlights the efficacy and safety profile of Tremelimumab, including observed side effects and its orphan drug designation for mesothelioma treatment. It also touches upon the combination therapies involving Tremelimumab and their impact on immune responses and patient survival rates, emphasizing the potential benefits of combining different tumor-killing strategies in cancer treatment. The information is sourced from research articles and clinical studies, offering a comprehensive understanding of Tremelimumab's role in cancer therapy.

Allergy and Clinical Immunology
Tremilumab therapy
Tremilumab therapy
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Introduction
• Tremilumab is a demonstrated therapeutic activity targeted
for several malignancies. Tremilumab (CP-675,206) a
humanized anti CTLA -4 is an IgG2 antibody having plasma
half life of 22 days
• The therapy has been evaluated on treatment of metastatic
melanoma.
• Phase 1 evaluation indicated maximum tolerable anti
tumour activity.
• In other phase I/II studies reported stable state of disease
occurring in section of melanoma patients treated with
therapy.
• Tremilumab has been utilized and tested on various cancers
both as an immunotherapy and combined therapy forms.
• Tremilumab is a demonstrated therapeutic activity targeted
for several malignancies. Tremilumab (CP-675,206) a
humanized anti CTLA -4 is an IgG2 antibody having plasma
half life of 22 days
• The therapy has been evaluated on treatment of metastatic
melanoma.
• Phase 1 evaluation indicated maximum tolerable anti
