CHE 430: Unsteady State Material Balance and Process Control
VerifiedAdded on 2022/10/06
|6
|926
|29
Homework Assignment
AI Summary
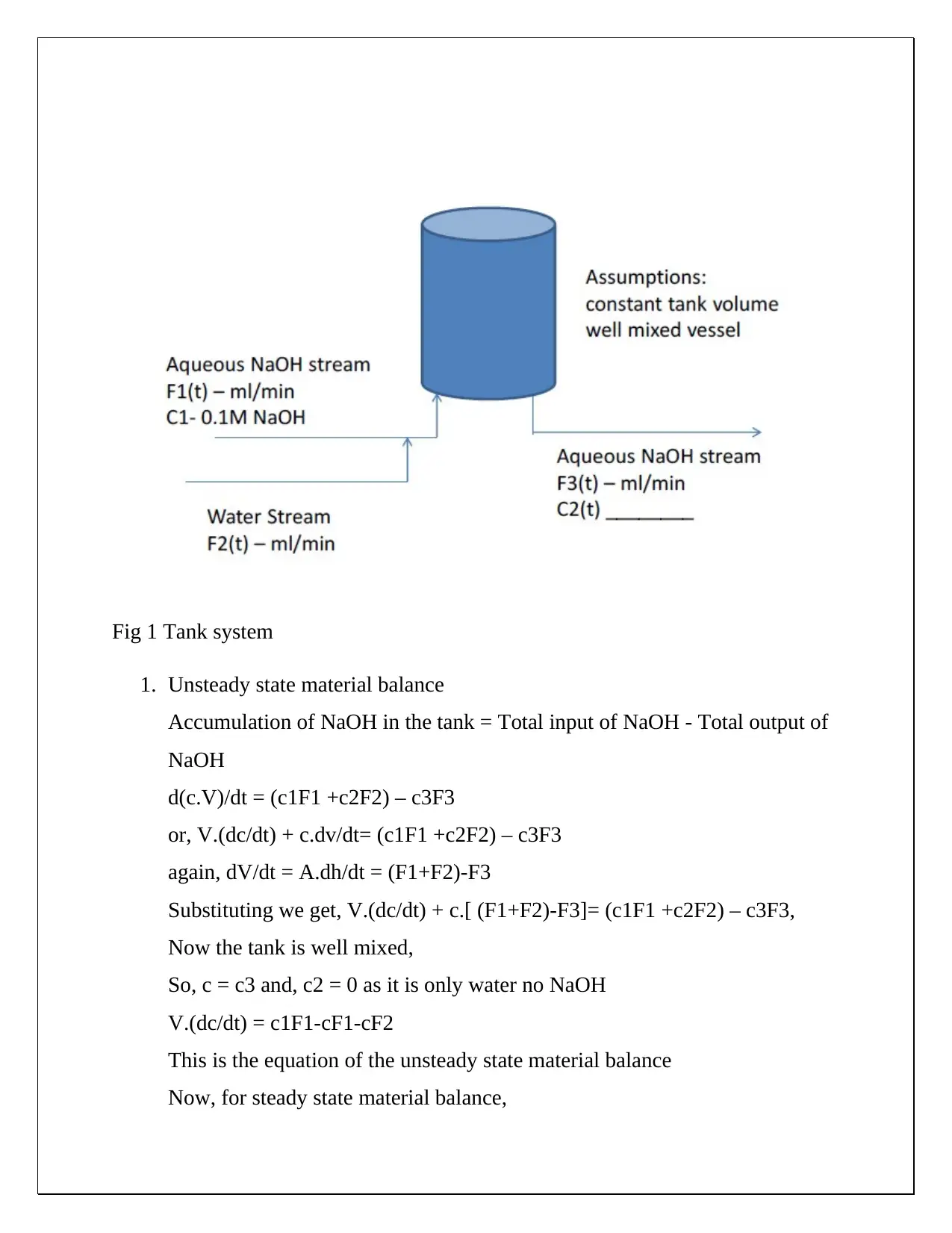
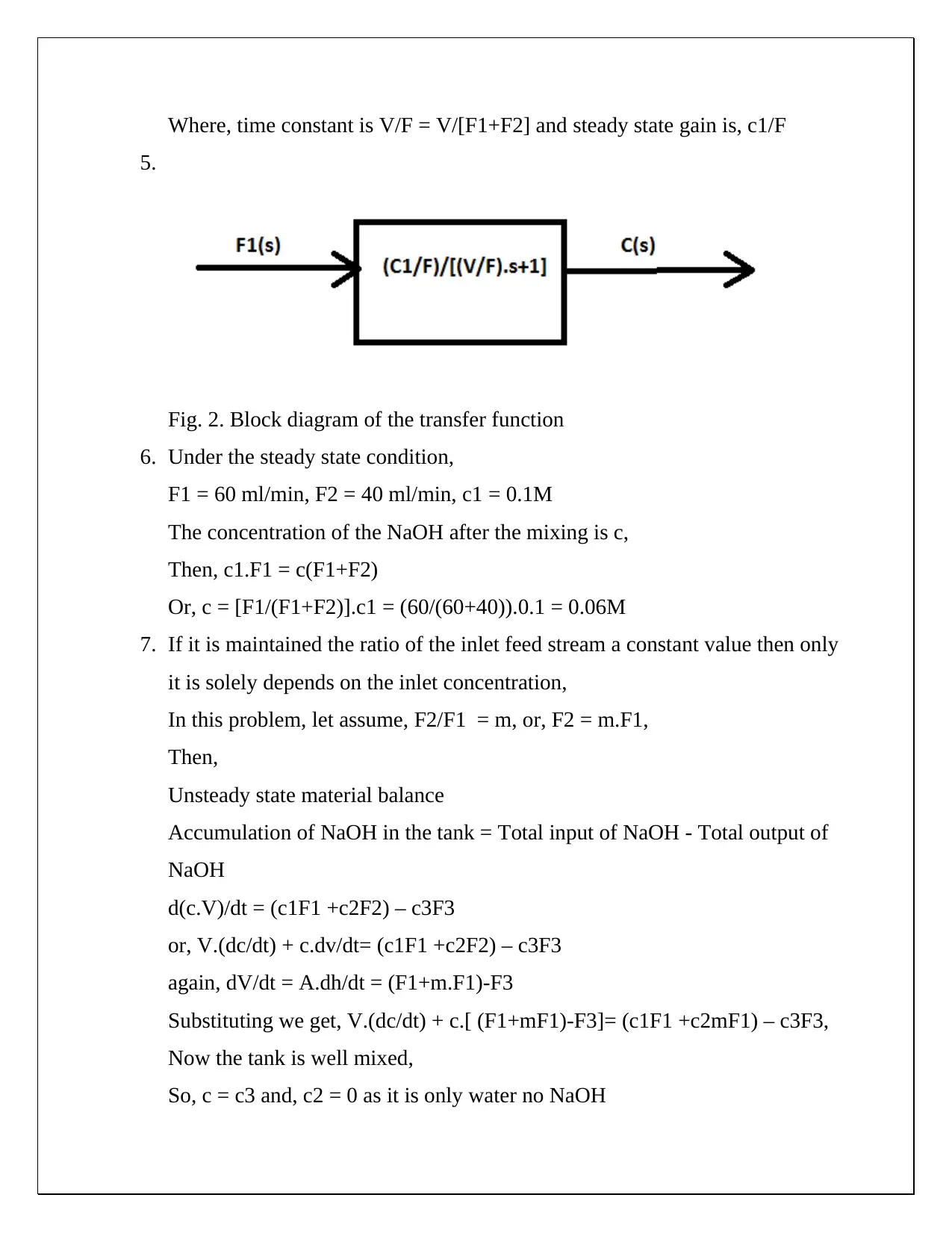
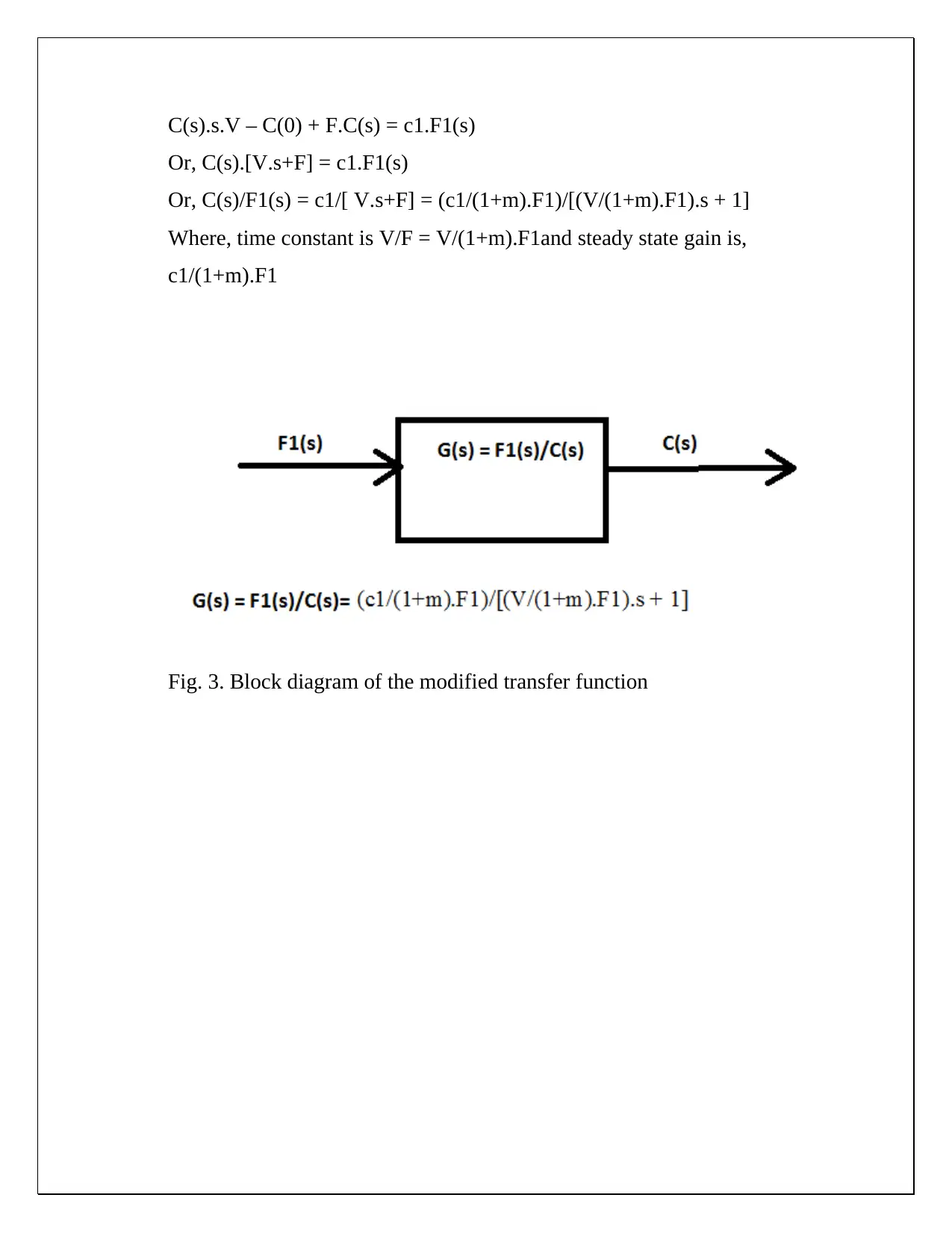
This document presents a comprehensive solution to a CHE 430 pre-lab assignment focused on unsteady state material balance. The solution begins by establishing unsteady and steady-state material balances for NaOH in a tank system, considering input and output flow rates. Non-linear terms are linearized, and deviation variables are defined to simplify the analysis. Laplace transforms are applied to derive transfer functions, steady-state gains, and time constants, allowing for the expression of outlet concentration in terms of flow disturbances. Block diagrams are created to visually represent the transfer functions. Furthermore, the solution calculates steady-state concentrations of NaOH at the mixing point and the mixer effluent under given flow rate and concentration conditions. The document also explores how to modify the process to ensure the outlet concentration depends solely on the inlet concentration. The solution provides detailed equations and calculations for both scenarios, including the derivation of modified transfer functions and block diagrams when the ratio of inlet feed streams is constant. The document demonstrates a strong understanding of chemical engineering principles related to process dynamics and control.
1 out of 6





![[object Object]](/_next/static/media/star-bottom.7253800d.svg)