tumour activity.
• In other phase I/II studies reported stable state of disease
occurring in section of melanoma patients treated with
therapy.
• Tremilumab has been utilized and tested on various cancers
both as an immunotherapy and combined therapy forms.
Function and mechanism
• Tremelumab aims at stimulating immune
system which attack on tumours. The cytotoxic
T lymphocytes can recognise and attack cancer
cells.
• It binds to proteins CTLA-4 expressed on the
surface of the activated T- Lymphocytes.
(Eroglu et al., 2015)
• Tremelumab aims at stimulating immune
system which attack on tumours. The cytotoxic
T lymphocytes can recognise and attack cancer
cells.
• It binds to proteins CTLA-4 expressed on the
surface of the activated T- Lymphocytes.
(Eroglu et al., 2015)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
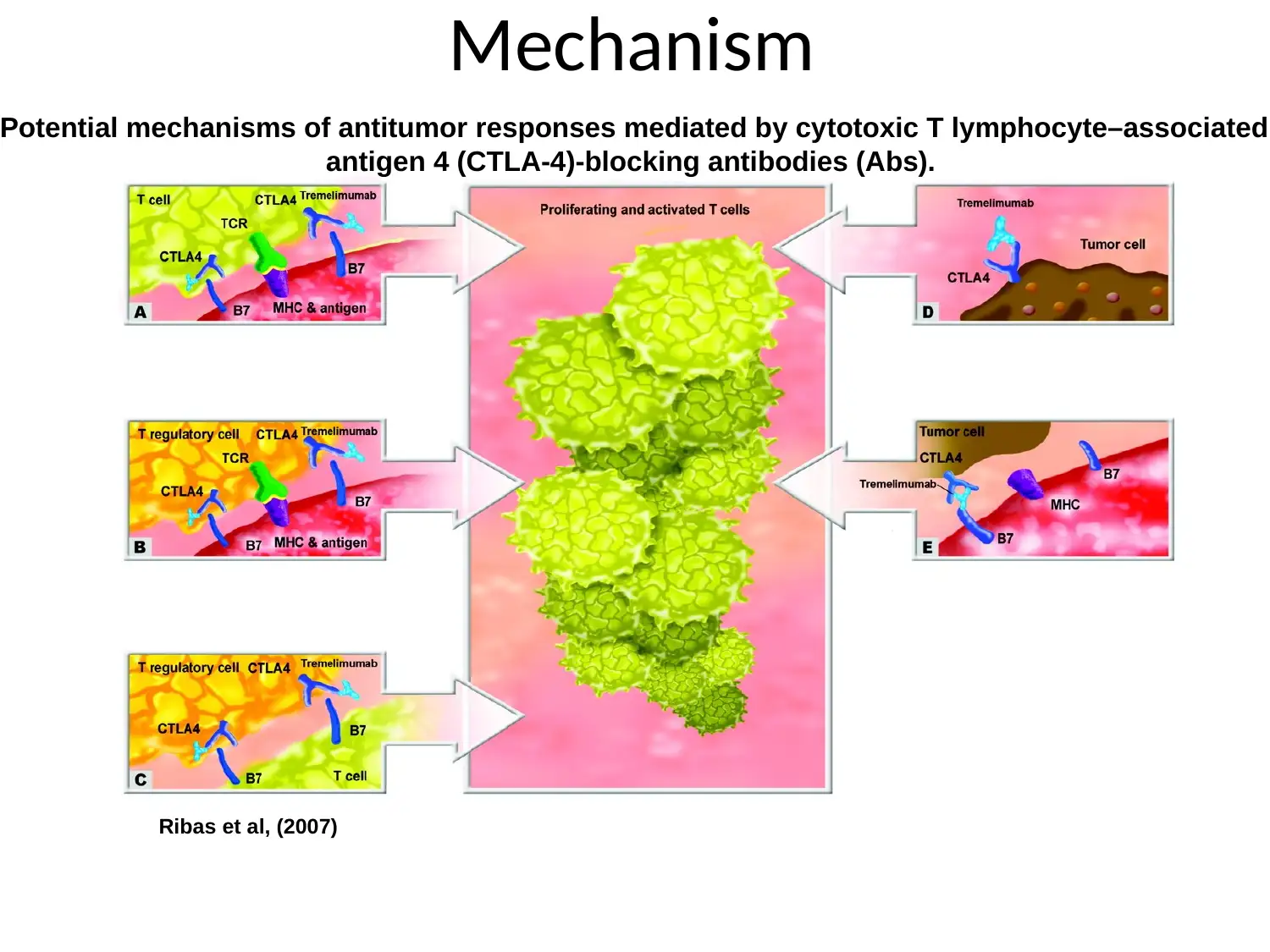
Mechanism
Ribas et al, (2007)
Potential mechanisms of antitumor responses mediated by cytotoxic T lymphocyte–associated
antigen 4 (CTLA-4)-blocking antibodies (Abs).
Ribas et al, (2007)
Potential mechanisms of antitumor responses mediated by cytotoxic T lymphocyte–associated
antigen 4 (CTLA-4)-blocking antibodies (Abs).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
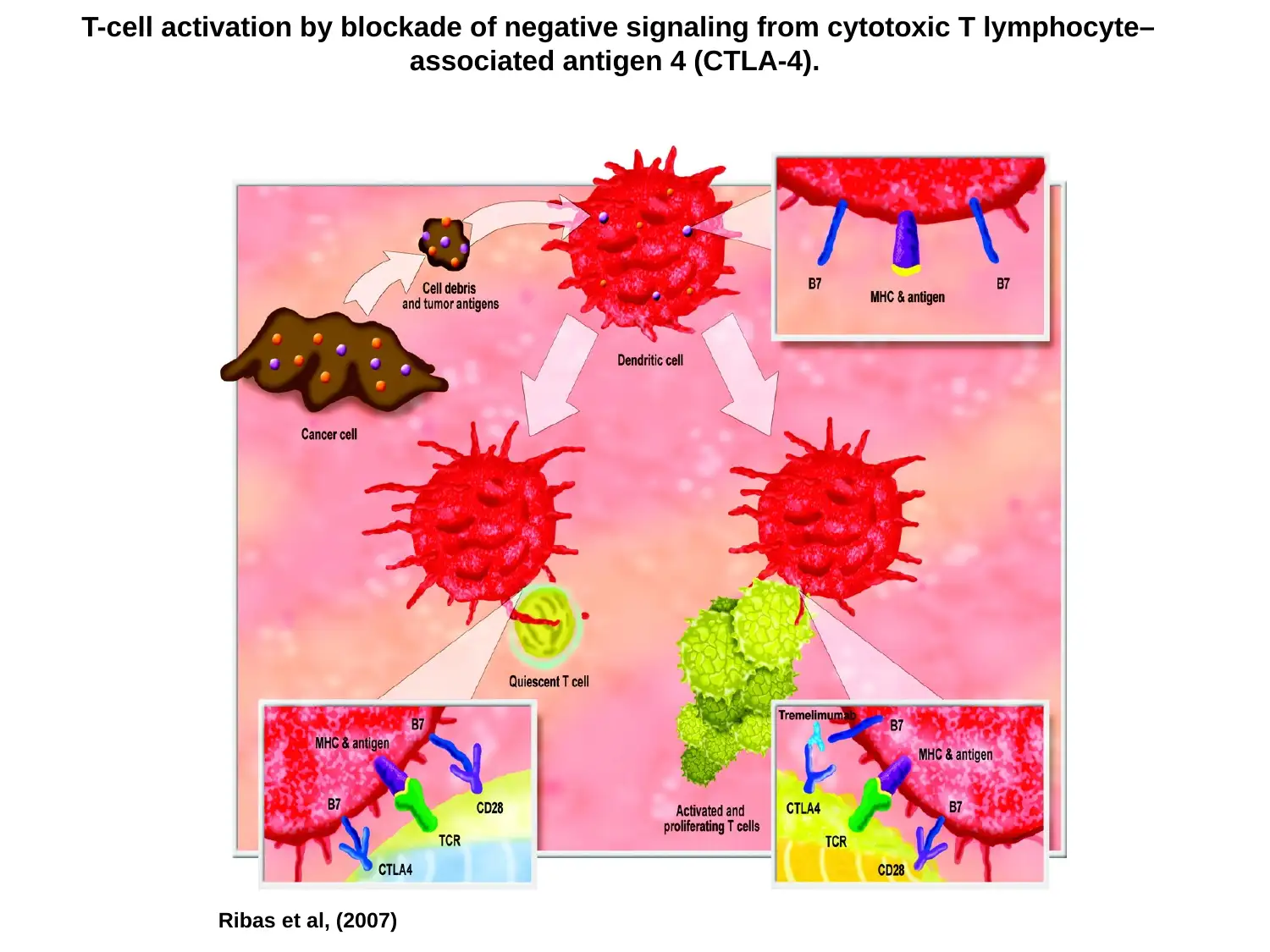
T-cell activation by blockade of negative signaling from cytotoxic T lymphocyte–
associated antigen 4 (CTLA-4).
Ribas et al, (2007)
associated antigen 4 (CTLA-4).
Ribas et al, (2007)
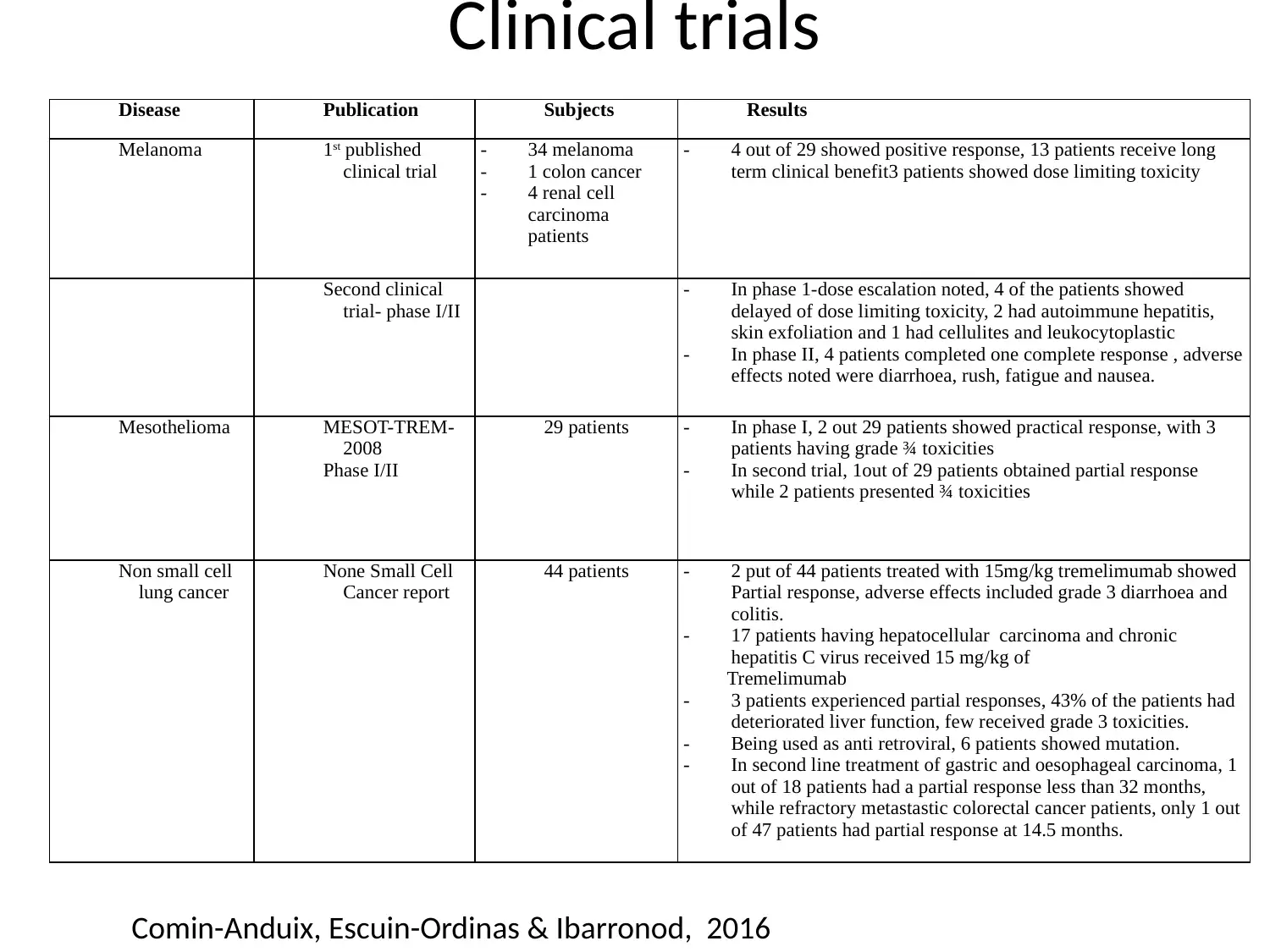
Clinical trials
Comin-Anduix, Escuin-Ordinas & Ibarronod, 2016
Disease Publication Subjects Results
Melanoma 1st published
clinical trial
- 34 melanoma
- 1 colon cancer
- 4 renal cell
carcinoma
patients
- 4 out of 29 showed positive response, 13 patients receive long
term clinical benefit3 patients showed dose limiting toxicity
Second clinical
trial- phase I/II
- In phase 1-dose escalation noted, 4 of the patients showed
delayed of dose limiting toxicity, 2 had autoimmune hepatitis,
skin exfoliation and 1 had cellulites and leukocytoplastic
- In phase II, 4 patients completed one complete response , adverse
effects noted were diarrhoea, rush, fatigue and nausea.
Mesothelioma MESOT-TREM-
2008
Phase I/II
29 patients - In phase I, 2 out 29 patients showed practical response, with 3
patients having grade ¾ toxicities
- In second trial, 1out of 29 patients obtained partial response
while 2 patients presented ¾ toxicities
Non small cell
lung cancer
None Small Cell
Cancer report
44 patients - 2 put of 44 patients treated with 15mg/kg tremelimumab showed
Partial response, adverse effects included grade 3 diarrhoea and
colitis.
- 17 patients having hepatocellular carcinoma and chronic
hepatitis C virus received 15 mg/kg of
Tremelimumab
- 3 patients experienced partial responses, 43% of the patients had
deteriorated liver function, few received grade 3 toxicities.
- Being used as anti retroviral, 6 patients showed mutation.
- In second line treatment of gastric and oesophageal carcinoma, 1
out of 18 patients had a partial response less than 32 months,
while refractory metastastic colorectal cancer patients, only 1 out
of 47 patients had partial response at 14.5 months.
Comin-Anduix, Escuin-Ordinas & Ibarronod, 2016
Disease Publication Subjects Results
Melanoma 1st published
clinical trial
- 34 melanoma
- 1 colon cancer
- 4 renal cell
carcinoma
patients
- 4 out of 29 showed positive response, 13 patients receive long
term clinical benefit3 patients showed dose limiting toxicity
Second clinical
trial- phase I/II
- In phase 1-dose escalation noted, 4 of the patients showed
delayed of dose limiting toxicity, 2 had autoimmune hepatitis,
skin exfoliation and 1 had cellulites and leukocytoplastic
- In phase II, 4 patients completed one complete response , adverse
effects noted were diarrhoea, rush, fatigue and nausea.
Mesothelioma MESOT-TREM-
2008
Phase I/II
29 patients - In phase I, 2 out 29 patients showed practical response, with 3
patients having grade ¾ toxicities
- In second trial, 1out of 29 patients obtained partial response
while 2 patients presented ¾ toxicities
Non small cell
lung cancer
None Small Cell
Cancer report
44 patients - 2 put of 44 patients treated with 15mg/kg tremelimumab showed
Partial response, adverse effects included grade 3 diarrhoea and
colitis.
- 17 patients having hepatocellular carcinoma and chronic
hepatitis C virus received 15 mg/kg of
Tremelimumab
- 3 patients experienced partial responses, 43% of the patients had
deteriorated liver function, few received grade 3 toxicities.
- Being used as anti retroviral, 6 patients showed mutation.
- In second line treatment of gastric and oesophageal carcinoma, 1
out of 18 patients had a partial response less than 32 months,
while refractory metastastic colorectal cancer patients, only 1 out
of 47 patients had partial response at 14.5 months.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Efficacy and Safety
• Tremilumab has not yet been approved but the
US Food and Drug Administration (FDA) to
manage cancer diseases.
• In the year 2015, it received orphan drug
designation to treat mesotherlima, this allowed
the pharmaceutical companies to initiate through
approval process.
• Orphan designation does not guarantee safe drug
or effective administration effect to the patient.
• Tremilumab has not yet been approved but the
US Food and Drug Administration (FDA) to
manage cancer diseases.
• In the year 2015, it received orphan drug
designation to treat mesotherlima, this allowed
the pharmaceutical companies to initiate through
approval process.
• Orphan designation does not guarantee safe drug
or effective administration effect to the patient.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Side effects
• Side effects have noted with mesothelioma
therapy has been identified and poses life
threatening side effects. It has been observed
to cause side effects such creation of holes on
intestines, small intestine obstruction, colon
inflammation and skin ulcers.
(Eroglu et al., 2015)
• Side effects have noted with mesothelioma
therapy has been identified and poses life
threatening side effects. It has been observed
to cause side effects such creation of holes on
intestines, small intestine obstruction, colon
inflammation and skin ulcers.
(Eroglu et al., 2015)
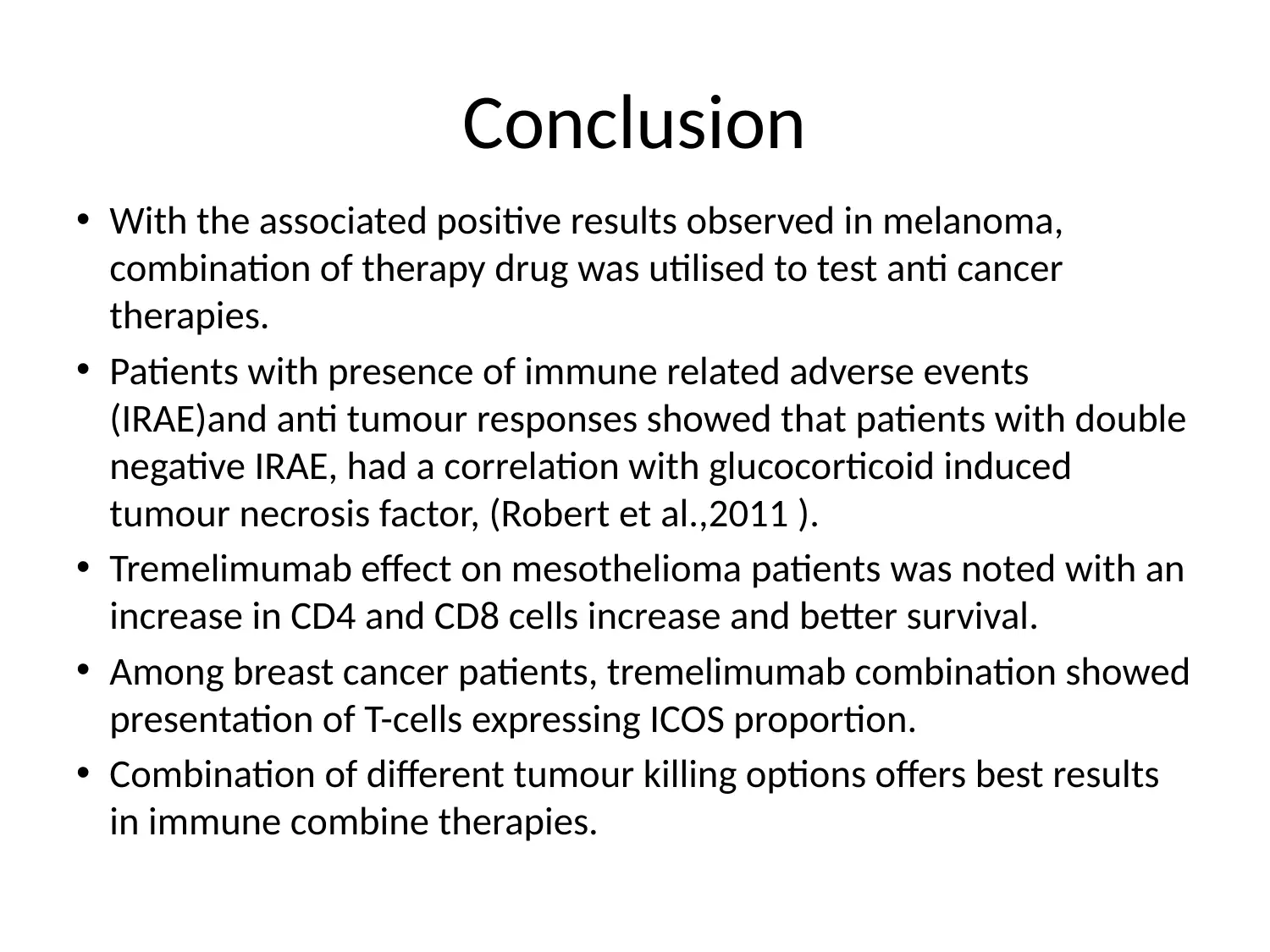
Conclusion
• With the associated positive results observed in melanoma,
combination of therapy drug was utilised to test anti cancer
therapies.
• Patients with presence of immune related adverse events
(IRAE)and anti tumour responses showed that patients with double
negative IRAE, had a correlation with glucocorticoid induced
tumour necrosis factor, (Robert et al.,2011 ).
• Tremelimumab effect on mesothelioma patients was noted with an
increase in CD4 and CD8 cells increase and better survival.
• Among breast cancer patients, tremelimumab combination showed
presentation of T-cells expressing ICOS proportion.
• Combination of different tumour killing options offers best results
in immune combine therapies.
• With the associated positive results observed in melanoma,
combination of therapy drug was utilised to test anti cancer
therapies.
• Patients with presence of immune related adverse events
(IRAE)and anti tumour responses showed that patients with double
negative IRAE, had a correlation with glucocorticoid induced
tumour necrosis factor, (Robert et al.,2011 ).
• Tremelimumab effect on mesothelioma patients was noted with an
increase in CD4 and CD8 cells increase and better survival.
• Among breast cancer patients, tremelimumab combination showed
presentation of T-cells expressing ICOS proportion.
• Combination of different tumour killing options offers best results
in immune combine therapies.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References
Comin-Anduix, B., Escuin-Ordinas, H., & Ibarrondo, F. J. (2016). Tremelimumab:
research and clinical development. OncoTargets and therapy, 9, 1767.
Eroglu, Z., Kim, D. W., Wang, X., Camacho, L. H., Chmielowski, B., Seja, E., ... & Hwu,
W. J. (2015). Long term survival with cytotoxic T lymphocyte-associated antigen 4
blockade using tremelimumab. European Journal of Cancer, 51(17), 2689-2697.
Ribas, A., Hanson, D. C., Noe, D. A., Millham, R., Guyot, D. J., Bernstein, S. H., ... &
Gomez-Navarro, J. (2007). Tremelimumab (CP-675,206), a cytotoxic T
lymphocyte–associated antigen 4 blocking monoclonal antibody in clinical
development for patients with cancer. The oncologist, 12(7), 873-883.
Robert, C., Thomas, L., Bondarenko, I., O'day, S., Weber, J., Garbe, C., ... &
Davidson, N. (2011). Ipilimumab plus dacarbazine for previously untreated
metastatic melanoma. New England Journal of Medicine, 364(26), 2517-2526.
Comin-Anduix, B., Escuin-Ordinas, H., & Ibarrondo, F. J. (2016). Tremelimumab:
research and clinical development. OncoTargets and therapy, 9, 1767.
Eroglu, Z., Kim, D. W., Wang, X., Camacho, L. H., Chmielowski, B., Seja, E., ... & Hwu,
W. J. (2015). Long term survival with cytotoxic T lymphocyte-associated antigen 4
blockade using tremelimumab. European Journal of Cancer, 51(17), 2689-2697.
Ribas, A., Hanson, D. C., Noe, D. A., Millham, R., Guyot, D. J., Bernstein, S. H., ... &
Gomez-Navarro, J. (2007). Tremelimumab (CP-675,206), a cytotoxic T
lymphocyte–associated antigen 4 blocking monoclonal antibody in clinical
development for patients with cancer. The oncologist, 12(7), 873-883.
Robert, C., Thomas, L., Bondarenko, I., O'day, S., Weber, J., Garbe, C., ... &
Davidson, N. (2011). Ipilimumab plus dacarbazine for previously untreated
metastatic melanoma. New England Journal of Medicine, 364(26), 2517-2526.
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

